Abstract
Alternaria genus includes many plant pathogens on numerous hosts, causing leaf spots, rots and blights. Alternaria blight has been observed as one of the important fungal diseases of pistachio (Pistacia vera L.) as well as its wild relatives (P. terebinthus, P. lentiscus, P. khinjuk, P. atlantica, P. mutica) in Turkey. Alternaria species were sampled from Pistacia spp. hosts from different geographic regions in Turkey during field trips in late spring to early fall of 2013. Alternaria blight symptoms were observed mainly on fruits and rarely on leaves. Four hundred and twenty two of the isolates were morphologically defined as A. alternata, A. tenuissima, A. arborescens and also intermediate morpho-species between A. alternata/A. arborescens. Pathogenicity of the isolates was confirmed with host inoculations on detached fruits. Mating types of 270 isolates of Alternaria spp. from the collection were identified using a PCR-based mating type assay that amplifies either a MAT1-1 or a MAT1-2 fragment from the mating locus. Although a strongly clonal population structure was expected due to the putative asexual reproduction of these fungi, both idiomorphs were detected at equal frequencies at several different spatial scales. The distribution of mating types within each geographic region, within host species as well as in overall collection was not significantly different from 1:1. Amplified fragments of partial idiomorph sequences were obtained for representative isolates. Parsimony trees were depicted based on sequence data of mating type genes for these representative isolates as well as some other Alternaria species obtained by Genebank. Several point mutations presented a few clusters which are supported by high bootsrapped values. The Alternaria blight disease agents both from cultivated and wild hosts were pathogenic on pistachio which may cause difficulties to control the disease because of extensity of pathogen sources. Besides, equal mating type distribution of the pathogen at both geographic and host species levels suggests a potential for sexual reproduction of Alternaria spp. in Turkey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Turkey is one of the centers of origin of pistachio (Pistacia vera L.) and many wild relatives (Pistacia spp.) grow sympatrically with cultivated pistachio in Turkey (Zohary 1952; Whitehouse 1957). Alternaria blight of pistachio is one of the most economically significant diseases of this important crop. Alternaria spp. from pistachio has been reported from in Egypt (Wasfy et al. 1974), California/USA (Michailides et al. 1995), South Africa (Swart and Blodgett 1998), Australia (Ash and Lanoiselet 2001). The disease causes premature defoliation, blight symptoms on leaves and the staining of nutshells, thus reducing fruit quality (Michailides 2005). Under favourable climatic and environmental conditions, the disease is highly destructive. Turkey is the third largest producer of pistachio in the world and severe disease epidemics have been observed in recent years in Turkey. The disease has been observed on many wild relatives of pistachio in Turkey and this may contribute to pathogen variability and provides an inoculum source for epidemics on cultivated pistachio. A. alternata, A. tenuissima and A. arborescens are all small-spored Alternaria species that are primarily responsible for the disease worldwide and these pathogens have been isolated from both fruits and leaves (Pryor and Michailides 2002). There is no detailed information about the disease and its pathogens in Turkey and no report about Alternaria blight of wild Pistacia spp. worldwide.
It has been known that morphology based identifications for section Alternaria is highly difficult due to plasticity in their morphological characters which are also affected by environmental conditions and species boundaries are not well-resolved within this section (Rotem 1994; Simmons 2007; Tymon et al. 2016; Ozkilinc et al. 2017). Still, traditional identification based on morphology is used as a first attempt to characterize pathogen isolates. But, molecular and/or phylogenetic tools should be used to confirm species reconginition as applied in some previous studies (Peever et al. 2004; Andrew et al. 2009; Stewart et al. 2014; Tymon et al. 2016; Ozkilinc et al. 2017).
The mating type locus (MAT) controls sexual reproduction in many fungi and the structure of MAT locus differs among ascomycete fungi (Turgeon 1998). A single mating type locus carries either of the two alternate alleles, MAT1-1 or MAT1-2, termed idiomorphs rather than alleles due to their significant sequence dissimiliarity (Turgeon and Yoder 2000). Even though the Alternaria genus comprises mostly asexual species, mating type genes have been identified in several Alternaria spp. (Arie et al. 2000; Berbee et al. 2003; Stewart et al. 2011) as well as in other putatively asexual ascomycete fungi (Sharon et al. 1996; Paoletti et al. 2005; Groenewald et al. 2006). The MAT1 locus of asexual Alternaria species was shown to be functional when used to transform a MAT1-deficient mutant of a closely related sexual species (Arie et al. 2000; Berbee et al. 2003). The mating type locus of several Alternaria species has been characterized and primers designed to estimate mating type ratios in populations via PCR (Arie et al. 2000; Berbee et al. 2003; Stewart et al. 2011). Both mating types from A. alternata and only MAT1-2 from A. arborescens were reported in small scale samples from several hosts (Arie et al. 2000; Berbee et al. 2003; Stewart et al. 2011). A 1:1 ratio of mating types was found among 90 isolates of A. alternata sampled from diverse hosts and geographic locations (Armitage et al. 2013). Sequence variation detected at the mating type locus can be used to detect recombination and to estimate phylogenies. Rate of evolution and sequence variation at the MAT locus can vary at the species or genus level. For example, MAT sequence variation of Cochliobolus samples was low within species but high between species (Turgeon 1998). Recently, Stewart et al. (2011) evaluated sequences of mating type genes in Alternaria species considering nucleotide diversity, substitution ratios and codon usage statistics. Purifying selection and biased codon usage were detected at the MAT1 locus in Alternaria spp. and it was explained by a recent sexual past, cryptic sexual present or additional important cellular role of the mating type genes (Stewart et al. 2011).
Mating type ratios in fungal populations are widely used indicators of reproductive mode. For example, distribution of MAT idiomorphs in a global collection of Aspergillus fumigatus, 43% of isolates carried the MAT1-1 idiomorph and 57% carried the MAT1-2 idiomorphs (Paoletti et al. 2005). Detection of a near 1:1 distribution ratio of MAT idiomorphs for a worldwide collection of 2035 Mycosphaerella graminicola isolates was concluded as evidence of sexual reproduction (Zhan et al. 2002). Mating type distribution of Alternaria blight disease agents should be informative to learn about pathogen population biology. There is no information about mating type ratios and the distribution of mating types for Alternaria pathogens in Turkey. Besides, there is no information about mating types of Alternaria pathogens specifically from wild Pistacia hosts anywhere in the world. Determination of mating type distributions over different locations and hosts may reflect reproductive behaviour of the pathogen and selection pressures on mating types. Moreover, diversity of mating types of these pathogens in cultivated and wild Pistacia ecosystems will give an insight to understand disease and pathogen evolution in a broad ecological range.
The objectives of this research were (i) to identify the pathogens causing Alternaria blight of Pistacia spp. in Turkey using morphological criteria; (ii) to confirm their pathogenicity on pistachio; (iii) to determine mating types of the Alternaria spp. isolates within and among populations sampled from wild and cultivated pistachio in different geographic locations; (iv) to compare sequence variation of each mating type idiomorph. This study will give first information about the pathogens causing Alternaria blight in wild and cultivated Pistacia spp. in Turkey and could be useful to improve disease management through increased knowledge of pathogen biology.
Materials and methods
Fungal Sampling
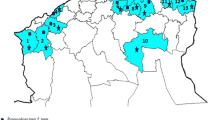
Collecting trips were carried out from late spring (May-June) to early fall (August-September) of 2013 across Turkey. Pistachio and its wild relatives occur mostly in Southeastern, Mediterrenean, Agean and South Marmara regions of Turkey with both cultivated species and wild relatives with sympatric distribution in these regions (Fig. 1). Sampling was carried out from both pistachio trees (P. vera) and wild relatives (P. terebinthus, P. lentiscus, P. khinjuk, P. atlantica, P. mutica). Sampling information showing host and region are shown in Supplementary Table. Thus, sampling was done from 332 trees at 287 different locations in 17 cities taking place in four geographic regions (Supplemantary Table). Dark brown to black blighted lesions were seen commonly on fruits and rarely on leaves. Each plant sample were treated seperately to discriminate pathogen sampling origin. Several fruits showing Alternaria bight symptoms were collected from a tree and kept in a paper bag and were taken to the laboratory within 24-48 h to isolate pathogens. Fruits were washed with tap water and then cut into small pieces (such as about 0.5-1 cm3 dimensions) bearing disease symptoms. These pieces were surface sterilized with 3% NaOCl solution for three minutes, then, rinsed in sterile distilled water three times and plated on potato dextrose agar (PDA) (Merck). Plates were incubated 3-5 days under cool white fluorescent light at 23 °C and 12 h photoperiod. Alternaria colonies were subcultured for single spore isolation. Cultures were maintained as single spore colonies and stored in filter papers at −20 °C. Four hundred-twenty two isolates were obtained. A specific code was given each isolate from one tree. Codes represented sampling province, orchard number and tree number. Additional isolation number if there is one more isolation per tree (Supplemantary Table). G or GY symbols were added if sampling was done in the late season (Supplemantary Table).
Map of Turkey showing cities where Pistacia spp. samples diseased with Alternaria blight were collected. Thin lines show city borders, thick lines show borders of geographic regions. Map source: https://tr.wikipedia.org/wiki/Dosya:Turkey_region_map_with_province_borders.svg (creative commons)
Morphological identification
Colony type and sporulation pattern was recorded for all isolates using the morphological criteria of Pryor and Michailides (2002) and Simmons (2007). Isolates were grown on PDA and weak-PDA for 7-10 days in incubators providing a 12 h dark/12 h light cycle at 20 ± 2 °C (Pryor and Michailides 2002). Moreover, conidiophore lengths were measured for some representative isolates following the criteria of Simmons (2007) and Tymon et al. (2016). Isolates were grown on potato carrot agar at 23 °C at 16 h dark and 8 h light cycle during 5-7 days prior to condiophore length measurements (Simmons 2007; Tymon et al. 2016). Morphologies were also observed and recorded for reference isolates A. arborescens (EGS 39-128), A. alternata (EGS 34-016 and EGS34-039) and A. tenuissima (EGS 34-015), kindly provided by Prof. Dr. B.M. Pryor (Arizona State University, Dept. of Plant Pathology, Tucson, Arizona/U.S.A.) and compared with the pistachio isolates sampled from Turkey.
Pathogenicity
Pathogenicity of the isolates was tested on pistachio fruits (cultivar Ohadi) obtained from pistachio trees grown in orchards of Pistachio Research Institute (Gaziantep, Turkey). Healthy fruits were washed with tap water and sterilized with 3% NaOCl solution for three minutes, rinsed in sterile distilled water three times then stored between filter papers until completely dry. Fruits were placed on plastic mesh above moistened filter papers in the bottom of clean plastic boxes to maintain humidity. Each box was divided in three zones using aluminum foil strips. Fifteen fruits were placed per box with five fruits per zone. One box was prepared for one fungal isolate. The fruits were sprayed with conidial suspensions of 1 × 106 conidia ml−1 produced from 7 to 10 days old cultures grown on PDA, and controls were sprayed with sterile dH2O. Three hundred fifty-eight isolates were tested. Due to low sporulation of 54 of the isolates, mycelium was harvested directly from the culture plate and applied to the fruit surface using a sterile rod to test their pathogenicity. Following inoculation, boxes were sealed and put into growth chambers with 12 h dark/light cycle at 20 ± 2 °C. Disease symptoms were evaluated after 7 days. The isolates originating from pistachio leaves (coded as 33-34, 35-37/Y1, 35-45, 35-22, 45-64, 17-13-GY), leaf stalk (coded as 35-37/leaf stalk) and one randomly selected isolate from pistachio fruit (coded as 35-13) (Supplemantary Table) were also tested for pathogenicity to pistachio leaves. Tests were performed on detached leaves of cv. Ohadi. For each isolate, four fully expanded and healthy leaves were used. Two leaves (one with abaxial side up the other with adaxial side up) were placed in plastic boxes prepared as described above but not divided in zones. For the leaf inoculation, 20 μl of a conidial suspension (106 conidia ml−1) were placed in two different spots on each leaf. Following inoculation, boxes were sealed and placed into growth chambers with a 12 h dark/light cycle at 20 ± 2 °C. As control, 20 μl of sterile distilled water were placed in two different spots on leaves and incubated in the same conditions. Leaves were monitored until the symptoms appeared and the experiment was ended at day 7th after inoculation.
Fungal DNA isolation
For DNA extraction, fungal mycelia were grown in potato dextrose broth (PDB) on a rotary shaker at 120 rpm at room temperature for 5-7 days. Growth medium was removed using a vacuum pump and mycelia were freeze-dried for 48 h. Dried mycelia were powdered using sterile metal rod before DNA isolation. Fungal DNA isolation was performed using the i-genomic Plant DNA isolation kit (Invitrogen). DNA concentrations were estimated using a Nanoquant spectrophotometer (NanoQuant Infinite M200, Tecan, Austria) and DNAs adjusted to 10-15 ng μl−1 concentrations.
Mating type determination
Two hundred and seventy isolates were chosen from the collection considering to represent different geographic location and host origin. The PCR-based mating-type marker system developed for A. alternata by Stewart (2011) was used to identify mating types in A. alternata, A. tenuissima and A. arborescens populations sampled from Pistacia spp. Primers ALMAT-L (GCAAGATTCTAGGCCCAACG) /AA-MAT1-867 (TGCGGTGGGGAGTAGTGT) and AAMAT2-1691 (CAGCACCCCGACTAC AAGTAT)/AsM1-8 (GGTCGTGAGTCGTGATCG) were used to amplify MAT1-1 and MAT1-2 genes, respectively (Stewart 2011). These primers were designed designed to the MAT1-1 [GenBank: AB009451] and MAT1-2 [GenBank: AB009452] idiomorphs of the Japanese pear pathotype of A. alternata (Arie et al. 2000) by Stewart (2011). Several isolates of A. alternata isolated from citrus with known mating type (SH-MIL-34 s, SH-MIL-11 s, SH-MIL-22 s for MAT1-1, and SH-MIL-14 s for MAT1-2), kindly provided by T.L. Peever (Washington State University, Plant Pathology Dept., Pullman/WA, U.S.A.), were used as positive controls. These isolates, plus A. arborescens isolate EGS39-128 (MAT1-2) and the other two reference isolates (EGS 34-016 and EGS 34-015 for MAT1-2) used in morphological identifications were included in the analysis. Twenty five μL PCR reaction mixtures contained 10-15 ng of template DNA, 1X PCR buffer (Applied Biological Materials Inc., Canada), 1.5 mM MgCl2 (Applied Biological Materials Inc., Canada) 200 μM dNTPs, and 1 μM of each primer. PCR conditions were 95 °C for 5 min, 35 cycles at 95 °C for 20 s, 58 °C for 30 s, and 72 °C for 30 s followed by a final step at 72 °C for 10 min. PCR amplifications were conducted separately for each MAT primer pair. Reactions were carried out with a Bio-Rad T100 thermocycler (USA). PCR products were detected on 1.5% agarose gel and visualized under UV light using a Vilber Lourmat Quantum ST4 1100 gel documentation system (Vilber Lourmat, France). Each isolate gave product ̴ 640 bp for MAT1-1 and ̴ 880 bp for MAT1-2. No isolate produced both amplicons. Negative control reactions without template DNA gave no amplicon. Mating-type distributions were tested for deviation from the expected ratios of 1:1 using chi-square goodness-of-fit tests.
Sequences of MAT1-1 and MAT1-2 idiomorphs
MAT1-1 and MAT1-2 amplicons were sequenced for 16 mating type 1 and 22 mating type 2 isolates from the collection, respectively. Representative isolates of A. alternata, A. tenuissima and A. arborescens morphospecies carrying either the MAT1-1 or MAT1-2 idiomorph were chosen to represent Alternaria species sampled from Pistacia spp. Seven isolates were chosen as positive control isolates including the MAT1-1 isolates SH-ML-11 s, SH-ML-34 s, and SH-ML-22 s, and MAT1-2 isolates EGS 39-128, EGS 34-016, EGS 34-015, and SH-ML-14 s. PCR amplicons were sequenced using an ABI 3500xL Genetic Analyzer (Applied Biosystems, MedSanTek Lab, Turkey). Sequences of both strands were obtained for each amplified fragment and edited in Bioedit v7.0.53 for Windows software (Hall 1999) and aligned using clustalW implemented in BioEdit software. Each consensus sequence was also used as a query to search similarities using BLASTn of the Basic Local Alignment Search Tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi) (Altschul et al. 1990). Coding regions were identified using NCBI database Blastn-tools. Number of haplotypes, Tajima’s neutrality test and substitution rates were estimated using DnaSP v.5 (Rozas et al. 2003). MAT1-1 and MAT1-2 sequences of different Alternaria species deposited in Genebank were obtained to compare phylogenies based on mating type sequences within the sampled isolates. Alternaria species Genebank were A. brassicae (AB444165.1 and AY042091), A. brassicicola (AB444163.1 and AB444162.1), A. citriarbusti (AB444181.1 and AB444168.1), A. citrimacularis (AB444184.1 and AB444185.1), A. gaisen (AB444187.1), Lewia infectoria (AB444188.1), A. longipes (AB444192.1), A. perangusta (AB444194.1), A. solani (AB444198.1 and AB444196.1), A. colombiana (AB444186.1), A. limicola (AB444189.1), A. limoniasperae (AB444190.1), A. toxicogenica (AB444201.1). Stemphylum sp. (AY339861.1 and AY340942) was selected as outgroups. ClustalW implemented in BioEdit v7.0.53 was used for multiple sequence alignment for each data set. Parsimony analysis was performed in PAUP* ver. 4.0b10 (Swofford 2003). Analyses were conducted by 20 replicate heuristic searches consisting of 1000 stepwise random addition replicates with branch swapping by the tree-bisection-reconnection algorithm. The trees were rooted with outgroup. Branch stability for each dataset was evaluated by 1000 bootstrap replications using a heuristic search with simple sequence addition to produce a strict-rule consensus tree with nodal support values. Nodes with greater than 70% bootstrap support values were considered significant. Trees were visualized using Acrhaeopteryx v.09901 beta (Han and Zmasek 2009).
Results
Morpho-species of Alternaria causing Alternaria blight of Pistacia spp.
Among total 422 isolates of Alternaria spp. sampled from Pistacia spp. throughout Turkey, 330 were identified morphologically as A. alternata, 88 as A. tenuissima, 1 isolate as A. arborescens and 3 isolates as A. alternata/A. arborescens intermediates (Supplementary Table). Isolate 02-28 showed typical arborecens type branching pattern and had a conidiophore length of 201 μm. Three isolates (02-29-1, 07-17-2/GY, and 31-09) showed intermediate characters between A. alternata and A. arborescens in terms of sporulation pattern. Conidiophore lengths were 127 and 171 μm for isolate 02-29-1 and 31-09, respectively. Conidiophore lengths were measured for representative isolates randomly chosen from collection which were A. alternata or A. tenuissima and conidiophore lengths of these isolates were less than 100 μm.
Pathogenicity of Alternaria spp. isolates from Pistacia spp.
All isolates, inoculated as spore suspension or as mycelium, caused typical Alternaria blight symptoms. Brown to dark lesions were observed on fruit shell. Thus, all of the isolates tested were confirmed as pathogenic to pistachio fruits. Isolates tested on leaves were also pathogenic on pistachio leaves and caused necrotic lesions.
Distribution of mating types
Mating type of 270 isolates of Alternaria spp. were determined. On average, across all populations analyzed, mating type frequencies did not differ significantly from a 1:1 ratio (Table 1). Distribution of mating types according to the geopraphic region and host species did not differ from 1:1 in each of the category, as well (Table 1). Mating type distribution of isolates based on morphospecies were evaluated for A. alternata/A.tenuissima, A. arborescens and intermediate types. Mating type of isolate 02-28, the only A. arborescens morphospecies identified in the sample was MAT1-2. Isolates represented as intermediate morpho-species were evaluated seperately. Isolate 02-29-1 was MAT1-2, isolates 31-09 and 07-17-2_GY were MAT1-1. The mating types ratio in the overall sample was 126:138 for isolates A. alternata/A. tenuissima (Chi-square value = 0.545, P = 0.460).
Sequence analysis of mating type idiomorphs
Five hundred sixty-eight bp of sequence data was obtained for MAT1-1 isolates representing A. alternata, A. tenuissima, and A. alternata/arborescens morphospecies from our collection. Nineteen variable sites including 13 parsimony-informative sites were observed. The sequenced region included both intron (349-568 bp) and exon (89-568 bp). One singleton mutation and 1 parsimony informative mutation were observed in the exon. Nine haplotypes were observed among 19 isolates based on MAT1-1 sequence data. 712 bp sequence data was obtained for MAT1-2 for 26 A. alternata/tenuissima/arborescens samples from our collection and 9 haplotypes were detected. 50 variable sites including 28 parsimony informative sites were recorded. Sequenced MAT1-2 region included both exon (1-356 bp) and intron (357-712 bp) regions. 10 singleton and 10 parsimony informative mutations were seen in exon part. The estimated Transition/Transversion bias was R = 5.73 for MAT1-1 region and R = 3.59 for MAT1-2 region. Samples presented insignificant negative Tajima’s values for both MAT1-1 and MAT1-2 regions which indicates possible population expansion. Sequences of MAT1-1 and MAT1-2 genes for our collection and reference isolates used in this study were deposited in Genebank (accession numbers from KY559241 to KY559286).
Sequence comparison were made within MAT1-1 and MAT1-2 isolates of Alternaria spp. and parsimony based trees were depicted (Fig. 2a-b). Moreover, several sequence data of MAT1 region of Alternaria spp. deposited in NCBI used for comparison. One most parsimonious tree was obtained for MAT1-2 and one of the tree was arbitrarily selected among two most parsimonious trees for MAT1-1. For both idiomorphs, sequences of these additional Alternaria species were highly distinct from our small spored Alternaria sequences (Fig. 2). Several number of mutations supported few clusters within the samples. Moreover, parsimony based analysis of MAT1-1 and MAT1-2 sequences indicated a distinction between morpho-species of A. alternata/tenuissima and A. arborescens.
Phylogenetic trees inferred from Maximum Parsimony (MP) analysis of sequences from mating type 1 (a) and mating type 2 (b) for Alternaria spp. Information about isolates from Pistacia spp. were provided by Supplementary Table. Morphospecies of the isolates were presented in parantheses. Reference isolates used for morphology are EGS39.128 (A. arborescens), EGS 34.16 (A. alternata) and EGS34.15 (A. tenuissima). SH-ML coded isolates were used as reference which their mating types were known. Sequences of MAT1-1 and MAT1-2 genes obtained in this study deposited in Genebank (accession numbers from KY559241 to KY559286). Sequence data of other Alternaria species were obtained from NCBI-genebank. Branch lengths are proprotional to number of substitutions. Bootstrap support values (1000 replicates) above 70% are shown
Discussion
This is the first comprehensive work characterizing the casual agent of Alternaria blight of cultivated pistachio and its wild relatives in Turkey on a large geographic and host scale. Most of the fungi causing this disease on both cultivated and wild hosts were classified morphologically as A. alternata. Even though the same disease mainly effects leaves of pistachio in U.S.A. (Pryor and Michailides 2002), the disease mainly occurs on fruits rather than leaves in Turkey. Only seven isolates were isolated from leaves among four hundred-twenty two isolates in the collection. This could be due the fact that Septoria and Septoria-like pathogens are very common disease agents on leaves of pistachio in Turkey (Crous et al. 2013) and probably competition does not allow for Alternaria infection on leaves. Because Alternaria pathogens obtained both from leaves and fruits have a capability to infect pistachio leaves as shown in our experiments.
A. alternata was found as the most common pathogen of this disease. Only one typical A. arborescens was found in a large geographic and host scale sampling collection. These three morpho-species were reported as Alternaria blight pathogen of pistachio in U.S.A. and frequency of these morpho-species did not present extreme differences in pathogen samples from both leaves and fruit parts (Pryor and Michailides 2002). Moreover there were three A. alternata / A.arborescens intermediate type isolates within the collection. Morphologically intermediate types were reported in small-spored Alternaria species collection from a wide host ranges (Andrew et al. 2009). Tymon et al. (2016) used morphology based keys to distinguish small-spored Alternaria species from potato. According to branching pattern, length of conida and conidiaphore, moprhospecies A. alternata, A. tenuissima and A. arborescens were determined, but, restriction digest assay using ApaI restriction site in the OPA1-3 regions was distinguished the isolates either A. alternata/tenuissima or A. arborescens (Tymon et al. 2016). Besides, inconsistent between morphology and molecular based assay was observed for few isolates of A. alternata/tenuissima and A. arborescens (Tymon et al. 2016). Defining A. alternata and A. tenuissima as a distinct morpho-species is difficult because morphological characters are highly overlapping and vulnerable to show plasticity or absence/inadequate of key morphological characters. Besides, these two morhop-species can not be distinguished by molecular phylogenetics, as well. (Ozkilinc et al. 2017; Ozkilinc H., unpublished data). In a recent study, A. alternata and A. tenuissima obtained from a diverse host range were considered as one species group in phylogenetic analysis comparing two sections of Alternaria genus (Ozkilinc et al. 2017). While A. alternata and A. tenuissima can not be distinguished based on molecular and phylogenetic approaches, A. arborescens is recognized as a distinct species from A. alternata/tenuissima (Tymon et al. 2016; Ozkilinc et al. 2017; Ozkilinc H. unpublished data). Some phylogenetic and molecular approaches were applied on an isolate collection used in this study and will be discussed in a future publication (Ozkilinc H. unpublished data).
Equal ratios of mating types grouped based on geographic region, host as well as across all collection could be an evidence that balancing selection is acting on these idiomorphs. In some other haploid, heterothallic fungi, similarly, the ratio of mating type idiomorphs was used as evidence of sexuality (Zhan et al. 2002; Paoletti et al. 2005). Even though indirect indication of sexuality cannot be a proof of sexuality (Schurko et al. 2009), our data is also a contribution to a previous results of indirect evidence for possible sexual reproduction in the Alternaria genus, specifically in small-spored Alternaria species (Linde et al. 2010; Stewart et al. 2011, 2014). Thus, these populations may not really asexual or some kind of selection on MAT locus may maintain both idiomorphs in equal frequency which requires detailed investigation of cellular roles of mating type gene products.
While there are report for mating types of A. alternata/tenuissima in literature, there has been only one report for A. arborescens which is for EGS39-128 isolate carrying mating type MAT1-2. We also detected MAT1-2 type for A. arborescens isolate coded as 02-28 within the collection. Among A. alternata/arborescens intermediate type isolates, two of these isolates (31-09 and 07-17-2-GY) were MAT1-1 type and the other one was MAT1-2 type. Moreover, it was detected a genetically distinct cluster among A. alternata isolates based on sequences of MAT1-2 region (Fig. 2b) and the isolates were from wild Pistacia species in this cluster (Supplementary Table) which indicated diversity in populations from native hosts.
Mutations occuring in the mating type idiomorphic sequences revealed several different haplotypes within small-spored Alternaria species from Pistacia hosts. Partial sequences of MAT1-2 idiomorph presented more mutational sites, especially in the exon, comparing to the MAT1-1 region which indicates different evolutionary rates on these two idiomorphs. Additionaly, mutations in MAT1-2 presented transversional bias which may indicate a directional selection on mating type genes.
A. arborescens and intermediate isolates clustered as a distinct group. Close phylogenetic relationship between A. arborescens and intermediate isolates was also observed in another work (Ozkilinc H., unpublished data). Besides, A alternata/A. tenuissima isolates indicated at least two distinct clusters within populations according to the both mating type idiomorphs. These small-spored Alternaria species are distinctly grouped from the other Alternaria species based on mating type sequences. The clustering implied that these regions could be phylogenetically informative within Alternaria genus, but species level distinction is required more genetic information to resolve species boundaries within small-spored Alternaria species.
Our results showed that both pistachio and wild relatives bear both mating types of Alternaria species causing Alternaria blight at equal frequencies across Turkey. Even though absence of teleomorph evidence for these species, sexual reproduction still could be questioned due to some indirect evidences such as equal frequencies of mating type genes, recombination detection and genetically distinct clusters. On the other hand, different mating types and genetic variations in sequences of mating type genes indicate for a high diversity for these pathogens in Turkey as expected in the origin centers of hosts as well its pathogens. Moreover ongoing research about genetic variability in other gene region will give more insights about the pathogen genetic variability and phylogenetic lineages (Ozkilinc H., unpublished data).
References
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Armitage, A. D. (2013). Alternaria alternata leaf spot pathogens: genetics, evolutionary history and diagnostics. PhD thesis, University of Warwick.
Andrew, M., Peever, T. L., & Pryor, B. M. (2009). An expanded multilocus phylogeny does not resolve morphological species within the small-spored Alternaria species complex. Mycologia, 101, 95–109.
Arie, T., Kaneko, I., Yoshida, T., Noguchi, M., Nomura, Y., & Yamaguchi, I. (2000). Mating-type genes from asexual phytopathogenic ascomycetes Fusarium oxysporum and Alternaria alternata. Molecular Plant-Microbe Interactions, 13, 1330–1338.
Ash, G. J., & Lanoiselet, V. M. (2001). First report of Alternaria alternata causing late blight of pistachio (Pistacia vera) in Australia. Plant Pathology, 50, 803.
Berbee, M. L., Payne, B. P., Zhang, G., Roberts, R. G., & Turgeon, B. G. (2003). Shared ITS DNA substitutions in isolates of opposite mating type reveal a recombining history for three presumed asexual species in the filamentous ascomycete genus Alternaria. Mycological Research, 107, 169–182.
Crous, P. W., Quaedvlieg, W., Sarpkaya, K., Can, C., & Erkılıç, A. (2013). Septoria-like pathogens causing leaf and fruit spot of pistachio. IMA Fungus, 4, 187–199.
Groenewald, M., Groenewald, J. Z., Harrington, T. C., Abeln, E. C. A., Crous, P. W. (2006). Mating type gene analysis in apparently asexual Cercospora species is suggestive of cryptic sex. Fungal Genetics and Biology, 43(12), 813–825.
Hall, T. A. (1999). BioEdit: A user-friendly biological sequence alignment editor and analysis. Nucleic Acids Symposium Series, 41, 95–98.
Han, M. V., & Zmasek, C. M. (2009). phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinformatics, 10, 356.
Linde, C. C., Liles, J. A., & Thrall, P. H. (2010). Expansion of genetic diversity of randomly mating founder population of Alternaria brassicicola infecting Cakile maritime in Australia. Appplied and Environmental Microbiology, 76, 1946–1954.
Michailides TJ. (2005) Pest, disease, and physiological disorders management: above ground fungal diseases. In Beede R. H, Freeman M. W, Haviland D. R, Holtz, B. A., & Kallsen, C. E. (Eds.), Pistachio Production Manual (pp: 214–232). Davis, CA.
Michailides, T. J., Morgan, D. P., & Doster, M. A. (1995). Diseases of Pistachio in California and their significance. Acta Horticulturae (ISHS), 419, 337–343.
Ozkilinc H., Rotondo F., Pryor B. M., Peever T.L. (2017). Contrasting species boundaries between sections Alternaria and Porri of the genus Alternaria. Plan Pathology, accepted on May 26 of 2017, DOI.
Paoletti, M., Rydholm, C., Schwier, E. U., Anderson, M. J., Szakacs, G., Lutzoni, F., Debeaupuis, J. P., Latgé, J. P., Denning, D. W., & Dyer, P. S. (2005). Evidence for sexuality in the opportunistic fungal pathogen Aspergillus fumigatus. Current Biology, 15, 1242–1248.
Peever, T. L., Su, G., Carpenter-Boggs, L., Timmer, L. W. (2004). Molecular systematics of citrus-associated Alternaria spp. Mycologia, 96(1), 119–134.
Pryor, B. M., & Michailides, T. J. (2002). Morphological, pathogenic and molecular characterization of Alternaria Isolates associated with Alternaria late blight of pistachio. Phytopathology, 92, 406–416.
Rotem, J. (1994). The genus Alternaria: biology, epidemiology and pathogenicity. Minnesota: American Phytopathological Society Press, St Paul.
Rozas, J. J., Sanchez-DelBarrio, J. C., Messeguer, X., & Rozas, R. (2003). DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics, 19, 2496–2497.
Sharon, A., Yamaguchi, K., Christiansen, S., Horwitz, B. A., Yoder, O. C., Turgeon, B. G. (1996). An asexual fungus has the potential for sexual development. MGG Molecular & General Genetics, 251(1), 60–68.
Simmons, E. G. (2007). Alternaria: An Identification Manual. Utrecht, Netherlands: CBS Fungal Biodiversity Centre.
Schurko, A. M., Neiman, M., & Logsdon Jr., J. M. (2009). Signs of sex: what we know and how we know it. Trends in Ecology & Evolution, 24, 208–217.
Stewart, J.E. (2011). Mating system and speciation of the citrus brown spot pathogen, Alternaria alternata. PhD Dissertation, WSU.
Stewart, J. E., Kawabe, M., Abdo, Z., Arie, T., & Peever, T. L. (2011). Contrasting Codon Usage Patterns and Purifying Selection at the Mating Locus in Putatively Asexual Alternaria Fungal Species. PLOS ONE, 6(5), e20083. https://doi.org/10.1371/journal.pone.0020083.
Stewart, J. E., Timmer, L., Lawrence, C. B., Pryor, B. M., & Peever, T. L. (2014). Discord between morphological and phylogenetic species boundaries: incomplete lineage sorting and recombination results in fuzzy species boundaries in an asexual fungal pathogen. BMC Evolutionary Biology, 14(1), 38. https://doi.org/10.1186/1471-2148-14-38.
Swart, W. J. & Blodgett J. T. (1998). Fungi associated with diseased pistachio trees in South Africa. Combined Congress of the Southern African New Crop Research Association, South African Crop Production Society and the Southern African Weed Science Society, 118.
Swofford, D. L. (2003). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sinauer Associates, Sunderland, Massachusetts.
Turgeon, B. G., & Yoder, O. C. (2000). Proposed nomenclature for mating type genes of filamentous ascomycetes. Fungal Genetics and Biology, 31, 1–5.
Turgeon, B. G. (1998). Application of mating type gene technology to problems in fungal biology. Annual Review of Phytopathology, 36, 115–137.
Tymon, L. S., Peever, T. L., & Johnson, D. A. (2016). Identificaiton and enumeration of small-spored Alternaria species associated with potato in the U.S. Northwest. Plant Disease, 100, 465–472.
Wasfy, E. H., Ibrahim, I. A., & Elarosi, H. M. (1974). New Alternaria disease of pistachio in Egypt. Phytopathologia Mediterranea, 13, 110–111.
Zhan, J., Kema, G. H. J., Waalwijk, C., & McDonald, B. A. (2002). Distribution of mating type alleles in the wheat pathogen Mycosphaerella graminicola over spatial scales from lesions to continents. Fungal Genetics and Biology, 36, 128–136.
Zohary, M. (1952). A monographical study of the genus Pistacia. Palestine Journal of Botany, 5, 187–228.
Whitehouse, W. E. (1957). The Pistachio nut-A new crop for the Western United States. Economic Botany, 11, 281–321.
Acknowledgements
This research is supported by TUBITAK, TUBITAK-TOVAG-112O554 project. The authors would like to thank Professor Tobin Peever (Dept. of Plant Pathology, Washington State University, Pullman, USA) for his advice in morphological analyses and contribution by providing reference isolates for mating type analyses and valuable comments; Professor Barry M. Pryor (Arizona State University, Dept. of Plant Pathology, Tucson, Arizona/U.S.A) for providing reference isolates for morphological description; Pistachio Research Institute for providing some facilities needed; Mr. A. Aktan for technical assistance in field trips.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Sampling location, isolate list, morpho-species definition and mating type infromation for Alternaria spp. from Pistacia spp. in Turkey (PDF 309 kb)
Rights and permissions
About this article
Cite this article
Ozkilinc, H., Sarpkaya, K., Kurt, S. et al. Pathogenicity, Morpho-Species and Mating Types of Alternaria spp. causing Alternaria blight in Pistacia spp. in Turkey. Phytoparasitica 45, 719–728 (2017). https://doi.org/10.1007/s12600-017-0624-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-017-0624-8