Abstract
Plectosphaerella cucumerina has been described recently as the causal agent of a leaf spot on wild rocket (Diplotaxis tenuifolia). Eight seed samples of wild rocket obtained from commercial seed lots used for sowing by farms severely affected by P. cucumerina, were assayed for the presence of the pathogen. Isolations were carried out on subsamples of seeds (400) unwashed or disinfected in 1% sodium hypochloride. The pathogenicity of the isolates of P. cucumerina obtained was tested in two trials carried out on wild rocket; four out of eight samples of rocket seeds were contaminated by P. cucumerina. Eleven isolates of P. cucumerina were obtained from 7,200 not disinfected seeds tested, while none was isolated from an equal number of disinfected seeds. All isolates were pathogenic on wild rocket. The results obtained indicate that rocket seeds are a potential source of inoculum for P. cucumerina. The possibility of isolating the pathogen from seeds, albeit from a low percentage of them, supports the hypothesis that the rapid spread of this new disease of rocket recently observed in Italy is due to the use of infected propagation material. Measures for prevention and control of the disease are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild rocket (Diplotaxis tenuifolia) is now widely cultivated and increasingly used in the Mediterranean cuisine both as a component of mixed salad and to decorate dishes. During spring 2012, symptoms of an unusual leaf spot disease were observed in several commercial greenhouses in southern Italy (near Salerno) and in northern Italy (near Bergamo) on plants of D. tenuifolia cv. Selvatica. The causal agent of the disease was identified as Plectosphaerella cucumerina (Garibaldi et al. 2012). The first symptoms on leaves of affected plants consisted of small (1 mm) black-brown spots of irregular shape, later coalescing into larger spots, 1 cm in diameter. Spots were surrounded by a yellow–gray halo, and were located mostly on the foliar limb, rib and petiole. Affected leaves were often distorted, appearing hook-like. The disease was severe under 75–90% r.h., at an air temperature of 20–26°C, and caused severe production losses. Affected tissues rotted quickly after packaging, during transit and commercialization of processed rocket (Garibaldi et al. 2012). The same pathogen is associated with root and collar rots of horticultural crops in Italy (Carlucci et al. 2012; Matta & Garibaldi 1981) and very recently has been observed on endive (Garibaldi et al. 2013). On wild rocket (D. tenuifolia), the disease has not yet been reported in other countries.
Circumstantial evidence from surveys in the area affected by the disease suggested that the sudden appearance of this disease may have been due to the transmission of the pathogen by seeds.
The present study was undertaken to ascertain the extent of and variation in occurrence of P. cucumerina in rocket seeds.
Materials and methods
Seed infection evaluation
Two seed samples of D. tenuifolia were obtained from the commercial farm where the disease was first observed (Salerno) and six seed lots were obtained from commercial farms located in Lombardy, where the disease was observed later (Gilardi et al. 2012) (Table 1) a total of eight seed samples were assayed for the presence of P. cucumerina (Table 2).
Subsamples represented by 400 seeds were tested on 90-mm-diam petri plates (ten seeds per plate) containing potato dextrose agar (PDA) to which was added streptomycin sulphate at 25 mg l −1, by following the method described by Mathur & Kongsdal (2003). Isolations were carried out on seeds only washed in distilled water (not disinfected) or disinfected by soaking for 1 min in 1% sodium hypochloride and dried. Plates were incubated for 10 days with 12 h per day of fluorescent light at 22°C. Forty plates were prepared per trial. Each sample was checked at least twice. Seeds infected by P. cucumerina were surrounded by a whitish-orange mycelium. The identification of the colonies of P. cucumerina were confirmed by microscopic observation of hyaline elliptical and ovoid conidia borne on phialides that developed from a whitish-orange mycelium produced on PDA (Palm et al. 1995) and by analysis of internal transcribed spacer (ITS) region (Garibaldi et al. 2012).
Isolates used and their preservation
The isolates obtained from seeds were coded as reported in Tables 3, 4 and 5. Two strains of P. cucumerina, obtained from infected leaves from Salerno (southern Italy) (coded RS-CC1, GenBank Accession No. AB469880) and PLC-27 from Bergamo (northern Italy), respectively, were used as controls. The different strains were maintained on PDA at 8°C.
Production of inoculum and pathogenicity test
The various isolates of P. cucumerina were grown in petri plates on PDA to which was added 25 mg l −1 of streptomycin sulphate, incubated for 7 days at 23°C with 12 h per day of fluorescent light. Spore suspensions were prepared from the single isolates (Table 5). The concentration of spores was determined by hemacytometer and adjusted with deionized water to 1 × 106 CFU (colony forming units) ml−1.
Seeds of Diplotaxis tenuifolia cv. Selvatica (Suba), previously disinfected by soaking for 1 min in 1% sodium hypochloride (disinfected) and washed in distilled water were sown in a steamed soil mixture [with steamed mix soil of 50% Tecno2 (70% white peat and 30% clay) and 50% of Tiesse3 (60% white peat, 20% clay 20% perlite); Turco Silvestro terricci, Bastia d’Albenga, SV] in 2 l pots and maintained at 25°C, with 12 h per day of fluorescent light. Three replicates were used, each consisting of 10–15 plants.
Thirty-day-old plants were artificially inoculated by spraying with a spore suspension (1 × 106 CFU ml−1) of the different isolates. As comparison, the isolates of P. cucumerina coded RS-CC1 and PLC-27 obtained from wild rocket in Salerno and in Lombardy, respectively, were used. Control plants were sprayed with water.
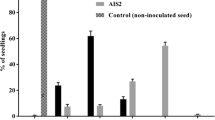
Typical symptoms of P. cucumerina started to be visible 8 days after artificial inoculation. Plants were checked for disease development and the percent of infected leaves was evaluated. The data were expressed as percent of infected leaves 15 days after the artificial inoculation (Table 5). P. cucumerina was consistently reisolated from the lesions. Data were statistically processed by means of variance analysis (ANOVA) and Tukey’s test (P < 0.05).
Results and discussion
Four out of eight samples of wild rocket seeds, used for sowing in farms severely affected by P. cucumerina, were contaminated by the pathogen (Tables 3 and 4) and eleven isolates were obtained out of 7,200 seeds which had not been disinfected. Seed disinfection with sodium hypochlorite reduced seed infection to below detection level so that from disinfected seeds it was not possible to isolate any strain of P. cucumerina.
Eleven isolates of P. cucumerina obtained from the different seed lots, were coded (Tables 3 and 4), maintained in culture and tested in two trials for their pathogenicity on D. tenuifolia. The two trials provided consistent results. All eleven isolates obtained from seeds were pathogenic on the cultivar Selvatica (Table 5); inoculated plants showed typical symptoms. The virulence of the isolates obtained from seeds was similar to that of isolates obtained in the field from infected plants of D. tenuifolia.
The recent outbreak of P. cucumerina on wild rocket represents a potential threat to rocket production in Italy. The disease has been detected on wild rocket, widely grown for processing. Identifying the primary source of inoculum is of critical importance for effective disease management.
This paper provides evidence that P. cucumerina is frequently seed-transmitted (four samples out of eight were contaminated), which suggests that seeds may be important in disseminating the pathogen.
The results of this study do not provide information on the effects of P. cucumerina on the quality and germination ability of rocket seeds. Our findings indicate that rocket seeds are a potential source of inoculum for development of P. cucumerina. The fast spread of the disease that occurred first in southern Italy in 2012, rapidly moving in a few months to northern Italy (Gilardi et al. 2012), permits us to hypothesize that the pathogen was introduced into Italy through infected seeds.
Further research should be carried out to determine the epidemiological significance of seedborne inoculum as well as efficient methods to eliminate this threat to rocket production. The use of P. cucumerina-free certified propagation material will become an essential prerequisite for worldwide distribution of this crop. Seed dressing with registered and effective fungicides should also represent one more option for disease management. Such treatments should also take into consideration the possible contamination of rocket seeds, as already reported, by Fusarium wilt agents (Garibaldi et al. 2004). The fact that no isolates were obtained from disinfected seeds allows us to speculate that the pathogen is an external contaminant of seeds. In such a case, seed disinfection should help in reducing the dissemination of the pathogen. In addition to the use of chemicals, other control methods should be exploited: a method based on the use of aerated steam, which proved effective in the control of seedborne diseases of cereals (Forsberg et al. 2005) and of legumes will be tested, as well as the use of biocontrol agents and natural products (Tinivella et al. 2009).
Since the conventional pathogen detection techniques may lack the sensitivity required to detect seedborne pathogens, the detection threshold of P. cucumerina in rocket seeds could be increased by using molecular techniques, such as PCR and RAPD, as already shown in the case of Fusarium wilt of basil (Chiocchetti et al. 2001), lettuce (Mbofung & Pryor 2010; Pasquali et al. 2007) and other vegetables (Lievens et al. 2012) and in the case of Phoma valerianellae in lamb’s lettuce seeds (Pellegrino et al. 2010). Interestingly, it should be noted that PCR and Real-Time PCR methods have been developed already for the detection and quantification of P. cucumerina, when used as a biocontrol agent of potato cyst nematodes (Globodera spp.) (Atkins et al. 2003).
References
Atkins, S. D., Clark, I. M., Sosnowska, D., Hirsch, P. R., & Kerry, B. R. (2003). Detection and quantification of Plectosphaerella cucumerina, a potential biological control agent of potato cyst nematodes, by using conventional PCR, Real-Time PCR, selective media, and baiting. Applied and Environmental Microbiology, 69, 4788–4793.
Carlucci, A., Raimondo, M. L., Santos, J., & Phillips, A. J. L. (2012). Plectosphaerella species associated with root and collar rots of horticultural crops in southern Italy. Persoonia, 28, 34–48.
Chiocchetti, A., Sciaudone, L., Durando, F., Garibaldi, A., & Migheli, Q. (2001). PCR detection of Fusarium oxysporum f. sp. basilici on basil. Plant Disease, 85, 607–611.
Forsberg, G., Johnsson, L., & Lagerholm, J. (2005). Effects of aerated steam seed treatment on cereal seed-borne diseases and crop yield. Journal of Plant Diseases and Protection, 112, 247–256.
Garibaldi, A., Gilardi, G., Pasquali, M., Keiji, S., & Gullino, M. L. (2004). Seed transmission of Fusarium oxysporum of Eruca vesicaria and Diplotaxis muralis. Journal of Plant Diseases and Protection, 111, 345–350.
Garibaldi, A., Gilardi, G., Ortu, G., & Gullino, M. L. (2012). First report of Plectosphaerella cucumerina on greenhouse cultured wild rocket (Diplotaxis tenuifolia) in Italy. Plant Disease, 96, 1825.
Garibaldi, A., Gilardi, G., Ortu, G., & Gullino, M.L. (2013). First report of Plectosphaerella cucumerina on field grown endive (Cichorium endivia) in Italy. Plant Disease, 97, in press.
Gilardi, G., Ortu, G., Gullino, M. L., & Garibaldi, A. (2012). Una nuova malattia della rucola selvatica causata da Plectosphaerella cucumerina. Protezione delle Colture, 5, 31–33.
Lievens, B., Hanssen, I. M., & Rep, M. (2012). Recent developments in the detection and identification of formae speciales and races of Fusarium oxysporum: from pathogenicity testing to molecular diagnostics. In M. L. Gullino, J. Katan, & A. Garibaldi (Eds.), Fusarium wilts of greenhouse vegetable and ornamental crops (pp. 47–55). St. Paul, MN, USA: APS Press.
Mathur, S. B., & Kongsdal, O. (2003). Common laboratory seed health testing methods for detecting fungi (1st revised ed.). Zurich, Switzerland: International Seed Testing Association.
Matta, A., & Garibaldi, A. (1981). Malattie delle piante ortensi (1st revised ed.). Bologna, Italy: Edagricole.
Mbofung, G. C. Y., & Pryor, B. M. (2010). A PCR-based assay for detection of Fusarium oxysporum f. sp. lactucae in lettuce seed. Plant Disease, 94, 860–866.
Palm, M. E., Gams, W., & Nirenberg, H. I. (1995). Plectosporium, a new genus for Fusarium tabacinum, the anamorph of Plectosphaerella cucumerina. Mycologia, 87, 397–406.
Pasquali, M., Dematheies, F., Gullino, M. L., & Garibaldi, A. (2007). Identification of race 1 of Fusarium oxysporum f. sp. lactucae on lettuce by inter-retrotransposon sequence-characterised amplified region technique. Phytopathology, 97, 987–996.
Pellegrino, C., Gilardi, G., Gullino, M. L., & Garibaldi, A. (2010). Detection of Phoma valerianellae in lamb’s lettuce seeds. Phytoparasitica, 38, 159–165.
Tinivella, F., Hirata, L. M., Celan, M. A., Wright, S. A. I., Amein, T., Schmitt, A., et al. (2009). Control of seed-borne pathogens on legumes by microbial and other alternative seed treatments. European Journal of Plant Pathology, 123, 139–151.
Acknowledgments
This work was carried out within the framework of the projects “Seed health: development of seed treatment methods, evidence for seed transmission and assessment of seed health (TESTA)”, funded by the European Union Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 311875 and “Plant and food biosecurity (PLANT FOOD SEC)”, funded by the European Union Seventh Framework Programme (FP7/2007-2013) under Grant Agreement 261752.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gilardi, G., Garibaldi, A. & Gullino, M.L. Seed transmission of Plectosphaerella cucumerina, causal agent of leaf spot of Diplotaxis tenuifolia in Italy. Phytoparasitica 41, 411–416 (2013). https://doi.org/10.1007/s12600-013-0302-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-013-0302-4