Abstract
The high load of protozoan parasites in marine bivalves often leads to mass mortalities of the hosts. On the west coast of Korea in the Yellow Sea, the protozoan parasite Perkinsus olseni has been identified as the agent causing mass mortality of Manila clam Ruditapes philippinarum. During August and September 2004, mass mortality of clam occurred at Hwangdo (HD) tidal flat in Anmyeondo Island on the west coast, resulting in a 50% reduction in the clam landings. Shortly after the mortality event, we examined pathology, and the fitness of the survived clams from HD to elucidate the impacts of P. olseni infection. Histology revealed that clams collected from HD in October 2004 were infected by P. olseni. In histology, P. olseni could be observed from all types of tissues of clams from HD, and severe inflammation was observed in the gills. Ray's fluid thioglycollate medium assay (RFTM) indicated that the infection intensity in clams from HD (1.738 × 106 cells/g gills in October and 1.476 × 106 cells/g gills in December) was significantly higher than the levels in clams from the neighboring tidal flats (0.001 to 0.622 × 106 cells/g gills, P < 0.05). Condition index (CI) and the total carbohydrate levels in clams from HD in October were significantly lower than those values in clams from other tidal flats (P < 0.05). In October, a negative correlation was observed between P. olseni infection intensity and CI in clams from HD, suggested that a high load of P. olseni causes substantial impacts on the host condition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Occurring widely in low intertidal or shallow subtidal on the west coast of Korea, Manila clam Ruditapes philippinarum is one of the key species in the tidal flat ecosystem linking the primary production to the upper trophic level (Koh and Khim 2014). Manila clams are often cultured at a commercial scale at licensed tidal flats by sowing 1.5 to 2.5 cm in shell length (SL) juveniles. Clams are harvested 2 to 3 years after the sowing, as they reach 3 to 4 cm in SL (Park et al. 2006; Ahn et al. 2016). In 2004, Korea produced 27,570 MTs of Manila clam, and approximately 75% of the national landings were originated from Taean coast on the west coast. The Taean coast includes numerous sandy-mud tidal flats with high benthic primary production, which support the clam to grow (Lee et al. 2013; Park et al. 2014). In 2005, the Manila clam landings from the Taean coast dropped dramatically to 12,000 MT, approximately one-half of the landings recorded in the area in 2004 (KOSIS 2019). Such dramatic drops in the Manila clam production were linked to the mass mortalities of clams in tidal flats Taean in 2005 summer, where the mortality was recorded as high as 50% (NFRDI, unpublished data).
Numerous studies have demonstrated that the fitness of marine bivalves is often impaired by biotic and abiotic factors, including habitat destruction, oil spill, thermal stress, summer hypoxia, low food availability, harmful algal blooms, and a high burden of parasitism (Soletchnik et al. 2005; Mizuta et al. 2011; Burdon et al. 2014; Hong et al. 2016; Turra et al. 2016). Parasite infection has been identified as one of the leading causes responsible for deteriorating fitness, as high levels of parasitism often lead to mass mortalities of the host organisms. Various prokaryotic, eukaryotic, and metazoan parasites have been identified from cultured marine bivalves, including single-celled Marteilia, Bonamia, Haplosporidium, digenetic metazoan trematode, and crustacean sea spider as they induced lethal and/or sub-lethal impacts on the host organisms (Allam et al. 2002; Thieltges 2006; Miyazaki et al. 2010; Carrasco et al. 2015; de Montaudouin et al. 2016; Robledo et al. 2018). Thieltges (2006) reported that heavy infestation by trematode Gymnophallus choledochus induces mass mortality of the edible cockle Cerastoderma edule in the northern Wadden Sea. In Japan, the sea spider Nymphonella tapetis also caused the mortality of Manila clam in Tokyo Bay, resulting in a shutdown of the clam fishery in 2007 (Miyazaki et al. 2010; Yamada et al. 2017). In Korean waters, Manila clams are known to host protozoan and metazoan parasites, including the protozoan parasite Perkinsus olseni (Park and Choi 2001; Park et al. 2005; Kang et al. 2017) and the metacercaria stage of trematode Parvatrema duboisi on the mantle and Cercaria sp. in the gonad and the visceral mass (Ngo and Choi 2004; Le et al. 2015).
The epizootic protozoan Perkinsus olseni has been listed as notifiable mollusk disease by the World Organization for Animal Health (OIE), which is responsible for the decrease in clam populations in Europe and Asia (Allam et al. 2002; Pretto et al. 2014; Nam et al. 2018; Waki et al. 2018). According to Park et al. (2005) and Kang et al. (2017), P. olseni is the sole agent responsible for perkinsosis in Manila clams in Korean waters. Sub-lethal impact of P. olseni parasitism, such as low level of fitness, has been reported from clam culture grounds in Europe (see the review of Villalba et al. 2004). Slow growth and retarded gonad maturation as the sub-lethal impact of P. olseni infection in Manila clam in intertidal have been reported from the south coast of Korea (Lee et al. 2020).
In the summer of 2004, mass mortality of Manila clam occurred on Hwangdo (HD) tidal flat in Anmyeondo Island, where numerous adult and juvenile clams emerged from the sediment and perished. In an attempt to understand the mass mortality dynamics of clams, we examined pathologic condition, the energy reserve, and condition index in October and December, as the clams were in post-spawning.
2 Materials and Methods
2.1 Sampling Efforts
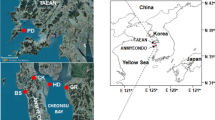
Anmyeondo Island (36°29′ N, 126°21′ E) encompasses well-developed tidal flats served as clam culture grounds (Fig. 1). In October and December 2004, clams with shell length ranging from 36.2 ± 3.2 mm (i.e., the longest axis of the shell, SL) were collected from Hwangdo (HD) tidal flat clam exhibited mass mortality in August and September 2004. For comparisons, clams were also collected from three other tidal flats in Anmyeondo Island (Nudong (ND), Gonam (GN), and Bangpo (BP), where no clam mass mortalities were observed in October and December in 2004 (Fig. 1). A total of 240 adult clams were collected from the four tidal flats and analyzed during the study (Table 1).
In the laboratory, the collected clams were acclimated in seawater at room temperature for 24 h to clear the sediments in the digestive tract. After measuring the SL, the soft body was removed from the shells, and excessive water on the tissue surface was removed, then weighed to mg using an electronic valance. Shells of each clam were dried at room temperature and weight to mg. Condition index (CI) was determined as the ratio of the wet tissue weight (g) to the dry shell weight (g).
2.2 Histology and Identification of Parasitic Organisms in Manila Clams
For histology, a 2–4 mm thick dorso-ventral section was made in the middle of the clam body. The body section containing the gonad, digestive gland, and gills was fixed in Davidson’s solution for 24 h. The tissues were dehydrated in a series of ethanol, embedded in paraffin, sliced at 5 μm, then stained with Harris hematoxylin, and counterstained with eosin Y. The remained tissue was lyophilized and stored at − 70 °C for the total carbohydrate analysis. The histology slides were examined under a compound light microscope to identify parasitic organisms, such as P. olseni (Park and Choi 2001; Ngo and Choi 2004) and the larval trematode (Shimura et al. 1982; Ngo and Choi 2004; Le et al. 2015; Jung et al. 2021).
2.3 Quantification of P. olseni Using RFTM Assay
Ray's fluid thioglycollate medium (RFTM) assay and 2 M NaOH digestion (Ray 1966; Choi et al. 1989) were used to determine the density of P. olseni in each clam. For the assay, one part of the gill tissue was excised from each clam and added to 5 ml of FTM supplemented with antibiotics (nystatin 200 unit/ml, chloramphenicol 100 ng/ml). After a week of incubation in the dark at room temperature, the gill tissues were digested in 2 M NaOH at 60 °C. The number of P. olseni hypnospores was then counted using a hemocytometer. Finally, the infection intensity was expressed as the number of P. olseni cells per gram gill tissue.
2.4 Biochemical Composition of the Manila Clams
The total carbohydrate in the tissue was determined using a phenol–sulfuric acid solution, according to Taylor (1995). Lyophilized clam tissue was pulverized, and 20–25 mg of subsample was taken and further homogenized in phosphate-buffered saline (PBS, 0.15 M NaCl, pH 7.3) using an ultrasonicator. The homogenized tissue was centrifuged, then the phenol–sulfuric acid solution was added to the supernatant. After recording the optical density (OD) at 480 nm using a spectrophotometer, the carbohydrate level was referred from the standard material, dextrose (anhydrous, SIGMA).
2.5 Statistical Analysis
Spatial variation in CI, P. olseni infection intensity among 4 populations of clams collected in October and December were compared using Kruskal–Wallis one-way analysis of variance (ANOVA) followed by Tukey’s range test. Spatial variation in CI and the total carbohydrate content were also tested using one-way ANOVA and Tukey’s range test. Statistical analysis was carried out using SAS statistical package program (SAS Institute, NC, USA), and the significance level was set at alpha < 0.05.
3 Results
3.1 Identification of Parasitic Organisms Appeared in Histology
Histology revealed that in October, P. olseni infection was prevalent among the clams in the tidal flats in Anmyeondo Island; clams from HD tidal flat showed numerous P. olseni trophozoites clusters in the connective tissues of the gills exhibiting excessive inflammation (Fig. 2A). The basophilic P. olseni trophozoites were appeared as a small spherical cell (5–15 µm in diameter) with a large vacuole in histology. Under a high magnification in a light microscope, the trophozoite could be identified by its characteristic nucleus, which appeared as a signet ring (McLaughlin and Faisal 1998; Villalba et al. 2004). The massive infection by P. olseni resulted in hemocyte infiltrations in the gills, which led to swollen connective tissue and deterioration in the gill structures (Fig. 2A).
Photomicrographs of different types of parasites observed from Manila clams. A P. olseni in the gill connective tissues causing inflammation. Severe hemocyte infiltration surrounding the clusters of P. olseni trophozoites (*, asterisk) resulted in granulomas (arrowheads) and gill deformation. GL, gill lamella. B Matacercaria of Parvatrema sp. (arrowheads) in the mantle epithelium. OS, oral sucker, DT, digestive tubule. C Gonad castration caused by heavy infection with Cercaria sp. in the the connective tissue of the ovary. The gonad was fully occupied by the sporocysts of Cercaria sp. consisting of mature Cercaria sp. and germ balls (*, asterisk). ES, eyespot, CT, Cercaria tail, IL, intestinal lumen, DT, digestive tubule. D Sporocyst of Cercaria sp. containing germ balls (*, asterisk) in the testis. Scale bar = 200 μm
Histology also demonstrated that clams in the study sites were infected by the metacercariae stage of trematode Parvatrema duboisi in the mantle epithelia, characterized by the oral suckers (Fig. 2B). The sporocysts stage Cercaria sp. was also commonly identified from the ovaries, where the sporocysts contained the germinal balls and mature metacercaria characterized by eyespot and tail (Fig. 2C). The sporocysts stage of Cercaria sp. also occurred in the testis (Fig. 2D). It was notable that the larval P. duboisi occurred limitedly in the mantle tissue, whereas the sporocysts of Cercaria sp. distributed in the gonad and the visceral mass. In most cases, Manila clams infected by Cercaria sp. and Parvatrema sp. were co-infected by the protozoan parasite P. olseni.
In October, P. olseni infection prevalence of clams at HD tidal flat was 100%, and the prevalence remained 100% in December. At GN and ND, the infection prevalence ranged from 88.9 to 90.0% in October, and then it dropped to 40% in December at ND. Among the four sampling sites, BP showed the lowest P. olseni prevalence, ranging from 11.1% (December) to 53.3% (October), respectively. In October, Parvatrema sp. infection prevalence was highest at GN (40%), while the prevalence at other tidal flats ranged from 0.0 (BP) to 4.3% (HD) (Fig. 3). In December, the Parvatrema sp. infection prevalence ranged 5.6 (BP) to 26.7% (ND). The infection prevalence of Cercaria sp. was highest at ND (22.2%) in October, while the lowest prevalence was recorded at HD as 4.3%.
3.2 Quantification of P. olseni Infection Intensity
Figure 4 shows the mean and standard error of P. olseni infection intensity of clams determined using RFTM. In October, the infection prevalence ranged from 96.7% (ND) to 100% (HD, BP, and GN), indicating that most clams in tidal flats in Anmyeondo island were infected by P. olseni. RFTM indicated that P. olseni infection intensity of clams at HD tidal flat (1.739 × 106 cells/g gills) was significantly higher than the intensities measured from BP (0.134 × 106 cells/g gills), GN (0.622 × 106 cells/g gills), and ND (0.483 × 106 cells/g gills) (P < 0.05).
In December, P. olseni prevalence remained 100% at HD and GN, and 16.7% and 83.3% at BP and ND, respectively. At HD tidal flat, the infection intensity remained high as 1.476 × 106 cells/g gills, which was significantly higher than the intensities recorded from BP (0.001 × 106 cells/g gills), GN (0.385 × 106 cells/g gills), and ND (0.104 × 106 cells/g gills) (Kruskal–Wallis test, P < 0.05).
3.3 CI and the Total Carbohydrate
CI of clams collected in October showed a spatio-temporal variation during the sampling (Fig. 5). In October, CI ranged from 0.51 (HD and ND) to 0.56 (BP and GN), and the one-way ANOVA indicated that CIs of clams in HD and ND tidal flats were significantly lower than clams in BP and GN (P < 0.05). In December, CI ranged from 0.43 (HD and ND) to 0.48 (GN). Compared to October, CI determined in December was significantly lower than CI recorded in October (Student’s t test, P < 0.05).
Figure 6 shows the level of total carbohydrates in clams. In October, the mean total carbohydrate content varied from 7.34 (HD) to 17.86% (GN). ANOVA test indicated that in October, the total carbohydrate content of clams in HD was significantly lower than BP, GN, and ND (P < 0.05). In December, the total carbohydrate levels ranged from 8.00% (ND) to 13.18% (BP). In December, the total carbohydrate level recorded at ND was significantly lower than the levels determined from three other sites, including HD (P < 0.05).
The effect of P. olseni infection on host fitness was tested using a simple regression between the CI and P. olseni infection intensity determined by RFTM. In October, P. olseni infection intensity was negatively correlated with the CI at HD (r2 = 0.25, P < 0.01, Fig. 7). In contrast, there was no significant correlation between CI and the infection intensities at the three sites (BP, r2 = 0.14, GN, r2 = 0.001, ND, r2 = 0.07). In December, no clear correlation was observed between CI and P. olseni infection intensity among the four sampling sites, including HD (Fig. 7).
4 Discussion
4.1 Mass Mortality of Manila Clams
During the mass mortality incident at the HD tidal flat in 2004 summer, numerous clams emerged on the sediment surface. Such surfaced clams were exposed to the hot air temperature during low tide and possibly received a high degree of thermal stress. Histology revealed that the clams collected in October 2004, shortly after the mass mortality event, were infected by P. olseni, exhibiting inflammation and necrosis in the gills. It is believed that such a severe infection in the gills could disrupt the respiration and feeding while they were in the sediment. RFTM assay of HD clams indicated that P. olseni infection intensity reached 1.74 million cells per gram gills, which is considered to be a heavy infection (Park and Choi 2001). In contrast, P. olseni density was significantly lower in clams in three other tidal flats, where no apparent mass mortality occurred during the summer. It is believed that the high level of P. olseni is responsible, at least in part, for the mortality that occurred at HD tidal flat. According to the studies carried out in Japan, P. olseni infection leads lethal to sub-lethal impacts on juveniles to adult clams, especially when the infection intensity reaches over a million cells per gram host tissue (Shimokawa et al. 2010; Waki and Yoshinaga 2013; Waki et al. 2012, 2018). Perkinsus olseni infection-driven mass mortality of Manila clam also reported from clam culture grounds in Europe (Villalba et al. 2004; Pretto et al. 2014).
Nam et al. (2018) first examined a correlation between P. olseni infection and clam emerging phenomenon during a mass mortality occasion in late summer. In mid-August of 2015, numerous clams emerged on the sediment surface within a day in a tidal flat on the west coast of Korea. Most of the emerged clams remained on the surface and perished within a week, possibly due to desiccation and thermal stress while exposed to the atmosphere (Nam et al. 2018). Compared to the normal clams in the sediment, the surfaced clams exhibited a significantly low CI and low cell-mediated immune capacity. RFTM assay revealed that P. olseni infection level of the surfaced clams (1.98 × 106 cells/g wet tissue) was significantly higher than the level recorded from clam in the sediment (1.16 × 106 cells/g wet tissue). Perkinsus olseni infection intensity of clams determined from HD tidal flat in October was somewhat comparable to the level reported by Nam et al. (2018), suggesting that mortality of clams observed at HD tidal flat was associated with the high level of P. olseni. It is also believed that some other internal and external parameters, such as spawning activity and high air temperature, also exerted synergistic stresses with P. olseni. According to Yang (2011), Manila clams in HD tidal in August are mostly partially spawning or spent stages, and such stressful reproductive activity could deteriorate the cell-mediated immune capacity (Hong et al. 2016).
4.2 Impacts of P. olseni Infection
In October, a negative correlation between CI and P. olseni infection intensity was observed from clams in HD tidal flat, whereas clams in the neighboring tidal flats showed no significant association with the infection intensity. It was also noticeable that CIs of clams from HD in October were significantly lower than those of clams in other tidal flats, although such difference in CI was no longer observed in December. Such degraded fitness of clams infected by P. olseni was also reported from tidal flats in Incheon bay and Gomso bay on the west coast of Korea. Park et al. (2006) first reported a negative correlation between P. olseni infection intensity and CI of Manila clams in Gomso bay, where P. olseni infection prevalence stayed at 100% throughout the year, and the intensity reached 2.03 × 106 cells/g gills in late summer. Yang et al. (2012) also reported a high P. olseni infection and low CI in clams in Gomso bay.
Along with CI, clams in HD tidal showed a significantly low carbohydrate content level, suggesting that the carbohydrate metabolism of clams in HD tidal flat is substantially higher than clams in other tidal flats. Such a high level of carbohydrate metabolism is a symptom of physiological stress in marine bivalves (Scheurink and Steffens 1990; Mizock 1995). Robledo et al. (1995) reported that the carbohydrate content decreased in the mussel Mytilus galloprovincialis when they were severely infected by Marteilia refringens, a protozoan parasite of some commercially important marine bivalves. Coustaua et al. (1991) also reported trematode infection facilitated glycogen mobilization in Mytilus edulis, resulting in declined carbohydrate content. Therefore, we believe that the significantly low level of carbohydrate in clams in HD tidal flat is closely linked to the high level of P. olseni infection, as the parasite exerted a certain level of stress on the host metabolism. We also observed the larval trematode infection in Manila clams in this study, and the larval trematode infection may cause a certain level of negative impacts on the host health condition, as Coustaua et al. (1991) reported previously.
In conclusion, we surveyed parasite load and health condition of Manila clams in tidal flats in Anmyendo Island during late summer and early winter to understand the sub-lethal effects of the parasitism. In October 2004, clams collected from HD tidal flat on the west coast of Korea were heavily infected by P. olseni. Clams in HD tidal flat also demonstrated significantly low CI and total carbohydrate levels, suggesting that the major sub-lethal impact of P. olseni is a decrease in fitness, as was reported from other marine bivalves heavily infected by P. olseni.
References
Ahn HM, Ki HJ, Jeong HD, Lee HJ, Han HK, Park KJ, Song JH (2016) Comparison of growth, condition index and mortality of manila clam (Ruditapes philippinarum) between originated from China (Liaoning Dandong) and Chungnam (Taean) in Gochang tidal flats. Korean J Malacol 32:175–184. https://doi.org/10.9710/kjm.2016.32.3.175
Allam B, Paillard C, Ford SE (2002) Pathogenicity of Vibrio tapetis, the etiological agent of brown ring disease in clams. Dis Aquat Org 48:221–231. https://doi.org/10.3354/dao048221
Burdon D, Callaway R, Elliott M, Smith T, Wither A (2014) Mass mortalities in bivalve populations: a review of the edible cockle Cerastoderma edule (L.). Estuar Coast Shelf S 150:271–280. https://doi.org/10.1016/j.ecss.2014.04.011
Carrasco N, Green T, Itoh N (2015) Marteilia spp. parasites in bivalves: a revision of recent studies. J Invertebr Pathol 131:43–57. https://doi.org/10.1016/j.jip.2015.07.016
Choi KS, Wilson EA, Lewis DH, Powell EN, Ray SM (1989) The energetic coast of Perkinsus marinus parasitism in oysters: quantification of the thioglycollate method. J Shellfish Res 8:125–131
Coustaua C, Renauda F, Delay B, Robbins I, Mathieu M (1991) Mechanisms involved in parasitic castration: in vitro effects of the trematode Prosorhynchus squamatus on the gametogenesis and the nutrient storage metabolism of the marine bivalve mollusc Mytilus edulis. Exp Parasitol 73:36–43. https://doi.org/10.1016/0014-4894(91)90005-H
de Montaudouin X, Lucia M, Binias C, Lassudrie M, Baudrimont M, Legeay A, Raymond N, Jude-Lemeilleur F, Lambert C, Le Goïc N, Garabetian F, Gonzalez P, Hégaret H, Lassus P, Mehdioub W, Bourasseau L, Daffe G, Paul-Pont I, Plus M, Do VT, Meisterhans G, Mesmer-Dudons N, Caill-Milly N, Sanchez F, Soudant P (2016) Why is Asari (=Manila) clam Ruditapes philippinarum fitness poor in Arcachon Bay: a meta-analysis to answer? Estuar Coast Shelf Sci 179:226–235. https://doi.org/10.1016/j.ecss.2015.09.009
Hong HK, Donagy L, Kang CK, Kang HS, Lee HJ, Park HS, Choi KS (2016) Substantial changes in hemocyte parameters of Manila clam Ruditapes philippinarum two years after the Hebei Spirit oil spill off the west coast of Korea. Mar Pollut Bull 108:171–179. https://doi.org/10.1016/j.marpolbul.2016.04.033
Jung BK, Chang T, Shin H, Ryoo S, Hong S, Lee J, Song H, Cho J, Kim DG, Kim MJ, Won EJ, Han ET, Shin EH, Chai JY (2021) Parvatrema duboisi (Digenea: Gymnophallidae) life cycle stages in Manila clams, Ruditapes philippinarum, from Aphae-do (Island), Shinan-gun, Korea. Korean J Parasitol 59:83–88. https://doi.org/10.3347/kjp.2021.59.1.83
Kang HS, Yang HS, Reece KS, Cho YG, Lee HM, Kim CW, Choi KS (2017) Survey on Perkinsus species in Manila clam Ruditapes philippinarum in Korean waters using species-specific PCR. Fish Pathol 52:202–205. https://doi.org/10.3147/jsfp.52.202
Koh CH, Khim JS (2014) The Korean tidal flat of the Yellow Sea: physical setting, ecosystem and management. Ocean Coast Manage 102:398–414. https://doi.org/10.1016/j.ocecoaman.2014.07.008
KOSIS (2019) Korean Statistical Information Service. https://kosis.kr/eng/index/index.do/. Accessed 8 Apr 2019
Le TC, Kang HS, Hong HK, Park KJ, Choi KS (2015) First report of Urosporidium sp., a haplosporidian hyperparasite infecting digenean trematode Parvatrema duboisi in Manila clam, Ruditapes philippinarum on the west coast of Korea. J Invertebr Pathol 130:141–146. https://doi.org/10.1016/j.jip.2015.08.004
Lee HM, Kim HJ, Park KI, Choi KS (2020) Enhanced growth, gonad maturation, and low-level parasite infection in juvenile Manila clam Ruditapes philippinarum cultured in subtidal cages on the south coast of Korea. Aquaculture 526:735410. https://doi.org/10.1016/j.aquaculture.2020.735410
Lee S, Park I, Koo BJ, Ryu JH, Choi JK, Woo HJ (2013) Macrobenthos habitat potential mapping using GIS-based artificial neural network models. Mar Pollut Bull 67:177–186. https://doi.org/10.1016/j.marpolbul.2012.10.023
McLaughlin SM, Faisal M (1998) Histopathlogical alterations associated with Perkinsus spp. infection in the softshell clam Mya arenaria. Parasite 5:263–271. https://doi.org/10.1051/parasite/1998053263
Miyazaki K, Kobayashi Y, Toba M, Tsuchiya H (2010) Biology of Nymphonella tapetis Ohshima, 1927, a harmful pycnogonid endoparasitic on the commercial bivalve, Ruditapes philippinarum. Proc Jpn Soc Syst Zool 28:45–54. https://doi.org/10.19004/taxa.28.0_45
Mizock BA (1995) Alterations in carbohydrate metabolism during stress: a review of the literature. Am J Med 98:75–84. https://doi.org/10.1016/S0002-9343(99)80083-7
Mizuta K, Yamatogi T, Higano J, Tamaki A (2011) Measure for preventing mass mortality of the Manila clam Ruditapes philippinarum during summer months by means of suspended culture in the water column. Suisan Zoshoku 59:435–442. https://doi.org/10.11233/aquaculturesci.59.435
Nam KW, Jeung HD, Song JH, Park KH, Choi KS, Park KI (2018) High parasite burden increases the surfacing and mortality of the Manila clam (Ruditapes philippinarum) in intertidal sandy mudflats on the west coast of Korea during hot summer. Parasite Vector 11:1–7. https://doi.org/10.1186/s13071-018-2620-3
Ngo TTT, Choi KS (2004) Seasonal changes of Perkinsus and Cercaria infections in the Manila clam Ruditapes philippinarum from Jeju, Korea. Aquaculture 239:57–68. https://doi.org/10.1016/j.aquaculture.2004.06.026
Park KI, Figueras A, Choi KS (2006) Application of enzyme-linked immunosorbent assay (ELISA) for the study of reproduction in the Manila clam Ruditapes philippinarum (Mollusca: Bivalvia): II. Impacts of Perkinsus olseni on clam reproduction. Aquaculture 251:182–191. https://doi.org/10.1016/j.aquaculture.2005.06.003
Park KI, Choi KS (2001) Spatial distribution of the protozoan parasite Perkinsus sp. found in the Manila clams, Ruditapes philippinarum, in Korea. Aquaculture 203:9–22. https://doi.org/10.1016/S0044-8486(01)00619-6
Park KI, Park JK, Lee J, Choi KS (2005) Use of molecular markers for species identification of Korean Perkinsus sp. isolated from Manila clams Ruditapes philippinarum. Dis Aquat Organ 66:255–263. https://doi.org/10.3354/dao066255
Park J, Song SJ, Ryu J, Kwan BO, Hong S, Bae H, Choi JW, Khim JS (2014) Macrozoobenthos of Korean tidal flats: a review on species assemblages and distribution. Ocean Coast Manage 102:483–492. https://doi.org/10.1016/j.ocecoaman.2014.07.019
Pretto T, Zambon M, Civettini M, Caburlotto G, Boffo L, Rossetti E, Arcangeli G (2014) Massive mortality in Manila clams (Ruditapes philippinarum) farmed in the Lagoon of Venice, caused by Perkinsus olseni. Bull Eur Assoc Fish Pathol 34:43–53
Ray SM (1966) A review of the culture method for detecting Dermocystidium marinum with suggested modifications and precautions. Proc Natl Shellfish Assoc 54:55–69
Robledo JAF, Marquis ND, Countway PD, Record NR, Irish EL, Schuldt MM, Kingston SE, Bishop TJ, Messerman NA, Bowden TJ (2018) Pathogens of marine bivalves in Maine (USA): A historical perspective. Aquaculture 493:9–17. https://doi.org/10.1016/j.aquaculture.2018.04.042
Robledo JAF, Santarém MM, González P, Figueras A (1995) Seasonal variations in the biochemical composition of the serum of Mytilus galloprovincialis Lmk. and its relationship to the reproductive cycle and parasitic load. Aquaculture 133:311–322. https://doi.org/10.1016/0044-8486(95)00009-Q
Scheurink AJW, Steffens AB (1990) Central and peripheral control of sympathoadrenal activity and energy metabolism in rats. Physiol Behav 48:909–920. https://doi.org/10.1016/0031-9384(90)90248-3
Shimokawa J, Yoshinaga T, Ogawa K (2010) Experimental evaluation of the pathogenicity of Perkinsus olseni in juvenile Manila clams Ruditapes philippinarum. J Invertebr Pathol 105:347–351. https://doi.org/10.1016/j.jip.2010.08.007
Shimura S, Yoshinaga T, Wakbayashi H (1982) Three marine cercariae in the clam Tapes philippinarum from Lake Hamana, Japan: morphology and level of infection. Fish Pathol 17:129–137. https://doi.org/10.3147/jsfp.17.129
Soletchnik P, Lambert C, Costil K (2005) Summer mortality of Crassostrea gigas (Thunberg) in relation to environmental rearing conditions. J Shellfish Res 24:197–207. https://doi.org/10.2983/0730-8000(2005)24[197:SMOCGT]2.0.CO;2
Taylor KACC (1995) A modification of the phenol/sulfuric acid assay for total carbohydrates giving more comparable absorbances. Appl Biochem Biotech 53:207–214. https://doi.org/10.1007/BF02783496
Thieltges DW (2006) Parasite induced summer mortality in the cockle Cerastoderma edule by the trematode Gymnophallus choledochus. Hydrobiologia 559:455–461. https://doi.org/10.1007/s10750-005-1345-4
Turra A, Pombo M, Petracco M, Siegle E, Fonseca M, Denadai MR (2016) Frequency, magnitude, and possible causes of stranding and mass-mortality events of the beach clam Tivela mactroides (Bivalvia: Veneridae). PLoS One 11:e0146323. https://doi.org/10.1371/journal.pone.0146323
Villalba A, Reece KS, Ordas MC, Casas SM, Figueras A (2004) Perkinsosis in molluscs: a review. Aquat Living Resour 17:411–432. https://doi.org/10.1051/alr:2004050
Waki T, Shimokawa J, Watanabe S, Yoshinaga T, Ogawa K (2012) Experimental challenges of wild Manila clams with Perkinsus species isolated from naturally infected wild Manila clams. J Invertebr Pathol 111:50–55. https://doi.org/10.1016/j.jip.2012.05.009
Waki T, Takahashi M, Eki T, Hiasa M, Umeda K, Karakawa N, Yoshinaga T (2018) Impact of Perkinsus olseni infection on a wild population of Manila clam Ruditapes philippinarum in Ariake Bay, Japan. J Invertebr Pathol 15:134–144. https://doi.org/10.1016/j.jip.2018.03.001
Waki T, Yoshinaga T (2013) Experimental challenges of juvenile and adult Manila clams with the protozoan Perkinsus olseni at different temperatures. Fish Sci 79:779–786. https://doi.org/10.1007/s12562-013-0651-4
Yamada K, Miyazaki K, Tomiyama T, Kanaya G, Miyama Y, Yoshinaga T, Wakui K, Tamaoki M, Toba M (2017) Impact of sea spider parasitism on host clams: susceptibility and intensity-dependent mortality. J Mar Biol Assoc UK 98:735–742. https://doi.org/10.1017/S0025315417000200
Yang HS (2011) Annual variation of reproductive effort, biochemical composition and Perkinsus olseni infection in Manila clam, Ruditapes philippinarum, off the west coast of Korea. Jeju National University, Jeju, p 155
Yang HS, Park KI, Donaghy L, Adhya M, Choi KS (2012) Temporal variation of Perkinsus olseni infection intensity in the Manila clam Ruditapes philippinarum in Gomso bay, off the west coast of Korea. J Shellfish Res 31:685–690. https://doi.org/10.2983/035.031.0312
Acknowledgements
This work was supported by a grant from Jeju National University (2020) to KS CHOI.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, HM., Park, KI., Yang, HS. et al. Negative Impacts of Perkinsus olseni Infection in Manila Clam Ruditapes philippinarum Observed from Tidal Flats in Anmyeondo Island on the West Coast of Korea During Post-Spawning Period. Ocean Sci. J. 56, 307–316 (2021). https://doi.org/10.1007/s12601-021-00024-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12601-021-00024-0