Abstract
Thirty-six strains of Bipolaris oryzae collected from infected seed from different geographical locations in India were used for the analysis of genetic variability by using molecular markers. The molecular characterization using three marker systems, i.e., universal rice primers (URPs), inter-simple sequence repeat (ISSR) and random amplified polymorphic DNA (RAPD) was carried out, which revealed differences in strains from different geographical origins as well as by clustering of various B. oryzae strains which otherwise could not be revealed through conventional characterization. Out of 13 URPs, 20 ISSR and 30 RAPD primers screened, seven URPs, eight ISSR and 14 RAPD markers gave very good reproducibility banding patterns. Polymorphic bands ranging between 86.3% and 100% in URP, 81.8% and 100% in ISSR, and 91.6% and 100% were recorded in the case of RAPD markers. The total number of bands recorded was 1791 with an average of 255.86 in URP, 2541 with an average of 317.63 in ISSR, and 3226 with an average of 230.42 in RAPD markers. Maximum heterozygosity (Hn) was revealed by URP 30F (0.26), ISSR 6 (0.36) and RAPD marker OPB-1 (0.31). The polymorphism information content (PIC) values ranged between 0.69 and 6.43 in URP, 3.86 and 8.03 in ISS, and 2.58 and 8.55 in RAPD marker profiles. Maximum cophenetic correlation was found in URP (r = 1.000), followed by ISSR (r = 0.813) and RAPD (r = 0.784). The combined analysis of all three marker systems showed high cophenetic correlation (r = 0.848), which indicated a good fit of the data for genetic diversity analysis. Hence, combined use of three marker systems would be more sensitive and reliable in characterizing genetic variability in B. oryzae strains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is one of the most important cereal crops and feeds more than one third of the world's population (Burgos et al. 2013; Khush 1997). Agricultural population densities on Asia’s rice-producing lands are among the highest in the world and continue to increase at a remarkable rate (www.knowledgebank.irri.org/ericeproduction/Importance_of_Rice.htm). Rapid population growth puts increasing pressure on the already strained food-producing resources. Abiotic and biotic stresses put additional pressure in declining yields of rice, which is a great challenge for the scientists. Rice is susceptible to several leaf spot diseases including blast and brown spot, which cause significant yield losses worldwide. The rice brown spot pathogen, Bipolaris oryzae (Breda de Haan) Shoem, is one of the most important seedborne diseases of rice, which is second only to blast disease in economic importance among the foliar diseases (Ou 1985) causing severe grain yield losses in both wet and dry seasons. Brown spot has been noted to reduce yields from 6% to 90% in Asia and was a cause for the Bengal famine in India (Mew & Gonzales 2002). This disease is associated with low soil fertility and often referred to as ‘poor man’s disease’ (Lee 1992). High variability within the pathogen leads to a break in the susceptibility nature against certain fungicides, with the development of either quantitative or qualitative resistance against the fungicides. Tolerance or resistance stability of host varieties for B. oryzae is not predictable without knowing the genetic structure of the pathogen. Therefore, understanding variation and diversity in a population of the pathogen and mechanisms that further influence the genotypic changes in the pathogen population, is an important step in developing disease management strategies. Very importantly, pathogen variation is also useful in identifying and characterizing resistant rice germplasm.
One of the most positive features of genetic diversity studies is pathogen tracking, by using the genetic pattern of fungus isolates. Therefore, knowledge of the pathogen population variability is necessary for effective host breeding for resistance (Savary et al. 2011). Variability in morphology and pathology of B. oryzae isolates has been reported from various rice-growing countries (Burgos et al. 2013; Kumar et al. 2011a; Ouedraogo et al. 2004), but except for one report which was restricted to only one rice-growing state of India (Kumar et al. 2011a), no studies have been reported from India. Very little information is known about the molecular diversity of B. oryzae strains in India.
During the last two decades, several molecular marker techniques such as Random amplified polymorphic DNA (RAPD), Variable number of tandem repeats (VNTRs), and Restriction Fragment Length Polymorphisms (RFLPs), have been developed for assessing the genetic diversity variation of B. oryzae from different countries (Burgos et al. 2013; Kumar et al. 2011a; Weikert-Oliveira et al. 2002). Repeat sequences from Korean weedy rice, originally referred to as Universal Rice Primers (URPs), have been used for fingerprinting of diverse genomes of plants, animals, and microbes (Kang et al. 2002). URP-PCR has been used earlier in molecular analyses of only a few fungi (Aggarwal et al. 2008; Kang et al. 2002). Hence, the aim of our work was to characterize the isolates of B. oryzae isolated from O. sativa using three different molecular markers, viz., inter-simple sequence repeats (ISSR), RAPD and URP-PCR.
Materials and methods
Isolation and maintenance of B. oryzae
Thirty-six isolates of B. oryzae were isolated from infected rice seeds collected from different rice-growing states in India (Table 1). The isolates were purified by modified single spore isolation techniques (Akhtar et al. 2013), maintained on potato dextrose agar (PDA) medium (HiMedia, Bombay, India) with periodic sub-culturing, and stored at 4°C. Isolates were designated as DQDO.
Genomic DNA isolation and PCR amplification
Thirty-six isolates of B. oryzae were grown on potato dextrose broth (HiMedia) at 28 ± 1°C for 7 days. Fresh mycelial mats were filtered through autoclaved muslin cloth, and about 1 g mycelia was ground in a pre-chilled pestle and mortar with liquid nitrogen. Approximately 200 mg of powdered mycelia was taken in a sterile eppendorf tube and used (HiPurATM fungal DNA purification kit; HiMedia) for DNA extraction as per the manufacturer’s protocol. The purified DNA was quantified by Nanodrop spectrophotometer (Thermo Fisher Scientific, Mumbai, India) and the final concentration of the template was adjusted to 25 ng μl-1 for use in PCR analysis.
The sequences of URP primers (derived from the repeat sequences of Korean weedy rice) were adapted from Kang et al. (2002) and URP, ISSR and RAPD primers were synthesized from Promega Inc. (Pragati Biomedical, New Delhi, India). PCR amplifications were carried out in a reaction volume of 25 μl containing 25 ng of template DNA. The reaction mixture consisted 10x PCR buffer (100 mM Tris-HCl, 500 mM KCl, 0.8% (v/v) Nonidet P40), 10 mM dNTPs, 1 U Taq DNA polymerase, and 0.2 μM primer. All the PCR reaction components were obtained from Fermentas, Thermo Fisher Scientific, India. The amplifications were performed using a Thermal Cycler (GenePro PCR, Bioer, Portsmouth, NH, USA). For URP, ISSR and RAPD markers, PCR temperature profiles were as follows: initial DNA denaturation at 94°C for 4 min, followed by 35 PCR cycles at 94°C for 1 min, 1 min for primer specific annealing temperature (Table 2), 72°C for 2 min and a final cycle at 72°C for 10 min. All the amplified PCR products were resolved by electrophoresis on 1.6% agarose for 2.5 h in 1x TBE buffer at 80 V, stained with ethidium bromide and photographed using the Gel Documentation System (AlphaImager® Corporation, Santa Clara, CA, USA).
Data analysis
The resolved DNA bands were documented and processed for data analysis. The clear, unambiguous and reproducible bands present across the DNA samples were scored as ‘1’ and absence of bands was recorded as ‘0’. The genetic relationship among the 36 isolates was analyzed from the combined 0/1 matrix data of URP, ISSR and RAPD profiles using the software program NTSYSpc version 2.02e (Exeter Software, E. Setauket, NY, USA) (Rohlf 1998) and the Jaccard’s similarity coefficient and unweighted pair group method using arithmetic average (UPGMA) (Sneath & Sokal 1973). The resultant matrix data were also analyzed for correlation analysis and matrix correspondence test (Mantel 1967) using the same software. Polymorphism percentage was calculated as per the proportion of polymorphic bands over the total number of scored bands of respective primer. The practical utility of a marker for detecting genetic variation was estimated using GenAlEx 6.41, calculating (i) expected heterozygosity (Hn), (ii) effective multiplex ratio (EMR), (iii) polymorphism information content (PIC), (iv) marker index (MI) and (v) resolving power (RP) (Peakall & Smouse 2006; Prevost & Wilkinson 1999). The confidence level of the clustering in the dendrogram was estimated through bootstrap analysis using the PAST version 2.03 program with 1000 permutations (Hammer et al. 2001).
Results
DNA fingerprint analysis
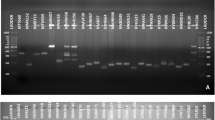
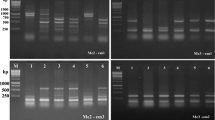
Genetic variation was detected among 36 strains of B. oryzae by using URP, ISSR and RAPD markers. Of the 13 URPs, 20 ISSR and 30 RAPD primers screened, seven URPs, eight ISSR and 14 RAPD markers gave very good reproducibility banding patterns (Fig. 1). The number of amplified products ranged from 9 to 23 in URP, 17 to 26 in ISSR and 12 to 25 in RAPD markers. The average number of amplified products obtained per primer was 16.86, 20.75 and 19.71 in URP, ISSR and RAPD, respectively. The total number of bands recorded was 1791, with an average of 255.86 in URP, 2541 with an average of 317.63 in ISSR, and 3226 with an average of 230.42 in RAPD markers. Polymorphic bands ranges recorded were between 86.3% and 100% in URP; 81.8% and 100% in ISSR; and 91.6% and 100% in the case of RAPD markers.
DNA fingerprinting profiles of 36 Bipolaris oryzae strains obtained with (a) URP 30 F, (b) ISSR 10 primers, and (c) RAPD primer OPA-9. Lanes with serial numbers 1–36 are fingerprinting profile of each B. oryzae strain (Table 1), NC – Negative control, M – 100 bp plus DNA ladder (Fermentas).
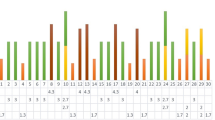
Maximum heterozygosity (Hn) was revealed by URP 30F (0.26) and URP 6R (0.24) and the least by URP 6F (0.11). In ISSR marker, maximum Hn was observed by ISSR 6 (0.36) and ISSR 15 (0.32) and the least by ISSR 1 (0.17). In the RAPD marker, the maximum Hn was revealed by OPB-1 (0.31) followed by OPA-9 and OPC-2 (0.30), and the least by OPC-4 (0.13) (Table 2). In URP-PCR, effective multiplex ratio (EMR) ranged from 8.0 to 23.0, in which URP 2F scored high EMR (23.0) followed by URP30F (19.0) and URP 17R (17). In ISSR-PCR, EMR ranged from 15.0 to 26.0, in which ISSR 15 scored high EMR (26.0) followed by ISSR -1, -18 and -19 (21.0), and the least by ISSR 20 (15.0). In RAPD marker, EMR ranged from 11.0 to 25.0, in which OPA-9 scored high EMR (25.0), followed by OPC-10 (23.0) and the least by OPC-4 (11.0). The polymorphism information content (PIC) values ranged from 0.69 to 6.43 in URP, 3.86 to 8.03 in ISSR, and 2.58 to 8.55 in RAPD marker profiles. URP2F scored the highest PIC values (6.43) among seven URPs, whereas ISSR 15 scored the highest PIC value (8.03) among eight ISSR primers and OPA-9 scored the highest PIC value (8.55) among 14 RAPD primers on genetic diversity analysis of B. oryzae (Table 2).
Based on band informativeness, the marker index (MI) values ranged from 0.88 to 5.06 in URP, 3.57 to 8.32 in ISSR, and 1.43 to 7.50 in RAPD marker studies. In URP-PCR, URP 2F showed the highest MI value (5.06) followed by URP 30F (4.94) and URP 17R (3.74). Among ISSR primers, ISSR 15 showed the highest MI value (8.32) followed by ISSR 6 (6.84) and ISSR 19 (6.10). In the case of RAPD marker, OPA-9 showed the highest MI value (7.50) followed by OPC-10 (6.44) and OPA-4 (6.38). The resolving power (RP) values ranged from 6.11 to 25.22 in URP, 10.89 to 24.67 in ISSR, and 5.39 to 19.78 in RAPD marker profiles. The highest RP was revealed by URP 30F (25.22) followed by URP 6R (15.33) and the lowest by URP 13R (6.11). In the case of ISSR markers, the highest RP value was marked by ISSR 15 (24.67) followed by ISSR 10 (20.28) and the lowest by ISSR 1 (10.89). Among RAPD markers, the highest RP value was scored by OPA-9 (19.78) followed by OPC-10 (15.33) and the lowest by OPC-4 (5.39) (Table 2).
Cluster analysis
Individual data analysis of URP marker showed the genetic similarity coefficient to be 32.1% to 87.3%. The least similarity difference (32.1%) observed between DQDO-16 and DQDO-27 strains collected from Haryana state, i.e., trans-gangetic plain region and Orissa state, i.e., eastern plateau and hills region, respectively. The highest similarity (87.3%) with bootstrap value 88% was observed between DQDO-3 and DQDO-4 strains, both collected from Chhattisgarh state, i.e., eastern plateau and hills region, and DQDO-12 and DQDO-13 strains (with bootstrap value 98%) collected from Uttar Pradesh, i.e., upper-gangetic plain regions. URP-based UPGMA analysis of all the strains was classified into three major clusters and subclusters with a few individual strains grouping – barring a few exceptions – based on geographical regions, i.e., strains from Orissa and Chhattisgarh as the first group, Uttar Pradesh and Haryana as the second group, New Delhi and Meghalaya as the third group, with a few strains from Orissa as a separate group (Fig. 2a).
Dendrogram based on UPGMA clustering of individual and pooled molecular data obtained from (a) URP, (b) ISSR, (c) RAPD, and (d) URP + ISSR + RAPD markers among 36 Bipolaris oryzae strains along with their place of collection. Correlation coefficient r, calculated by the Mantel test, represents goodness of fit of the dendrogram. The percent bootstrap values above 50 % are indicated in the major forks.
In the case of ISSR marker genetic similarity coefficient was relatively higher, ranging from 22.1% to 95.5% among all the strains and also compared with URP and RAPD. The least similarity difference was observed between DQDO-16 and DQDO-22 strains collected from Haryana state, i.e., the trans-gangetic plain region and Orissa state, i.e., eastern plateau and hills region, respectively. The highest similarity difference was observed between DQDO-29 and DQDO-36 strains (95.5% with bootstrap value 71%) collected from New Delhi state, i.e., trans-gangetic plain region and Meghalaya state, i.e., lower gangetic plain region. ISSR-based UPGMA analysis of all the strains was classified into four major clusters (12 strains belong to Chhattisgarh, Uttar Pradesh and Orissa as the first group, 11 strains belong to Haryana, New Delhi, Meghalaya and Uttar Pradesh as the second group, three strains from New Delhi and Haryana as the third group, and five strains from Tamil Nadu, Uttar Pradesh and Haryana as the fourth group) and subclusters with a few individual strains (two strains from Haryana and one strain from Orissa) grouping – barring a few exceptions – based on geographical regions (Fig. 2b).
Among all the marker systems, RAPD-based genetic similarity coefficient was relatively lower and ranged from 19.3% to 77.1% among all strains and also compared with URP and ISSR markers. The least similarity difference was observed between DQDO-16 and DQDO-29 strains collected from Haryana state, i.e., trans-gangetic plain region and New Delhi state, respectively. The highest similarity difference was observed between DQDO-30 and DQDO-31 strains (77.1% with bootstrap value 68%) both collected from New Delhi state, i.e., trans-gangetic plain region. RAPD-based UPGMA analysis of all the strains was classified into more than five major clusters and subclusters with very few individual strains grouped as a single strain (Fig. 2c).
Comparison of different marker systems
Three different aspects of the performance of marker systems (URP, ISSR and RAPD) were considered for comparison. Combined data analysis of URP, ISSR and RAPD showed genetic similarity ranging from 27.4% to 87.8%. The least similarity difference (27.4%) was observed between DQDO-16 and DQDO-22 strains collected from Haryana state, i.e., trans-gangetic plain region and Orissa state, i.e., eastern plateau and hills region, respectively. The highest similarity difference was observed between DQDO-29 and DQDO-30 strains (87.8% with bootstrap value 92%) both collected from New Delhi state, i.e., trans-gangetic plain region. Two major clusters were observed in combined data analysis, indicating all strains belong to the upper-gangetic plain (Uttar Pradesh state) as a single subcluster and other strains belong to the eastern plateau and hills region (Chhattisgarh state) and trans-gangetic plains region (Haryana state) grouped in subclusters. Similarly, we observed other major cluster strains belonging to the trans-gangetic plain region (Haryana and New Delhi states) and the eastern plateau and hills region (Orissa state) grouped in separate subclusters (Fig. 2d).
Arithmetic means of expected heterozygosity (Hn), polymorphic information content (PIC), effective multiplex ration (EMR), marker index (MI) and resolution power (RP) were calculated. The highest mean number of DNA markers (20.75), mean number of bands (317.63), mean polymorphic bands (19.75), arithmetic mean of Hn (0.27), EMR (19.75), MI (5.30) and RP (17.64) were detected with ISSR compared with URP and RAPD. The highest mean value of PIC (6.14) was observed in RAPD compared with URP and ISSR (Table 2). The Mantel test was carried out on three marker systems to obtain reliable estimates of genetic similarities among the B. oryzae strains tested with their cophenetic correlation values. Maximum cophenetic correlation was found in URP (r = 1.000) followed by ISSR (r = 0.813) and RAPD (r = 0.784) (Table 3). The combined analysis of all three marker systems showed high cophenetic correlation (r = 0.848), which indicated a good fit of the data for genetic diversity analysis. The results of the Mantel test showed a poor or fair correlation between any two marker systems (Table 3).
Discussion
Brown spot of rice caused by B. oryzae has been reported in all rice-growing countries in Asia, the Americas and Africa, but information on the variability of the pathogen’s population is limited. The first and most important factor in plant protection is sufficient information about the genetic structure of pathogen populations and more information would certainly be effective in selecting efficient strategies of pathogen control (Nagaty & El Assal 2011). In the present investigation, we collected 36 strains of B. oryzae from different geographical regions of India.
Molecular markers are used as important tools for the characterization of genetic diversity in the pathogen where morphological characteristics are either absent or not able to differentiate strains properly (Sharma et al. 1999). Nevertheless, fungal genetic characters are effectively influenced by various environmental and cultural conditions. Geographic region is also one of the major factors influencing the genetic structure of B. oryzae in India. This could be due mainly to various soil types and certain weed hosts serving as major sources of inoculum reservoir for B. oryzae (Biswas et al. 2008). Therefore, problems associated with studying different levels of genetic variability in B. oryzae have been addressed by the use of molecular marker techniques (Burgos et al. 2013; Kumar et al. 2011a; Ouedraogo et al. 2004).
The UPGMA cluster analysis based on Jaccard similarity coefficients of URP marker revealed three major genetic groups among the strains with URP 30F supported by moderate bootstrap confidence values compared with other URP primers. The critical identity of geographic populations grouped for a few isolates with few exceptions shows that the populations were not evident in the cluster analysis, indicating homogenizing effects of gene flow between populations coupled with host selection. URP marker use in the molecular analysis of a few other fungi, viz., Pleurosis, Chaetomium, Colletotrichum species, has been reported by earlier workers (Aggarwal et al. 2008; Kang et al. 2002; Kumar et al. 2011b). However, the applicability of this URP and ISSR marker system in the molecular analysis of B. oryzae has not been reported so far in the literature. The results obtained with the URP marker in either individual or combined data analysis with the ISSR and RAPD marker systems revealed differences at strain level which are not noticeable when applying conventional classification methods. Results in the present study are also in agreement with earlier workers regarding use of the URP marker system for genetic diversity analysis of fungi. This includes nucleotide alterations, insertions and deletions at initiation sites of fungi that may result in polymorphic DNA, which is detectable by the URP-PCR (Aggarwal et al. 2008; Kang et al. 2002; Kumar et al. 2011b).
ISSR markers are useful for studying genetic diversity in B. oryzae isolated from different geographical regions. These are rapid, reproducible and produce a large number of polymorphic bands and aid in the understanding of pathogen population dynamics, which can facilitate the development of effective control strategies. ISSR-based UPGMA analysis of all the strains were classified into four major clusters and subclusters. These results are in agreement with previous reports in which the ISSR marker showed good polymorphism within and between Alternaria spp. (Hong et al. 2006), Rhizoctonia spp. (Li et al. 2011) and Fusarium oxysporum f. sp. ciceris (Dubey et al. 2012).
Clustering based on UPGMA for 14 RAPD markers classified 36 B. oryzae strains into two major clusters and nine subclusters depending on percent similarity coefficient, although RAPD PCR analysis is widely used for discriminating among closely related organisms. In our study, with few exceptions most of the strains were able to cluster together in the dendrogram, according to their geographical origin, which strengthened the belief that RAPD-based variation is related to the geographical origin of the strains. Whereas earlier reports related to molecular diversity studies of B. oryzae are partially in agreement with our present study, i.e., even RAPD data revealed huge diversity within and between B. oryzae groups, there is a lack of grouping based on geographical origin of the strains (Kumar et al. 2011a; Motlagh and Anvari 2010; Weikert-Oliveira et al. 2002).
Compared with individual marker data analysis – with the exception of URP data – pooled data were found to be more informative and showed good fit (r = 0.848) with the data for diversity analysis (Table 3). The use of these molecular data would aid in programs for the crop improvement of B. oryzae resistance in varietal deployment strategies and provide a better understanding of the genetic variability of B. oryzae strains. The extensive genotypic difference among B. oryzae strains from India implies that this pathogen can adapt to erratic climatic conditions better than a population with minor variation (McDonald et al. 1989). Although the genetic variability here was detected by different molecular marker systems, much information about the inherent variability of this representative fungus in each geographical region is the basis for screening disease resistance germplasm in various rice breeding programs.
References
Aggarwal, R., Sharma, V., Kharbikar, L. L., & Renu, S. (2008). Molecular characterization of Chaetomium species using URP-PCR. Genetics and Molecular Biology, 31, 943–946.
Akhtar, J., Kandan, A., Singh, B., Dev, U., Chand, D., Kumar, J., et al. (2013). A simple modified technique for obtaining pure cultures of seed-borne fungi. Indian Journal of Plant Protection, 42, 156–159.
Biswas, S. K., Ratan, V., Srivastava, S. S. L., & Singh, R. (2008). Influence of seed treatment with biocides and foliar spray with fungicides for management of brown leaf spot and sheath blight of paddy. Indian Phytopathology, 61, 55–59.
Burgos, M. R. G., Katimbang, M. L. B., Dela Paz, M. A. G., Beligan, G. A., Goodwin, P. H., Ona, I. P., et al. (2013). Genotypic variability and aggressiveness of Bipolaris oryzae in the Philippines. European Journal of Plant Pathology, 137, 415–429.
Dubey, S. C., Priyanka, K., Singh, V., & Singh, B. (2012). Race profiling and molecular diversity analysis of Fusarium oxysporum f. sp. ciceris causing wilt in chickpea. Journal of Phytopathology, 160, 576–587.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 9–15.
Hong, S. G., Maccaroni, M., Figuli, P. J., Pryor, B. M., & Belisario, A. (2006). Polyphasic classification of Alternaria isolated from hazelnut and walnut fruit in Europe. Mycological Research, 110, 1290–1300.
Kang, H. W., Park, D. S., Park, Y. J., You, C. H., Lee, B. M., Eun, M. Y., et al. (2002). Fingerprinting of diverse genomes using PCR with universal rice primers generated from repetitive sequence of Korean weedy rice. Molecular Cells, 13, 281–287.
Khush, G. S. (1997). Origin, dispersal, cultivation and variation of rice. Plant Molecular Biology, 35, 25–34.
Kumar, P., Anshu, V., & Kumar, S. (2011a). Morpho-pathological and molecular characterization of Bipolaris oryzae in rice (Oryzae sativa). Journal of Phytopathology, 159, 51–56.
Kumar, N., Jhang, T., Satya, V., & Sharma, T. R. (2011b). Molecular and pathological characterization of Colletotrichum falcatum infecting subtropical Indian sugarcane. Journal of Phytopathology, 159, 260–267.
Lee, F. N. (1992). Brown spot. In R. K. Webster & P. S. Gunnell (Eds.), Compendium of rice diseases (pp. 14–17). APS: St. Paul, MN, USA.
Li, Y. Q., Lei, L. P., Dong, W. H., Wang, S. M., Naito, S., & Yang, G. H. (2011). Molecular diversity of binucleate Rhizoctonia AG-A in China. Phytoparasitica, 39, 461–470.
Mantel, N. (1967). The deduction of disease clustering and a generalized regression approach. Cancer Research, 27, 209–220.
McDonald, B. A., McDermott, J. M., Goodwin, S. B., & Allard, R. W. (1989). The population biology of host–pathogen interactions. Annual Review of Phytopathology, 27, 77–94.
Mew, T. W., & Gonzales, P. (2002). A handbook of rice seedborne fungi. Los Banos, Laguna, Philippines/Enfield, NH, USA: International Rice Research Institute/Science Publishers.
Motlagh, M. R. S., & Anvari, M. (2010). Genetic variation in a population of Bipolaris oryzae based on RAPD-PCR in north of Iran. African Journal of Biotechnology, 9, 5800–5804.
Nagaty, M. A., & El Assal, S. E. (2011). Molecular characterization and genetic relationships among some grape (Vitis vinifera L.) cultivars as revealed by RAPD and SSR markers. European Journal of Experimental Biology, 1, 71–82.
Ou, S. H. (1985). Rice diseases (2nd ed.). Kew, Surrey, UK: Commonwealth Mycological Institute.
Ouedraogo, I., Correll, J. C., Boza, E. J., Cartwright, R. D., Lee, F. N., & Sankara, P. (2004). Pathogenic, molecular, and genetic diversity among Bipolaris, Drechslera, and Exserohilum species on rice. In R. J. Norman, J. F. Meullenet, K. A. K. Moldenhauer, & B. R. Wells (Eds.), Rice research studies (pp. 111–119). Fayetteville, AR, USA: AAES Research Series 529.
Peakall, R., & Smouse, P. E. (2006). GENEALEX 6: Genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6, 288–295.
Prevost, A., & Wilkinson, M. J. (1999). A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theoretical and Applied Genetics, 98, 107–112.
Rohlf, F. J. (1998). NTSYS-pc: Numerical Taxonomy and Multivariate Analysis System. Version 2.02j. Biostatistics Inc. Setauket, NY, USA: Exeter Biological Software.
Savary, S., Nelson, A., Sparks, A. H., Willocquet, L., Duveiller, E., Mahuku, G., et al. (2011). International agricultural research tackling the effects of global and climatic changes on plant diseases in the developing world. Plant Disease, 48, 1–40.
Sharma, T. R., Prachi, S., & Singh, B. M. (1999). Applications of polymerase chain reaction in phytopathogenic microbes. Indian Journal of Microbiology, 39, 79–81.
Sneath, P. H. A., & Sokal, R. R. (1973). Numerical taxonomy: The principles and practice of numerical classification. San Francisco, CA, USA: W. H. Freeman and Co.
Weikert-Oliveira, R. C. B., Resende, M. A., Valerio, H. M., Caligiorne, R. B., & Paiva, E. (2002). Genetic variation among pathogens causing Helminthosporium diseases of rice, maize and wheat. Fitopathologia Brasileira, 27, 238–246. www.knowledgebank.irri.org/ericeproduction/Importance_of_Rice.htm.
Acknowledgments
The authors gratefully acknowledge the support and encouragement of the Director, NBPGR; and the financial support of the Indian Council of Agricultural Research (ICAR), New Delhi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kandan, A., Akhtar, J., Singh, B. et al. Molecular diversity of Bipolaris oryzae infecting Oryza sativa in India. Phytoparasitica 43, 5–14 (2015). https://doi.org/10.1007/s12600-014-0430-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-014-0430-5