Abstract
Omnivorous predatory Heteroptera are important biological control agents of pests in several crops. They can feed on plant food resources that may positively affect their biological characteristics. In the current paper, the influence of leaves and flowers on the predation rate of the omnivorous predator Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae) was investigated. Its predation rates were recorded on prey offered on (a) a single leaf of tomato, pepper or black nightshade (Solanum nigrum), or (b) a leaf of pepper or S. nigrum plus flowers of pepper or S. nigrum, respectively. In all cases the aphid Myzus persicae (Sulzer) (Homoptera: Aphididae) was used as prey at densities of 4, 8, 12, 16, 20 and 24 nymphs of the second instar. The experiments were conducted in petri dishes at 25 ± 1°C and prey consumption was evaluated after 24 h. The predation rate of M. pygmaeus was significantly higher on leaves of S. nigrum than on those of pepper at the prey density of 20 prey items. Therefore, the hypothesis that increased predation rates should occur on plants of lower suitability for development or reproduction was not supported under our experimental conditions. The flower availability did not alter the prey consumption among the prey densities on S. nigrum. However, the presence of a pepper flower caused a significant decrease in the predation rates on pepper leaves, at prey densities higher than eight prey items. Thus, pepper flowers can provide the predator with nutrient sources that may partially substitute for prey consumption, with practical implications in biological control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Omnivory, i.e., feeding on more than one trophic level, occurs widely in terrestrial plant-inhabiting arthropod communities (Polis & Strong 1996). The feeding on both prey and plant food resources is widespread among the predatory Heteroptera that are natural enemies of several arthropod pests in managed and natural ecosystems (Coll & Guershon 2002).
The omnivorous predator Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae) is considered to be among the major natural enemies of whiteflies, Lepidoptera and aphids in protected and open-field vegetable crops (Arnó et al. 2009; Perdikis et al. 2008; Urbaneja et al. 2009) and is commonly used in augmentative biological control programs in Europe (van Lenteren 2012). Feeding exclusively on the plant, the nymphs of this predator reached adulthood with a high survival rate, and females showed higher longevity (i.e., average female longevity was 25 days on eggplant and tomato) (Lykouressis et al. 2008; Perdikis & Lykouressis 2000, 2004a, b).
Despite the widely accepted evidence that plant-derived resources are an important diet component among predaceous mirid bugs (Eubanks & Styrsky 2005; Lundgren 2009; Pumariño et al. 2011), the ability of these predators to utilize plant food resources available in flowers has rarely been investigated. Braman & Beshear (1994) reported that the thrips predator Dicyphus rhododendri (Dolling) (Hemiptera: Miridae) can feed on pollen within flowers.
In addition, M. pygmaeus adults have been recorded in flowers of the weed Ecbalium elaterium (L.) (Cucurbitaceae) (Perdikis & Lykouressis 2000) and in several cases in pepper flowers (D. Perdikis and co-workers, personal observations). Furthermore, M. pygmaeus can complete its development in petri dishes, feeding on bee pollen pellets or stamens cut from fully blooming flowers of E. elaterium, without any other plant or prey source but only water offered on moistened cotton. In these cases nymphal development was completed in a similar period to that recorded when feeding on leaves of eggplant, which was rated as a more suitable plant than tomato or pepper (Perdikis & Lykouressis 2000, 2004a,b). The developmental duration of M. pygmaeus was significantly shortened when bee pollen pellets were offered together with eggplant leaves and it was similar to that recorded when the mirid fed on eggplant leaves infested with the highly suitable prey Myzus persicae (Sulzer) (Homoptera: Aphididae) (Perdikis & Lykouressis 2000). Maleki et al. (2006) reported also that pollen favored the development and the fecundity of M. pygmaeus. Vandekerkhove & De Clercq (2010) showed that development and reproduction were efficiently supported when bee pollen pellets replaced part of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs in the diet of M. pygmaeus. Further evidence can be derived from the study of Portillo et al. (2012), who showed that the longevity of M. pygmaeus females on broad bean plants without extrafloral nectaries was significantly longer in the presence than in the absence of cattail pollen. It is also noteworthy that cattail pollen supported the establishment of the predator on tomato crops (Put et al. 2012).
Parallel to the effects on the life history traits, i.e., development and reproduction, feeding on plant material may affect the prey consumption rate of an omnivorous predator. Foraging on low-quality plant food sources has been reported to result in increased prey consumption (Agrawal et al. 1999; Agrawal & Klein 2000; Eubanks & Denno 2000; Magalhães et al. 2005). On the other hand, plant feeding by predatory mirids is generally more intense in prey scarcity (Castañé et al. 2011).
Although regarded as efficiently exploiting plant resources, the prey consumption rates of M. pygmaeus on leaves of different plants have been but little researched (Perdikis et al. 1999; Pumariño et al. 2011). Similarly, although pollenivory can support development, survival and reproduction in M. pygmaeus, its influence on prey consumption rates by this predator has not been fully explored. In fact, according to Eubanks & Styrsky (2005), the effect of the omnivorous predators on prey population as influenced by the host plant quality is the most important unanswered question regarding omnivorous predators.
The current study focused on the effects of solanaceous plant food sources (leaves or leaves with flowers) on the predation rate of the omnivorous predator M. pygmaeus under a wide range of availability rates of a highly suitable natural prey, the aphid M. persicae. Our hypotheses were: (a) the mirid should increase its predation rates when foraging on leaves of lower suitability for survival and reproduction, and (b) the mirid should reduce its prey consumption when feeding on a flower that offers nutritional benefits to the predator.
Materials and methods
Predator and plants
Adults and nymphs of M. pygmaeus were collected from a tomato field in Co. Boeotia, central Greece, and bred on potted eggplants (cv. ‘Bonica’) infested with M. persicae. Pure culture of M. persicae was also kept on other potted eggplants.
Sweet pepper (cv. ‘Vidi’) and tomato (cv. ‘Arletta’) plants were grown from seeds, while Solanum nigrum L. (Solanaceae) plants – which best supports development and reproduction of M. pygmaeus in certain areas of Greece (Lykouressis et al. 2008) – were grown from seedlings collected at the campus of the Agricultural University of Athens, Greece.
Lone insect and plant breedings were maintained in wood-framed muslin cages (length 80 cm × width 80 cm × height 70 cm), in a glasshouse at 22.5 ± 3.0°C (mean ± S.D.), under natural lighting and without the use of any chemicals.
Predation assays
Two experimental schemes were followed: (a) only leaves of pepper or S. nigrum or a leaflet of tomato; and (b) one flower plus one leaf of pepper or S. nigrum.
Assays were conducted in plastic petri dishes (9 cm diam.), bearing a hole (3 cm diam) in the lid provided with fine muslin to prevent excessive humidity inside the dish. A layer of moistened cotton was placed on the bottom of each dish, the leaf/leaflet was placed upside down on it and the leaf/leaflet and/or flower petiole was covered with moistened cotton to maintain the organ turgor. The leaves were collected from young plants (6–8 leaves) and the flowers were collected at full bloom from mature plants.
Second instar nymphs of M. persicae were added as prey for the predator on the leaf/leaflet of each petri dish. The aphids were carefully dislodged and left undisturbed for 1 h to settle. Six prey densities were used: 4, 8, 12, 16, 20 and 24 aphids per leaf. These densities were selected based on a previous study showing that the predator’s fifth instar nymphs were satiated when 24 second instar nymphs of M. persicae were offered (Fantinou et al. 2008). Finally, a single 2-day-old fifth instar nymph of the predator was introduced into each dish.
After predator introduction, the dishes were introduced into a growth chamber at 25 ± 1°C, 65 ± 5% r.h. and 16:8 h L:D and the number of consumed aphids was recorded 24 h later. The assay was replicated ten times (predators) for each prey density.
The aphid mortality due to manipulations was examined as control (without predator) and it was found to be negligible.
Statistical analysis
Exploratory analysis showed the data residuals were not normally distributed. For this reason the data were analyzed with Generalized Linear Models with Poisson errors and log link function. The predation rate data recorded when M. pygmaeus fed on leaf (pepper, S. nigrum and tomato) without flower were analyzed with the factors: “host plant” and “prey density”. The data on the predation rates when the predator fed on pepper or on S. nigrum leaf with or without flower were analyzed with the factors: “flower availability or not” and “prey density”. Predation rates between S. nigrum and pepper leaf with flower were analyzed with the factors: “host plant” and “prey density”. In all cases, comparisons between means at each prey density were conducted using contrasts.
Analyses were performed using the statistical package JMP (SAS Institute 2008).
Results
Predation rate of M. pygmaeus on leaves without flowers
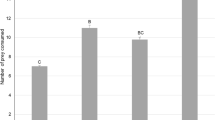
The predation rate of M. pygmaeus was recorded on leaves of pepper, S. nigrum and tomato at different prey densities. Data analyses showed that the effect of the host plant was significant (χ 2 2,162 = 6.04, P<0.047) (Fig. 1). Contrasts showed that predation rates on S. nigrum leaf were higher than on pepper leaf (χ 2 1=5.64, P<0.017) but between S. nigrum and tomato and also between tomato and pepper significant differences were not recorded (χ 2 1=0.35, P>0.55 and χ2 1=3.11, P>0.08, respectively). Among the host plants and at each prey density, a significantly higher predation rate was recorded on S. nigrum than pepper leaf at the prey density of 20 prey items.
Predation rates (mean±SE) of the omnivorous predator Macrolophus pygmaeus when feeding on different densities of its prey, 2nd instar nymphs of Myzus persicae, offered on a leaf of pepper, Solanum nigrum or tomato, in a petri dish for 24 h at 25 ± 1°C. Columns labeled with the same letter do not differ significantly among the three host plants within each prey density
Effect of flower supplement on the predation rate of M. pygmaeus at different levels of prey availability
The predation rate of M. pygmaeus was significantly lower on a pepper leaf with a flower than a pepper leaf without flower (χ 2 1,108 = 42.7, P<0.001) (Fig. 2a). Additionally, a significant interaction between the factors: “flower availability or not” and “prey density” was recorded (χ 2 5,108 = 13.1, P<0.02). Contrasts showed that predation rates on pepper leaf were higher than on pepper leaf with flower at the prey densities of 12, 16, 20 and 24 aphids (χ 2 1=30.94, P<0.001, χ 2 1=23.32, P<0.001, χ 2 1=7.71, P<0.001 and χ 2 1=25.70, P<0.001, respectively).
Predation rates (mean±SE) of the omnivorous predator Macrolophus pygmaeus when feeding on different densities of its prey, 2nd instar nymphs of Myzus persicae, offered on a leaf: (a) of pepper with or without a single flower, (b) on a leaf of Solanum nigrum with or without a single flower, and (c) on a leaf of pepper or a leaf of S. nigrum together with a single flower of each respective host plant, in a petri dish for 24 h at 25 ± 1°C. Columns labeled with the same letter do not differ significantly between the two treatments in each prey density
The predation rates on S. nigrum leaf with flower were similar to those without flower (χ 2 1,108 = 1.19, P>0.27), without differing significantly at each prey density (Fig. 2b).
Finally, the predation rates on pepper leaf with flower were significantly lower in comparison with those recorded on S. nigrum leaf plus flower (χ 2 1,108 = 64.34, P<0.001) (Fig. 2c). Significant differences were recorded between the treatments at the prey densities of 12, 16, 20 and 24 aphids (χ 2 1=32.44, P<.001, χ 2 1=21.09, P<0.001, χ 2 1=23.85, P<0.001 and χ 2 1=22.18, P<0.001, respectively).
Discussion
The predation rate of M. pygmaeus on leaves of S. nigrum was significantly higher in comparison with pepper when 20 prey items were used, whereas no significant differences were detected between S. nigrum and tomato leaves. The observed differences could be associated with evidence of the suitability of these plants as food sources for the predator. On leaves of S. nigrum and tomato cv. Arletta, which was also used here, without prey, the intrinsic rate of population increase of M. pygmaeus at 25°C was 0.0091 d-1 and 0.0021 d-1, respectively, with average fecundity of 9.30 eggs on S. nigrum and 5.71 eggs on tomato, whereas on pepper (cv. Vidi) no female oviposited (Lykouressis et al. 2008; Perdikis & Lykouressis 2004a,b). Therefore, despite the very low suitability of S. nigrum and tomato leaves to support the population increase of the predator in the absence of prey, both plants may be of a comparatively higher quality than pepper. These outcomes do not support the hypothesis that increased prey feeding should occur on lower quality host plants (i.e., pepper in our case), as shown in other studies (Eubanks & Denno 2000; Janssen et al. 2003; Magalhães et al. 2005). Likely, these outcomes might have been influenced by additional factors, such as trichome density, plant allelochemicals or plant chemical composition, which could reduce the overall quality of the plant food, also affect the quality of the prey and, finally, interfere with the foraging behavior of the predator (Coll & Ridgway 1995; De Clercq et al. 2000; Francis et al. 2001; Madadi et al. 2007).
In the combined treatments, where leaf with prey together with a single flower of S. nigrum or pepper were offered to the predator, there was a significant decrease in the prey consumption rates in relation to the aphid densities on pepper but not on S. nigrum. This suggests that pepper flowers and aphids might be partially substitutable in the M. pygmaeus diet. The pepper flowers are well known sources of pollen (Karapanos et al. 2009) but they produce very small quantities of nectar that do not exceed 10 μl per flower (Roldán-Serrano & Guerra-Sanz 2004). Therefore, the observed decrease in prey consumption when pepper flower was supplied might be due to the predator pollenivory. In the case of predatory mites, prey consumption was significantly decreased when pollen was supplied (Badii et al. 2004; Robinson et al. 2008; van Rijn & Sabelis 2005). Conversely, in a similar experiment, the presence of pollen did not alter the predation rates of Coleomegilla maculata DeGeer (Coleoptera: Coccinellidae) on a series of prey densities indicating very low or no pollen consumption (Hazzard & Ferro 1991).
Alternatively, the addition of a flower in the arena could increase the spatial complexity that, in sequence, might reduce the time available for prey searching and feeding by the foraging predator (Hoddle 2003). However, in our case prey feeding of M. pygmaeus on a S. nigrum leaf remained unchanged when a S. nigrum flower was added in the experimental arena. Considering that the flowers of both species are of similar size, the increase in spatial complexity most likely might not have been significantly involved in the observed lowering of the predation rate of the predator.
Plant feeding covers, at a variable rate, the nutritional needs of omnivorous predators and, thus, decreases their predation rates (Holt & Lawton 1994). Despite a lower per capita predation rate, plant food of high suitability may support higher predator numbers, which in the long term may cause a more intense adverse effect on the prey population (Nomikou et al. 2010; Robinson et al. 2008).
It was further documented here that when a pepper flower was available to the predator, prey consumption decrease was significant at higher prey densities. Wei & Walde (1997) reported a 20% reduction in prey consumption by Typhlodromus pyri Scheuten (Acari: Phytoseiidae) in the presence of pollen, a phenomenon that occurred only at higher prey densities. These authors stated that an upper limit may exist in prey availability above which prey consumption can be replaced by pollen feeding. Our results support this assumption and indicate that further research is required to quantify replacement of prey consumption in the presence of plant food resources, which might be useful knowledge in studying life history strategies of natural enemies (Lundgren et al. 2005).
One important shortcoming in the use of M. pygmaeus in pest control is its slow establishment rate (Castañé et al. 2006). In order to overcome this issue, the addition of food supplements such as eggs of E. kuehniella or pollen can benefit the population build-up of the predator (Put et al. 2012). Evidence of the present study indicates that the establishment of the predator on pepper crops can be enhanced if it is released when flowers have appeared on the crop. Furthermore, feeding on flowers was not found to affect the prey consumption at low prey densities, which usually is the case during the period of the predator’s establishment on a crop. However, the applicability of these results should be studied for a longer period and under pragmatic field conditions, considering that the ladybird omnivorous predator C. maculata consumed a smaller amount of pollen in the field than that expected by the laboratory experiments (Lundgren et al. 2005).
In conclusion, the evidence obtained indicates that: (i) feeding on leaves of tomato, pepper and S. nigrum affected prey consumption, but the hypothesis that increased predation rates should occur on plants of lower suitability was not supported by the results; and (ii) feeding on pepper flowers supplied on the leaves reduced prey consumption when prey was available at increased densities.
References
Agrawal, A. A., & Klein, C. N. (2000). What omnivores eat: direct effects of induced plant resistance on herbivores and indirect consequences for diet selection by omnivores. Journal of Animal Ecology, 69, 525–535.
Agrawal, A. A., Kobayashi, C., & Thaler, J. S. (1999). Influence of prey availability and induced host plant resistance on omnivory by western flower thrips. Ecology, 80, 518–523.
Arnó, J., Sorribas, R., Prat, M., Matas, M., Pozo, C., Rodríguez, D., et al. (2009). Tuta absoluta, a new pest in IPM tomatoes in the northeast of Spain. IOBC/WPRS Bulletin, 49, 203–208.
Badii, M. H., Hernandez-Ortiz, E., Flores, A. E., & Landeros, J. (2004). Prey stage preference and functional response of Euseius hibisci to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Experimental and Applied Acarology, 34, 263–273.
Braman, S. K., & Beshear, R. J. (1994). Seasonality of predaceous plant bugs (Heteroptera, Miridae) and phytophagous thrips (Thysanoptera, Thripidae) as influenced by host-plant phenology of native azaleas (Ericales, Ericaceae). Environmental Entomology, 23, 712–718.
Castañé, C., Alomar, O., Riudavets, J., & Gemeno, C. (2006). Reproductive traits of the generalist predator Macrolophus caliginosus. IOBC/WPRS Bulletin, 29, 229–234.
Castañé, C., Arnó, J., Gabarra, R., & Alomar, O. (2011). Plant damage to vegetable crops by zoophytophagous mirid predators. Biological Control, 59, 22–29.
Coll, M., & Guershon, M. (2002). Omnivory in terrestrial arthropods: mixing plant and prey diets. Annual Review of Entomology, 47, 267–297.
Coll, M., & Ridgway, R. L. (1995). Functional and numerical responses of Orius insidiosus (Heteroptera: Anthocoridae) to its prey in different vegetable crops. Annals of the Entomological Society of America, 88, 732–738.
De Clercq, P., Mohaghegh, J., & Tirry, L. (2000). Effect of host plant on the functional response of the predator Podisus nigrispinus (Heteroptera: Pentatomidae). Biological Control, 18, 65–70.
Eubanks, M. D., & Denno, R. F. (2000). Host plants mediate omnivore-herbivore interactions and influence prey suppression. Ecology, 81, 936–947.
Eubanks, M. D., & Styrsky, J. D. (2005). Effects of plant feeding on the performance of omnivorous “predators”. In: F. L. Wäckers, P. C. J. van Rijn & J. Bruin (Eds.) Plant-provided food and herbivore–carnivore interactions (pp. 148–177). Cambridge, UK: Cambridge University Press.
Fantinou, A., Perdikis, D., & Maselou, D. (2008). Prey killing without consumption: does predator show adaptive foraging behavior? Biological Control, 47, 187–193.
Francis, F., Lognay, G., Wathelet, J. P., & Haubruge, E. (2001). Effects of allelochemicals from first (Brassicaceae) and second (Myzus persicae and Brevicoryne brassicae) trophic levels on Adalia bipunctata. Journal of Chemical Ecology, 27, 243–256.
Hazzard, R. V., & Ferro, D. N. (1991). Feeding responses of adult Coleomegilla maculata (Coleoptera, Coccinellidae) to eggs of Colorado potato beetle (Coleoptera, Chrysomelidae) and green peach aphids (Homoptera, Aphididae). Environmental Entomology, 20, 644–651.
Hoddle, M. S. (2003). The effect of prey species and environmental complexity on the functional response of Franklinothrips orizabensis: a test of the fractal foraging model. Ecological Entomology, 28, 309–318.
Holt, R. D., & Lawton, J. H. (1994). The ecological consequences of shared natural enemies. Annual Review of Ecology and Systematics, 25, 495–520.
Janssen, A., Willemse, E., & van der Hammen, T. (2003). Poor host plant quality causes omnivore to consume predator eggs. Journal of Animal Ecology, 72, 478–483.
Karapanos, I. C., Mahmood, S., & Thanopoulos, C. (2009). Fruit set in solanaceous vegetable crops as affected by floral and environmental factors. The European Journal of Plant Science & Biotechnology, 2(Special Issue 1), 45–61.
Lundgren, J. G. (2009). Relationships of natural enemies and non-prey foods. Dordrecht: Springer International.
Lundgren, J., Huber, A., & Wiedenmann, R. N. (2005). Quantification of consumption of corn pollen by the predator Coleomegilla maculata (Coleoptera: Coccinellidae) during anthesis in an Illinois corn field. Agricultural & Forest Entomology, 7, 53–60.
Lykouressis, D., Giatropoulos, A., Perdikis, D., & Favas, C. (2008). Assessing the suitability of non-cultivated plants and associated insect prey as food sources for the omnivorous predator Macrolophus pygmaeus (Hemiptera: Miridae). Biological Control, 44, 142–148.
Madadi, H., Enkegaard, A., Brodsgaard, H. F., Kharrazi-Pakdel, A., Mohaghegh, J., & Ashouri, A. (2007). Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. Journal of Applied Entomology, 131, 728–733.
Magalhães, S., Janssen, A., Montserrat, M., & Sabelis, M. W. (2005). Host-plant species modifies the diet of an omnivore feeding on three trophic levels. Oikos, 111, 47–56.
Maleki, F., Ashouri, A., Mohaghegh, J., & Bandani, A. (2006). Effects of some diets on Macrolophus pygmaeus Rambur (Hemiptera: Miridae) fitness under laboratory conditions. Communications in Applied Biological Sciences Ghent University, 71, 393–397.
Nomikou, M., Sabelis, M. W., & Janssen, A. (2010). Pollen subsidies promote whitefly control through the numerical response of predatory mites. Biocontrol, 55, 253–260.
Perdikis, D., Kapaxidi, E., & Papadoulis, G. (2008). Biological control of insect and mite pests in greenhouse solanaceous crops. In H. Passam (Ed.) The fruiting species of the Solanaceae. The European Journal of Plant Science and Biotechnology, 2(Special Issue 1), 125–144.
Perdikis, D., & Lykouressis, D. (2000). Effects of various items, host plants and temperatures on the development and survival of Macrolophus pygmaeus Rambur (Hemiptera: Miridae). Biological Control, 17, 55–60.
Perdikis, D., & Lykouressis, D. (2004a). Macrolophus pygmaeus (Hemiptera: Miridae) population parameters and biological characteristics when feeding on eggplant and tomato without prey. Journal of Economic Entomology, 97, 1291–1298.
Perdikis, D. C., & Lykouressis, D. P. (2004b). Myzus persicae (Homoptera: Aphididae) as a suitable prey for Macrolophus pygmaeus (Hemiptera: Miridae) population increase on pepper plant. Environmental Entomology, 33, 499–505.
Perdikis, D. C., Lykouressis, D. P., & Economou, L. P. (1999). The influence of temperature, photoperiod and plant type on the predation rate of Macrolophus pygmaeus (Rambur) on Myzus persicae. Biocontrol, 44, 281–289.
Polis, G. A., & Strong, D. R. (1996). Food web complexity and community dynamics. American Naturalist, 147, 813–846.
Portillo, N., Alomar, O., & Wäckers, F. L. (2012). Nectarivory by the plant-tissue feeding predator Macrolophus pygmaeus Rambur (Heteroptera: Miridae): Nutritional redundancy or nutritional benefit? Journal of Insect Physiology, 58, 397–401.
Pumariño, L., Alomar, O., & Agustí, N. (2011). Development of specific ITS markers for plant DNA identification within herbivorous insects. Bulletin of Entomological Research, 101, 271–276.
Put, K., Bollens, T., Wäckers, F. L., & Pekas, A. (2012). Type and spatial distribution of food supplements impact population development and dispersal of the omnivore predator Macrolophus pygmaeus (Rambur) (Hemiptera: Miridae). Biological Control, 63, 172–180.
Robinson, K. A., Jonsson, M., Wratten, S. D., Wade, M. R., & Buckley, H. L. (2008). Implications of floral resources for predation by an omnivorous lacewing. Basic and Applied Ecology, 9, 172–181.
Roldán-Serrano, A. S., & Guerra-Sanz, J. M. (2004). Dynamics and sugar composition of sweet pepper (Capsicum annuum L.) nectar. Journal of Horticultural Science & Biotechnology, 79, 717–722.
SAS Institute. (2008). JMP version 8.0. Cary, NC, USA: SAS Institute Inc.
Urbaneja, A., Montón, H., & Mollá, O. (2009). Suitability of the tomato borer Tuta absoluta as prey for Macrolophus caliginosus and Nesidiocoris tenuis. Journal of Applied Entomology, 133, 292–296.
Vandekerkhove, B., & De Clercq, P. (2010). Pollen as an alternative or supplementary food for the mirid predator Macrolophus pygmaeus. Biological Control, 53, 238–242.
Van Lenteren, J. C. (2012). The state of commercial augmentative biological control: plenty of natural enemies but a frustrating lack of uptake. Biocontrol, 57, 1–20.
Van Rijn, P. C. J., & Sabelis, M. W. (2005). Impact of plant-provided food on herbivore–carnivore dynamics. In: F. L. Wäckers, P. C. J. van Rijn & J. Bruin (Eds.) Plant-provided food for carnivorous insects: A protective mutualism and its applications (pp. 223–266). Cambridge, UK: Cambridge University Press.
Wei, Q. C., & Walde, S. J. (1997). The functional response of Typhlodromus pyri to its prey, Panonychus ulmi: the effect of pollen. Experimental and Applied Acarology, 21, 677–684.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lykouressis, D., Perdikis, D. & Charalampous, P. Plant food effects on prey consumption by the omnivorous predator Macrolophus pygmaeus . Phytoparasitica 42, 303–309 (2014). https://doi.org/10.1007/s12600-013-0360-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-013-0360-7