Abstract
Antibacterial peptide fractions generated via proteolytic processing of snow crab by-products exhibited activity against Gram-negative and Gram-positive bacteria. Among the bacterial strains tested, peptide fractions demonstrated inhibitory activity against the Gram-negative bacteria such as Aeromonas caviae, Aeromonas hydrophila, Campylobacter jejuni, Listonella anguillarum, Morganella morganii, Shewanella putrefasciens, Vibrio parahaemolyticus and Vibrio vulnificus and against a few Gram-positive bacteria such as Listeria monocytogenes, Staphylococcus epidermidis and Streptococcus agalactiae. The principal bioactive peptide fraction was comprised mainly of proteins and minerals (74.3 and 15.5%, respectively). Lipids were not detected. The amino acid content revealed that arginine (4.6%), glutamic acid (5.3%) and tyrosine (4.8%) residues were represented in the highest composition in the antibacterial peptide fraction. The optimal inhibitory activity was observed at alkaline pH. The V. vulnificus strain, most sensitive to the peptide fraction, was used to develop purification methods. The most promising chromatography resins selected for purification, in order to isolate peptides of interest and to carry out their detailed biochemical characterization, were the SP-Sepharose™ Fast Flow cation exchanger and the Phenyl Sepharose™ High Performance hydrophobic interaction media. The partially purified antibacterial peptide fraction was analyzed for minimum inhibitory concentration (MIC) determination, and the value obtained was 25 μg ml−1. Following mass spectrometry analysis, the active peptide fraction seems to be a complex of molecules comprised of several amino acids and other organic compounds. In addition, copper was the main metal found in the active peptide fraction. Results indicate the production of antibacterial molecules from crustacean by-products that support further applications for high-value bioproducts in several areas such as food and health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antimicrobial peptides (AMPs) are known as important components of the innate immune system in a variety of organisms including both vertebrates and invertebrates [6, 22]. Generally, AMPs contain 15–45 amino acid residues [7] and possess cationic and amphipathic properties, which allow for interactions with the membrane of cells in order to neutralize and kill pathogenic microorganisms [28, 31, 32]. Although there are extensive studies on AMPs, only a few have been isolated and completely characterized from crustaceans [17, 24, 37] even though these organisms, living in a microbe-rich oceanic environment, are capable of producing AMPs as a first-line defense system [30, 33].
Schnapp et al. [35] reported in 1996 the isolation and the partial sequencing of a proline-rich 6.5-kDa antibacterial peptide from the shore crab Carcinus maenas while Relf et al. [33] isolated a cysteine-rich 11.5-kDa polypeptide from the same crab species in 1999. Another characterization was the penaeidin family of AMPs from the Pacific white shrimp Litopenaeus vannamei [11]. Thus, using the molecular approach of expressed sequence tag (EST) analysis, putative homologues of the 11.5-kDa polypeptide (from C. maenas) and the penaeidins have been characterized in other shrimp species, including L. setiferus [2, 15], Penaeus monodon [38] and P. japonicus [34]. Callinectin, a cationic 3.7-kDa peptide, was isolated and partially characterized from the blue crab, Callinectes sapidus [23]. Additionally, some peptide fragments generated from the C-terminal part of crustacean hemocyanin have been shown to possess antibacterial activities such as the cationic 1.9-kDa peptide named astacidin 1 [24]. Haug et al. [17] detected high antibacterial activity against several bacterial strains in haemocyte extracts of the spider crab, Hyas araneus, and further isolated and purified AMPs from these extracts [17, 37]. Also, Herbinière et al. [19] reported the isolation and the characterization of armadillin, a novel glycine-rich antibacterial peptide from the woodlouse Armadillidium vulgare (terrestrial isopod, crustacean). More recently, the isolation and characterization of two AMPs from the American lobster, Homarus americanus, were reported [3]. In most of the crustacean species studied so far, AMPs have shown cationic properties and have been located in the hemolymph and/or in the hemocytes [18]. Interestingly, antifungal peptides were generated from the C-terminus of shrimp hemocyanin which presented a negative net charge [12]. To our knowledge, only one other novel anionic antibacterial peptide, named scygonadin, was recently isolated from the seminal plasma of the mud crab, Scylla serrata [41].
The increase in antibiotic-resistant pathogenic bacteria has stimulated the search for alternative antibacterial agents from natural sources [18, 27]. Many antibacterial peptides show a high specificity for prokaryotes and a low toxicity for eukaryotic cells [36, 42]. Their mechanism of action is still largely unknown, although several seem to act by forming pores in the cell membrane and making susceptible bacteria leaky [13] allowing for many interesting activities (antibiotic, fungicidal, virucidal and tumoricidal). Based on these facts, such peptides are being developed for use as a novel class of antimicrobial agents, which could be extended to various food, biomedical and health applications [28].
Even though AMPs were isolated from other crustaceans, here we report, for the first time, the presence of AMPs originating from hydrolysates of snow crab by-products. In previous work published from our laboratory, it was mentioned that many bioactive compounds fractionated from snow crab by-products should be further characterized for their potential applications [4]. Hence, the detection of antibacterial activity in enzymatic hydrolyzed snow crab by-products fractions was performed using reference and environmental bacterial strains of interest in the food and health sectors. The most sensitive bacterial strain was used to pursue the development of more specific purification methods. The method developed was used for the purification of peptides of interest and to carry out their detailed biochemical characterization (molecular weight, amino acid composition). Determination of mineral elements and characterization of organic compounds, in the active partially purified fraction, were performed by several analyses using mass spectrometry.
Materials and Methods
Enzymatic Hydrolyzed Fractions of Snow Crab By-Products
Snow crab hydrolysate fractions were produced at the Aquatic Products Technology Centre (CTPA, MAPAQ, Gaspé, QC, Canada) according to the procedure described previously [4]. Briefly, 100 kg of water was added to 100 kg of grinded snow crab by-products (cephalothorax shells, digestive systems including hepatopancreas and hemolymph), the total volume was heated to 40°C, and 100 g of the proteolytic enzymes blend Protamex® was added to start the hydrolysis. After 60-min hydrolysis at 40°C, the temperature in the tank was gradually increased to a temperature of 85°C to inactivate the proteases before the liquid content was decanted and run through a two-phase separator to isolate insoluble fraction from the aqueous fraction. The aqueous fraction was centrifuged in order to separate lipids from proteins. The protein solution was then passed through multiple filtration steps to separate the proteins by molecular weight: 50-kDa retentate (>50 kDa), 1-kDa retentate (50–1 kDa), nano-filtration retentate (1 kDa–200 Da) and reversed osmosis retentate (<200 Da). Final fractions were kept frozen until analyses (−20°C).
Bacterial Strains and Growth Media
Reference strains used in this study are listed in Table 1. All strains were maintained in nutrient media with 10% glycerol at −80°C. Before the experiments, strains were sub-cultured at least two times, in their respective media.
Determination of the Inhibitory Spectrum
The resulting protein hydrolysate fractions, crude and concentrated (fivefold) by evaporation using a Titan vapor trap (Jouan, Winchester, USA), were evaluated for antibacterial activity against 24 bacterial strains on the basis of a slightly modified agar well diffusion assay described by Himelbloom et al. [20]. Briefly, each overnight culture was diluted 1:10 and 2 ml used to seed 200 ml of the selected culture media containing 0.75% (w/v) agar, corresponding to approximately 5 × 105 cells/ml. The plates were prepared by pouring the inoculated media and allowed to solidify, and 6-mm wells were punched. Aliquots (80 μl) of the peptide samples were added to the wells. The plates were incubated at 4°C for 2 h to permit diffusion into the solid media and thereafter at the appropriate temperature for 18 h. Clear zones around the wells indicated antibacterial activity. One bioactive peptide fraction (nano-filtration retentate; 1 kDa–200 Da) was selected for further analyses, based on this antibacterial activity.
Bioactive Peptide Fraction Analyses
Chemical Composition Determination
Initial bioactive peptide fraction (nano-filtration retentate; 1 kDa–200 Da) was characterized to determine its chemical composition as described previously [4]. Moisture and minerals (ash) were measured using the official methods of analysis of the Association of Official Analytical Chemists (No. 950.46 and No. 938.08) [1]. Lipids were analyzed using a method based on the Bligh and Dyer method [6]. Crude proteins were determined by the Kjeldahl method (nitrogen × 6.25) adapted from the official method of A.O.A.C. No. 988.05 [1].
Amino Acid Analyses
Two different methods were used for amino acid determination of the initial bioactive peptide fraction as described previously [4]. The EZ: faast amino acid analysis procedure was used for tryptophan determination requiring an alkaline hydrolysis. The EZ: faast amino acid analysis procedure (Phenomenex, Torrance, CA, USA) was used and consisted of a solid-phase extraction step followed by derivatization and liquid/liquid extraction. Derivatized samples were analyzed by gas chromatography with flame ionization detection (FID). The AccQ-Tag amino acid analysis procedure (Waters, Mississauga, ON, Canada) was used for the determination of amino acids resisting to acidic hydrolysis (including taurine). The AccQ-Tag method is a pre-column derivatization technique for peptide and protein hydrolysate amino acids. The amino acids were separated by reversed-phase high-performance liquid chromatography (HPLC) and quantified by fluorescence detection.
pH Studies
In order to measure peptide fractions (nano-filtration retentate; 1 kDa–200 Da) activity as a function of pH, fractions concentrated (fivefold) using a Titan vapor trap (Jouan, Winchester, USA), at a starting pH of 7.7, were adjusted with 2 N NaOH or 1 N HCl at pH 5, 6, 7, 8 and 9. Antibacterial activity was evaluated for each fraction using agar diffusion test (80 μl) as described previously. The bacterial strain Vibrio vulnificus LEA GN38 was used as sensitive strain.
Purification Process
The initial developmental work was performed using 1-ml resin columns. Several ion exchange chromatography (IEX) and hydrophobic interaction chromatography (HIC) resins were tested using HiTrap™ IEX Selection Kit and HiTrap™ HIC Selection Kit (GE Healthcare, Baie d’Urfé, QC, Canada). Bioactive peptide purification at a larger scale was achieved using 20-ml resin XK16/20 columns (GE Healthcare, Baie d’Urfé, QC, Canada) in order to generate more bioactive peptide material. Following each purification step, the antibacterial activity was followed by agar well diffusion assay and by size exclusion chromatography against V. vulnificus LEA GN38 . The scale-up of the purification was performed as follows:
Ion Exchange Chromatography
Bioactive fraction from the snow crab processing (580 ml), previously filtered (1.2-μm Glass microfibre filters, 70 mm, Whatman, VWR Canlab, Mississauga, ON, Canada; 0.45-μm HAWP nitrocellulose membrane filters, Millipore, Bedford, MA, USA) and adjusted at pH 9 with 2 N NaOH, was captured using a SP-Sepharose™ Fast Flow cation exchange column (GE Healthcare, Baie d’Urfé, QC, Canada). The column (GE Healthcare, Baie d’Urfé, QC, Canada) was connected to a FPLC system (AKTÄ Explorer 100, GE Healthcare, Baie d’Urfé, QC, Canada). The system was equipped with UV (215, 254, 280 nm), conductivity and pH monitors, which were connected to an autosampler and a fraction collector. The chromatograms were obtained and analyzed using the UNICORN 5.0 software. The column was equilibrated with 10 CV (CV = column volume) of 50 mM bicine buffer at pH 9 (buffer A). The peptide solution was applied to the column at a rate of 156 cm/h; then, the column was washed successively with buffer A alone and buffer A containing 1 M NaCl. Bioactive peptide fractions were eluted from the column with buffer A.
Hydrophobic Interaction Chromatography
The active fractions collected from the SP-Sepharose column were pooled (1,046 ml), and salt was added in order to obtain a concentration of 1 M NaCl. This solution was then applied to a Phenyl Sepharose™ High Performance hydrophobic interaction column (GE Healthcare, Baie d’Urfé, QC, Canada) connected to the same FPLC system (AKTÄ Explorer 100, GE Healthcare, Baie d’Urfé, QC, Canada). The column was equilibrated with buffer A containing 1 M NaCl. The peptide solution was applied to the hydrophobic interaction chromatography (HIC) column at the rate of 156 cm/h; the column was washed with the same equilibration buffer, followed by a second wash with buffer A. Bioactive peptide fractions were eluted with buffer A. The active fractions were pooled (275 ml) and concentrated (fivefold) using a Titan vapor trap (Jouan, Winchester, VI, USA). The material was stored at 4°C.
Analytical Methods
Total Protein Assay
Protein concentration in the purified fractions was determined using the Coomassie Plus Protein Assay kit (Pierce, Rockford, IL, USA). Bovine serum albumin was used as standard (bovine serum albumin, fraction V, Pierce, Rockford, IL, USA).
Molecular Weight Distribution
Molecular weight distributions of bioactive peptide fractions (crude and purified) were determined by size exclusion chromatography (SEC) on a Superdex Peptide HR 10/300 GL column (GE Healthcare, Baie d’Urfé, QC, Canada) using a FPLC system (AKTÄ Explorer 100, GE Healthcare, Baie d’Urfé, QC, Canada) based on the method described previously [4]. The mobile phase (isocratic) consisted of 50 mM sodium phosphate buffer containing 150 mM of NaCl at pH 7.0. Samples were eluted at a flow rate of 0.5 ml/min. The sample size loaded onto the SEC column was 50 μl, corresponding to approximately 50 μg of proteins/peptides according to the total protein assay described previously. Protein hydrolysates analyzed on the Superdex Peptide HR 10/300 GL column were detected by monitoring absorbance at 215 nm.
Minimum Inhibitory Concentration Determination of the Purified Fraction
Inhibitory activity measurement of the purified fraction was performed using a critical microdilution method [10] with slight modifications. Briefly, the purified sample (200 μg ml−1) was sterilized by filtration on a Millex PVDF Durapore 0.45-μm filter. Twofold serial dilutions of the sample were made in duplicate by repeated transfers of 125 μl in 125-μl volumes of Tryptic Soy Broth (TSB) supplemented with 0.08% cellobiose (w/v), 1.0% NaCl (w/v) and 2.0% Bacto-peptone (w/v) in a 96-well Falcon 3072 flat-bottom polystyrene microplate (Becton–Dickinson Labware, Franklin Lakes, NJ, USA). Each well was inoculated with 50 μl of an overnight culture of the indicator strain V. vulnificus LEA GN38 precisely adjusted at 2 × 105 CFU/ml. The microplate was placed in a Multiskan Ascent microplate reader (Labsystems, Helsinki, Finland) and incubated at 35°C for 16 h. Optical density (OD) was monitored at 600 nm every 10 min. The minimal inhibitory concentration (MIC) was defined as the lowest sample concentration that resulted in the absence of turbidity (OD600nm equal to negative control) after 16 h of incubation.
The mode of action of the purified sample was assessed according to Tome et al. [40] by adding 35 μl of sample in the inoculated wells after 5 and 6 h during the exponential growth phase in the microplate reader, followed by regular OD600nm readings.
Determination of Copper and Other Elements in Bioactive Peptide Fractions by Inductively Coupled Plasma-Mass Spectrometry
Metals and metalloids were measured in bioactive peptide fractions (crude and purified) diluted with acidified ultrapure water (NANOpure Infinity system, Barnstead Int., Dubuque, IO, USA) by inductively coupled plasma interfaced to a quadrupole mass spectrometer (ICP-MS, Agilent 7500c, Agilent, Japan) equipped with a micro flow nebuliser. The element quantification was performed in normal mode with a seven point’s calibration plot of standard solution Multi-Element IV (Fluka Chemie Gmbh, Switzerland) with a concentration range from 0.125 to 200 ng ml−1 for the heavy metals, arsenic and selenium. Potassium, calcium and magnesium were quantified with a seven point’s calibration plot of standard solution Trace metal III (JT Baker, Philisburg, NJ, USA) with a concentration range from 31 to 1,000 ng ml−1. Performance of the method was assessed by the analysis of a quality control (QC) solution of known metal concentration and a procedural blank. Control of the system, acquisition and data processing were carried out with the Agilent 7500 ICP-MS ChemStation® software (revision C).
Characterization of Organic Compounds in the Active Fraction by Electrospray Ionization-Mass Spectrometry (ESI–MS) and Liquid Chromatography-Electrospray Ionization-Mass Spectrometry
Structural information of the main compounds present in the active fraction was studied by mass spectrometry with electrospray ionization (ESI) with sample introduction by direct infusion or following chromatographic separation by liquid chromatography. The LC–MS system consisted of a liquid chromatograph Surveyor LC coupled via an atmospheric pressure ionization interface to an ion trap mass spectrometer with MSn capabilities (Surveyor LC linked to a LCQ Advantage, ThermoFinnigan, San Jose, CA, USA). Direct infusion was achieved by diluting the active fraction in water and infusing directly the solution in the ESI source via the syringe pump. Detection of ions was performed in positive mode with a mass scan range from 60 to 2,000 amu. In MSMS (MS2) experiment, the precursor ion (m/z = 578) was fragmented with a collision-induced dissociation energy of 40 (arbitrary unit) and the positive product ions were scanned from 155 to 600 amu. Chromatography was performed on a Discovery RP-C18 5 cm × 2.1 mm column (Supelco, Bellefonte, PA, USA), equipped with a pre-column, with an isocratic elution with 96% water and 4% acetonitrile in 6 min with a flow rate of 0.4 ml min−1. Injection volume of the active fraction diluted in water was 10 μl in full loop mode. Detection of ions was performed in positive mode with a mass scan range from 100 to 2,000 amu. System control, data acquisition and data processing were performed with the Xcalibur 1.3 software (ThermoFinnigan, San Jose, CA, USA).
Qualitative Analysis of Copper Associated to Organic Compounds in the Active Fraction by LC-ICP-MS
Distribution of copper associated with organic molecules was determined using the same RP-C18 column and chromatographic conditions as in liquid chromatography-electrospray ionization-mass spectrometry (LC–ESI–MS), on a LC1100 liquid chromatograph (Agilent, Japan) coupled to the Agilent 7500c ICP-MS equipped with a micro flow nebuliser. The signal at a mass-to-charge ratio of 63 was monitored for the detection of copper. Control of the system, acquisition and data processing were carried out with the Agilent 7500 ICP-MS ChemStation® software (revision C).
Results
Detection of Antibacterial Activity in Enzymatic Hydrolyzed Snow Crab By-Product Fractions
All snow crab hydrolysate fractions previously produced [4] were analyzed for the determination of their inhibitory spectrum against bacterial strains of interest in environmental, food and health sectors. On the basis of clear zones obtained by the agar well diffusion assay, active peptide fractions were selected (data not shown). Mainly, low molecular weight fractions corresponding to nano-filtration retentate (ranging from 200 Da to 1 kDa) exhibited antibacterial activity against several bacteria. Growth inhibition of many Gram-negative bacteria, such as Aeromonas caviae, Aeromonas hydrophila, Campylobacter jejuni, Listonella anguillarum, Morganella morganii, Shewanella putrefasciens, Vibrio parahaemolyticus and Vibrio vulnificus, was noted as well as for a few Gram-positive bacteria, such as Listeria monocytogenes, Staphylococcus epidermidis and Streptococcus agalactiae (Table 1).
Bioactive Peptide Fraction Analyses
The antibacterial peptide fraction (nano-filtration retentate) was characterized to determine its chemical composition (moisture, ash, crude proteins and lipids). As seen in a previous work [4], this fraction is comprised mainly of proteins and minerals (74.3 and 15.5%, respectively), expressed on a dry weight basis, and lipids were not detected.
The antibacterial peptide fraction was also analyzed for its amino acid content. The total amino acids measured in the nano-filtration retentate fraction were determined in previous work [4]. The three most important amino acids were arginine (4.6%), glutamic acid (5.3%) and tyrosine (4.8%) residues, which are amino acids with polar side chains (including not charged and charged). The content of residues with polar side chains was higher (27.0%) than that of other amino acid residues with non-polar side chains (23.5%).
An absence of inhibition zone in the agar diffusion assay was noticed at pH 5. All other pH values demonstrated the presence of inhibition zone. However, antibacterial activities increased at alkaline pH (Table 2).
Purification Process
The initial developmental work for the purification process was performed using 1-ml resin columns. Several ion exchange chromatography (IEX) and hydrophobic interaction chromatography (HIC) resins were tested as described previously. Screening of various resins permitted to develop the peptide purification process which could then be used at a larger scale using 20-ml resin XK16/20 columns in order to generate more bioactive peptide material. IEX and HIC using SP-Sepharose™ Fast Flow cation exchange column and Phenyl Sepharose™ High Performance hydrophobic interaction column appeared as promising purification and polishing steps. Because of the ionic resin used in this study (SP-Sepharose); these antibacterial peptides, recovered in the flow through, seem to possess anionic properties, which are considerably less frequent than cationic properties for these kinds of peptides [28].
Purification results are given in Table 3. The total protein concentration in the starting material (580 ml) was 323 μg ml−1. According to Table 3, approximately 12% of total proteins were lost during the first purification step. Only 7% of total proteins could be recovered after the last purification step. However, even if the total protein concentration was low (50 μg ml−1), biological activity shows an improved inhibition zone (diameter of 10 mm) in comparison with the SP-Sepharose step (diameter of 9 mm), which could indicate that the active peptides may have been concentrated. Only the purified antibacterial peptide fraction was analyzed for MIC determination, and the value obtained was 25 μg ml−1.
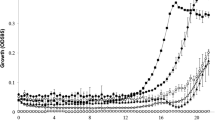
Figure 1 shows the effect of bioactive peptide fraction (Phenyl-Sepharose eluate (248 μg ml−1)) on the growth of V. vulnificus LEA GN38. The addition of 35 μl of the peptide fraction in the wells after 5 h of incubation stopped the growth but did not decrease the optical density at 600 nm (OD600nm). However, the peptide fraction addition in the wells after 6 h rapidly caused a drop in the OD600nm for approximately 1 h and then caused complete growth inhibition.
Effect of adding 35 μl of the partially purified peptide (Phenyl-Sepharose eluate (248 μg/ml)) on the growth of V. vulnificus LEA GN38, after 5 (circles) and 6 (squares) h of incubation; triangles represent control growth of the tested microorganism. Data points represent the average of three experiments run in duplicate
Figure 2 shows the FPLC profile obtained for all purification steps using size exclusion chromatography. The FPLC profile of peptide fraction loaded on the IEX chromatography column: (a) was characterized by the presence of low molecular weight molecules around 800 Da and less. The presence of low molecular weight proteins could be explained by the enzymatic hydrolysis treatment performed during the processing of snow crab by-products [4]. After a first purification step using an IEX column, the FPLC profile was quite similar to the initial one (b) but was characterized by lower protein amounts (Table 3), which could explain the weaker inhibition zone in (Table 3). Finally, the results of the last purification step, using HIC, showed only one peak around 800 Da on the FPLC profile (c), which was active as indicated in Table 3.
Mineral Determination
Following the purification method development, minerals were also analyzed. Minerals constitute an important portion of the starting antibacterial peptide fraction (15.5%, expressed on a dry weight basis) [4]. Table 4 shows minerals present in the antimicrobial peptide fractions following the purification process. The initial antibacterial peptide fraction was rich in K (154 μg g−1), Mg (147 μg g−1) and Cu (65 μg g−1). All mineral contents decreased following each purification steps, but the Cu content recovered within the purified fraction was higher at 3.25 μg g−1 in comparison with other minerals, suggesting a possible role of this metal in antibacterial properties. However, it is unlikely that the antibacterial activity observed was caused solely by the presence of copper in solution, because no antibacterial activity was detected when a copper sulfate solution (CuSO4), at the same concentration, was tested (data not shown).
Characterization of Active Fraction by Mass Spectrometry with Electrospray Ionization
The total ion current chromatogram obtained from the qualitative analysis of the purified active fraction by LC–ESI–MS shows a main peak eluting at 0.77 min (Fig. 3a, upper chromatogram), suggesting a polar compound with low affinity for the reversed phase. However, two compounds are revealed with ion chromatogram traced with two major ions detected with mass-to-charge ratios of 578 and 173 (Fig. 3a, middle and lower chromatograms, respectively). Thus, the active fraction would be comprised of a polar compound eluting at 0.8 min and a more hydrophobic molecule, with a greater affinity to the non-polar C18 stationary phase than to the aqueous solvent at 96%, eluting at 3 min with a large tailing due to the isocratic conditions. As no unambiguous structural information from the mass spectra at both retention times was evidenced, we cannot exclude that these two chromatographic peaks correspond to two groups of organic molecules, with different polarity, that form clusters in the ion source. The direct infusion in the ESI source of the active peptide fraction produced a mass spectrum of positive ions dominated by a repetitive fragmentation pattern (Fig. 4a). The heavier ion in the mass spectrum has a m/z ratio of 1873, and thereafter, a successive loss of 185 is observed down to the ion with a mass of 208. By isolating the ion with m/z = 578 as the precursor ion in MS2 mode, we observed the loss of two 185 units 578 → 393 and 393 → 208 (Fig. 4b). A good candidate to the identity of the 185 unit is the sodium adduct of a hexose (162 + 23) [10]; as discussed by these authors, an oligosaccharide coordinated with a sodium ion will fragment along three main pathways: loss of the metal ion, glycosidic bond cleavage and cross-ring cleavage. The transition 578 → 415 illustrates the loss of a hexose, whereas the transitions 415 → 393 and 230 → 208 illustrate the loss of a Na+ cation. The cross-ring cleavage produces a loss of an ion with m/z = 127 (e.g. 578 → 451, Fig 4a) and also the loss of an ion with m/z = 58 (e.g. 451 → 393, Fig 4a). The ion with m/z = 208 could correspond to a hexose with two sodium atoms as the ESI process is known to favor sodium adduct formation over protonation if background alkali concentration is important [25].
Copper is the main metal found in the active peptide fraction (Table 4). The qualitative analysis by liquid chromatography coupled to ICP-MS shows that copper is associated with the more polar compound or group of compounds, detected under the same chromatographic conditions by LC–ESI–MS (Fig. 3b) at retention time of 0.8 min. The ionization process in the argon plasma of the ICP-MS destroys completely the molecular structure leaving only ionized elements, thus the signal monitored with m/z = 63 can only be attributed to Cu+. No copper was detected within the time interval corresponding to the more hydrophobic group of molecules. Hence, the bioactive peptide fraction purified according to our purification strategy contains amino acids, as explained earlier, but also copper and two groups of organic molecules with different polar characteristics.
Discussion
Detection of Antibacterial Activity in Enzymatic Hydrolyzed Snow Crab By-Product Fractions
Investigations into potential utilization of the fractions produced by enzymatic hydrolysis of snow crab by-products were undertaken in the present study in order to identify and characterize AMPs. A main fraction composed of low molecular weight molecules exhibited antibacterial activity against several bacteria. A major activity was detected against Gram-negative bacteria (A. caviae, A. hydrophila, C. jejuni, L. anguillarum, M. morganii, S. putrefasciens, V. parahaemolyticus and V. vulnificus), while the growth of only a few Gram-positive bacteria was inhibited (L. monocytogenes, S. epidermidis and S. agalactiae). The bacterial strain V. vulnificus was chosen as indicator strain due to the higher and clearer inhibition detected and reproducibility. In addition, Vibrio such as V. vulnificus and V. parahaemolyticus are pathogenic bacteria causing food poisonings. Moreover, the bacterial species Vibrio being widespread in the marine environment and several shell diseases in crustaceans are attributed to Vibrio species [29]. It is possible that the snow crab could develop a defense mechanism against these bacteria. Such biological activity has already been postulated for peptides resulting from the spider crab (Hyas araneus) from the cool waters of Norway [18] as well as for peptides from blue crab (Callinectes sapidus) from the Atlantic Ocean [23]. Furthermore, the microbial flora identified in certain crabs from cool water is composed of Vibrio bacterial strains [14].
Bioactive Peptide Fraction Analyses
The bioactive peptide fraction (nano-filtration retentate) was characterized and was mainly composed of proteins and minerals (74.3 and 15.5%, respectively). According to amino acids analysis, this fraction would contain a strong proportion of amino acids with polar side chains charged (glutamic acid and tyrosine) and uncharged (arginine). AMPs vary substantially in their amino acid sequences. In spite of variations in structure and size, the majority of antimicrobial peptides are amphiphilic, displaying both hydrophilic and hydrophobic surfaces [39].
Cationic antimicrobial peptides are known to possess small numbers of acidic residues (glutamate or aspartate) and excess numbers of cationic (arginine or lysine and/or histidine) residues and around 30–50% hydrophobic residues [16]. Anionic antimicrobial peptides, which are less known in comparison with cationic peptides, possess a considerable amount of acidic residues. The presence of aspartic acid would play an important role in biological activity [9]. Among the major classes of AMPs, there are peptides with an overrepresentation of one or two amino acids [39], which include peptides rich in proline, glycine, cysteine, histidine or tryptophan [28]. Also, the amino acid composition dictates the charge of a protein or a peptide at a given pH. As seen for the bioactive peptide fraction, the biological activity increased at alkaline pH (from 6 to 9 values).
Purification Process and Purified Fractions Analysis
After identifying sensitive bacterial strains and following an initial biochemical characterization of the antibacterial peptide fraction, development of specific purification methods was pursued in order to isolate bioactive peptides. Antibacterial peptides present in this fraction were initially from a complex medium, and a purification step was required to remove other peptides and to minimize the loss of peptides of interest as well as preserving their biological activity. In the case of unknown peptides such as snow crab AMPs, the purification work requires finding the appropriate resin, pH and polarity conditions. AMPs characteristics are known to be mainly cationic and less often anionic, as well as hydrophobic or amphipathic. Bioactive peptide fraction analyses, presented earlier, have helped to establish preliminary conditions.
The resin selected for the purification of snow crab AMPs was the SP-Sepharose™ Fast Flow, a cation exchange resin, which allowed for the separation of cationic and anionic peptides. Active peptides, recovered in the flow through, were then injected on a Phenyl Sepharose™ High Performance hydrophobic interaction column. Following this purification scheme, bioactive peptides seemed to possess anionic and hydrophobic properties. The last purified fraction showed an antimicrobial effect against V. vulnificus LEA GN38 strain, corresponding to a MIC value of 25 μg ml−1, and this effect was due specifically to the presence of molecules of low molecular weight (around 800 Da). The MIC value obtained in the present study is lower in comparison with MIC values obtained for other AMPs originating from hydrolysates of oyster [26], which were most sensitive for Gram-positive bacteria, with MIC values between 40 and 60 μg ml−1.
Other studies have been pursued in order to elucidate the structure and mechanism of action of snow crab AMPs. For instance, the LC–ESI–MS analysis of the active purified fraction demonstrated the presence of more than one peak. It appears that the active peptide fraction is a mixture of amino acids and other organic compounds. Mineral content results in the active fraction demonstrated a potential role of copper in antibacterial properties. The nature of minerals could be important for the antibacterial activity. For instance, the need for co-factors, such as Zn, Cu, Fe and Co, for biological activity is often required for anionic peptides [8, 9]. Since the active peptide fraction was extracted from crustacean biomass and copper is the main metal in the purified fraction (Table 4), one hypothesis could be that the active peptide fraction consisted of glycosylated hemocyanin fragments [21]. Hemocyanins are known as respiratory proteins in the form of metalloproteins containing two copper atoms that reversibly bind a single oxygen molecule (O2) [5]. In addition, some peptide fragments generated from the C-terminal part of crustacean hemocyanin have been shown to possess antimicrobial activities [12, 24]. In most of the crustacean species studied, the antimicrobial activity has been located in the hemolymph and/or in the haemocytes, which were included in the snow crab by-products hydrolysates [4].
Further studies are necessary in order to understand the structure and mechanism of action involved in the antibacterial activity of the active peptide fraction, the identity of the organic molecules involved and the role of copper. The results obtained in this work show, for the first time, that snow crab by-products clearly contain molecules possessing a significant antibacterial activity. The field of study represented here holds the promise for developing new AMPs originated from snow crab by-products. Finding new anti-infectious agent offers a different biocidal mechanism to conventional antibiotics, which are becoming ineffective against the spread of multidrug-resistant pathogens. They can have many applications in public health such as biopreservatives in food, in animal feed, as well as in the medical area.
References
A.O.A.C (2002) Official methods of analysis of the Association of Official Analytical Chemists. 17th edn. In: Horwitz W (ed) Association of official analytical chemists. Washington, Methods no 950.46 and 938.08 and 988.05
Bartlett TC, Cuthbertson BJ, Shepard EF, Chapman RW, Gross PS, Warr GW (2002) Crustins, homologues of an 11.5-kDa antibacterial peptide, from two species of Penaeid shrimp, Litopenaeus vannamei and Litopenaeus setiferus. Mar Biotechnol 4:278–293
Battison AL, Summerfield R, Patrzykat A (2008) Isolation and characterization of two antimicrobial peptides from haemocytes of the American lobster Homarus americanus. Fish Shellfish Immunol. 25:181–187
Beaulieu L, Thibodeau J, Bryl P, Carbonneau M-É (2009) Characterization of enzymatic hydrolysated snow crab (Chionoecetes opilio) by-product fractions: a source of high-valued biomolecules. Biores Technol 100:3332–3342
Beltramini M, Colangelo N, Giomi F, Bubacco L, Di Muro P, Hellmann N, Jeanicke E, Decker H (2005) Quaternary structure and functional properties of Penaeus monodon hemocyanin. FEBS J 272:2060–2075
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Boman HG (2003) Antibacterial peptides: basic facts and emerging concepts. J Intern Med 254:197–215
Brogden KM, Ackermann M, McCray PB, Tack BF (2003) Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents 22:465–478
Brogden KA, De Lucca AJ, Bland J, Elliot S (1996) Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci USA 93:412–416
Cancilla MT, Wong AM, Voss LR, Lebrilla CB (1999) Fragmentation reactions in the mass spectrometry analysis of neutral oligosaccharides. Anal Chem 71:3206–3218
Destoumieux D, Bulet P, Loew D, Dorsselaer AV, Rodriguez J, Bachère E (1997) Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). J Biol Chem 272:28398–28406
Destoumieux-Garzon D, Saulnier D, Garnier J, Jouffrey C, Bulet P, Bachère E (2001) Antifungal peptides are generated from the C-terminus of shrimp hemocyanin in response to microbial challenge. J Biol Chem 276:47070–47077
Elsbach P, Weiss J (1998) Role of the bactericidal/permeability-increasing protein in host defence. Curr Opin Immunol 10:45–49
Faghri MA, Pennington CL, Cronholm LS, Atlas RM (1984) Bacteria associated with crabs from cold waters with emphasis on the occurrence of potential human pathogens. Appl Env Microbiol 47:1054–1061
Gross PS, Bartlett TC, Browdy CL, Chapman RW, Warr GW (2001) Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific white shrimp, Litopenaeus vannamei, and the Atlantic white shrimp, L. setiferus. Dev Comp Immunol 25:565–577
Hancock REW, Brown KL, Mookherjee N (2006) Host defense peptides from invertebrates—emerging antimicrobial strategies. Immunobiol 211:315–322
Haug T (2004) Marine bioprospecting: marine invertebrates and algae—a potential source for the discovery of novel antibiotics. Doctor scientiarum thesis, University of Tromso, Norway
Haug T, Kjuul AK, Stensvag K, Sandsdalen E, Styrvold OB (2002) Antibacterial activity in four marine crustaceans decapods. Fish Shellfish Immunol 12:371–385
Herbinière J, Braquart-Varnier C, Grève P, Strub J-M, Frère J, Dorsselaer AV, Martin G (2005) Armadillidin: a novel glycine-rich antibacterial peptide directed against gram-positive bacteria in the woodlouse Armadillidium vulgare (terrestrial isopod, crustacean). Dev Comp Immunol 29:489–499
Himelbloom B, Nilsson L, Gram L (2001) Factors affecting production of an antilisterial bacteriocin by Carnobacterium piscicola strain A9b in laboratory media and model fish systems. J Appl Microbiol 91:506–513
Idakieva K, Stoeva S, Voelter W, Gielens C (2004) Glycosylation of Rapana thomsiana hemocyanin. Comparison with other prosobranch (gastropod) hemocyanins. Comp Biochem Physiol Part B 138:221–228
Iwanaga S, Lee BL (2005) Recent advances in the innate immunity of invertebrates animals. J Biochem Mol Biol 38:128–150
Khoo L, Robinette DW, Noga EJ (1999) Callinectin, an antibacterial peptide from blue crab, Callinectes sapidus, hemocytes. Mar Biotechnol 1:44–51
Lee SY, Lee BL, Söderhäll K (2003) Processing of an antibacterial peptide from hemocyanin of the freshwater crayfish Pacifastacus leniusculus. J Biol Chem 278:7927–7933
Leitner A, Emmert J, Boerner K, Lindner W (2007) Influence of solvent additive composition on chromatographic separation and sodium adduct formation of peptides in HPLC-ESI MS. Chromatographia 65:649–653
Liu Z, Dong S, Xu J, Zeng M, Song H, Zhao Y (2008) Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control 19:231–235
Marr AK, Gooderham WJ, Hancock REW (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6:468–472
Marshall SH, Arenas G (2003) Antimicrobial peptides: a natural alternative to chemical antibiotics and a potential for applied biotechnology. Elect J Biotechnol 6:271–284
Noga EJ, Smolowitz R, Khoo LH (2000) Pathology of shell disease in the blue crab, Callinectes sapidus Rathbun, (Decapoda: Portunidae). J Fish Dis 23:389–399
Patat SA, Carnegie RB, Kingsbury C, Gross PS, Chapman R, Schev KL (2004) Antimicrobial activity of histones from hemocytes of the Pacific white shrimp. Eur J Biochem 271:4825–4833
Patrzykat A, Douglas SE (2003) Gone gene fishing: how to catch novel marine antimicrobials. Trends Biotechnol 21:362–369
Powers JPS, Hancock REW (2003) The relationship between peptide structure and antibacterial activity. Peptides 24:1681–1691
Relf JM, Chisholm JRS, Kemp GD, Smith VJ (1999) Purification and characterization of a cystein-rich 11.5-kDa antibacterial protein from the granular haemocytes of the shore crab, Carcinus maenas. Eur J Biochem 264:350–357
Rojtinnakorn J, Hirono I, Itami T, Takahashi Y, Aoki T (2002) Gene expression in haemocytes of kuruma prawn, Penaeus japonicus, in response to infection with WSSV by EST approach. Fish Shellfish Immun 13:69–83
Schnapp D, Kemp GD, Smith VJ (1996) Purification and characterization of a proline-rich antibacterial peptide, with sequence similarity to bactenecin-7, from the haemocytes of the shore crab, Carcinus maenas. Eur J Biochem 240:532–539
Smith VJ, Chisholm JRS (2001) Antimicrobial proteins in crustaceans. Adv Exp Med Biol 484:95–112
Stensvag K, Haug T, Sperstad SV, Rekdal O, Indrevoll B, Styrvold OB (2008) Arasin 1, a proline-arginine-rich antimicrobial peptide isolated from the spider crab, Hyas araneus. Dev Comp Immunol 32:275–285
Supungul P, Klinbunga S, Pichyangkura R, Jitrapakdee S, Hirono I, Aoki T, Tassanakajon A (2002) Identification of immune-related genes in hemocytes of black tiger shrimp (Penaeus monodon). Mar Biotechnol 4:487–494
Tincu JA, Taylor SW (2004) Antimicrobial peptides from marine invertebrates. Antimicrob Agents Ch 48:3645–3654
Tome E, Gibbs PA, Teixeira PC (2008) Growth control of Listeria innocua 2030c on vacuum-packaged cold-smoked salmon by lactic acid bacteria. Int J Food Microbiol 121:285–294
Wang KJ, Huang WS, Yang M, Chen HY, Bo J, Li SJ, Wang GZ (2007) A male-specific expression gene, encodes a novel anionic antimicrobial peptide, scygonadin, in Scylla serrata. Mol Immunol 44:1961–1968
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Acknowledgments
The authors wish to thank E. Gagnon et Fils Lté (Grande-Rivière, Québec, Canada) for providing the snow crab by-products, Mr. Piotr Bryl, Mrs. Marie-Élise Carbonneau, Mrs. Nadine Renaud, Mr. Michel Parisé and Mrs. Diane Ouellet (Aquatic Products Technology Centre, Gaspé, Québec, Canada) for their scientific input and technical expertise. We thank Mrs. Christine Barthe (MAPAQ) for providing several bacterial strains. Thanks also to Mrs Maria Pacheco-Oliver (Biotechnology Research Institute, National Research Council, BRI-NRC) for her presubmission review of this paper. Lucie Beaulieu would like to thank the Innovation and Technologies Directorate of Ministère de l’agriculture, des pêcheries et de l’alimentation (MAPAQ, Québec, Canada) and Ministère du développement économique, de l’innovation et de l’exportation (MDEIE, Québec, Canada) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beaulieu, L., Thibodeau, J., Desbiens, M. et al. Evidence of Antibacterial Activities in Peptide Fractions Originating from Snow Crab (Chionoecetes opilio) By-Products. Probiotics & Antimicro. Prot. 2, 197–209 (2010). https://doi.org/10.1007/s12602-010-9043-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-010-9043-6