Abstract
A local isolate of Metarhizium anisopliae (Hypocreales: Clavicipitaceae), Bacillus thuringiensis subsp. kurstaki and chlorantraniliprole were assessed against six field populations of tomato fruitworm Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) in a series of laboratory bioassays. Two dose rates of B. thuringiensis (0.5, 1 μg g−1), one of both M. anisopliae (1.3 × 106 conidia ml−1) and chlorantraniliprole (0.01 ppm) were applied alone and in combination with each other against 2nd, 3rd, 4th and 5th larval instars. The mortality was observed every 24 h until pupation. The bioassays were carried out at 25°C and 75% r.h. The highest mortality was observed in Rawalpindi with the lowest pupation rate by applying the combined concentrations of B. thuringiensis and chlorantraniliprole. The lowest mortality was observed in population from Gujranwala among all the tested populations. The antagonistic interaction was noted where the high dose rate of B. thuringiensis was combined with M. anisopliae; however, the remaining interactions enhanced the mortality and reduced the percent pupation. The overall results demonstrated that all the treatments gave significant control of the larval instars of H. armigera. The population from Gujranwala proved least susceptible whereas the one from Rawalpindi was highly susceptible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Helicoverpa armigera
Hübner (Lepidoptera: Noctuidae) is a cosmopolitan polyphagous insect pest of economically important crops (Cherry et al. 2000; Wakil et al. 2009a; b; 2010). Synthetic insecticides continue to be the main controlling agents but several cases of resistance and reduced susceptibility of H. armigera to insecticides and environmental and human health concerns have been reported worldwide (Gunning et al. 1998; Martin et al. 2000; Qaim et al. 2008). The injudicious and repeated use of insecticides resulted in the development of resistance in H. armigera populations in different localities of Punjab province, Pakistan (Ahmad et al. 2001; 2003). This situation prompted the researchers to test the safe alternatives like Metarhizium anisopliae, Bacillus thuringiensis and a new chemical insecticide against geographically distinct populations. Similarly, Purwar & Sachan (2006) emphasized eco-friendly alternatives because they have been showing good results for the protection of agricultural crops.
Among the alternatives, entomopathogenic fungi are getting serious attention due to their environmental safety and pest selectivity (Carner & Yearian 1989). The efficacy of entomopathogenic fungi is well documented by Nguyen et al. (2007), who reported promising results of seven strains of Metarhizium anisopliae, Beauveria bassiana and Paecilomyces fumosoroseus against different larval stages of H. armigera. The fungal spores germinate and penetrate the cuticle by making germ tubes and proliferate in the hemolymph, which later produce new propagules (Zimmermann 2007).
Bacillus thuringiensis
Berliner is a spore-forming gram positive bacterium which is considered as an effective insecticide harmless to natural enemies, quite safe to mammals and environmentally acceptable (Entwistle et al. 1993). B. thuringiensis toxins bind to specific receptors located on the brush border membrane of midgut columnar cells, which eventually leads to cell death (Bravo et al. 2004).
Chlorantraniliprole (Coragen®) powered by Rynaxypyr is a reduced risk new class of chemistry, the anthranilic diamides, which has an excellent environmental profile due to low mammalian toxicity and low residual effect. It works through ingestion, contact, ovicidal and ovi-larvicidal activity (Lahm et al. 2007). The muscle contraction is controlled by managing the balance of calcium levels in the muscle cells through ryanodine receptors. The chlorantraniliprole makes the ryanodine receptors open and release all the stored calcium, which causes the death of insects by the rapid cessation of feeding, lethargy, regurgitation and muscle paralysis (Cordova et al. 2007).
Considering the significance of these promising alternatives and the paucity of data on the interaction of chlorantraniliprole with microbial agents, the present study was designed to evaluate the separate and combined effects of M. anisopliae, B. thuringiensis and chlorantraniliprole on the mortality of 2nd, 3rd, 4th and 5th larval instars of H. armigera collected from different geographical localities of Punjab province, Pakistan; the pupation rate of the larvae was also assessed.
Materials and methods
Rearing of study insects
Six populations of H. armigera were collected from Gujranwala, Sheikhupura, Faisalabad, Lahore, Sargodha and Rawalpindi districts of Punjab, Pakistan, and reared on artificial diet in the IPM Laboratory, Department of Agricultural Entomology, University of Agriculture, Faisalabad. These larvae were reared in 32-well plastic trays (6 cm in diameter × 5.5 cm in depth) until pupation. The adults were kept in plastic jars (15 cm in diameter × 19 cm in depth) lined with coarse tissue paper as nappy liner for egg laying. They were provided with 10% honey solution in a 5-ml test tube plugged with cotton and placed vertically on the top of the jar. The eggs were surface sterilized with 0.5% sodium hypochlorite followed by two changes of distilled water and were placed in plastic bags for hatching (Marzban et al. 2009). The newly emerged larvae were fed on artificial diet (Wakil et al. 2011) at 25 ± 2°C, 70 ± 5% r.h. synchronized at a photoperiod of 14:10 (L:D) hours.
Chlorantraniliprole
It is a novel insecticide in the anthranilic diamide class powered by Rynaxypyr which is a semi-viscous liquid off-white in color. It contains (20% w v−1) chlorantraniliprole (200 ml l −1) and (80% w v−1) other ingredients (800 ml l −1) provided by DuPont™ Operations Private Limited, Pakistan.
Bacillus thuringiensis toxin
The wettable powder (WP) commercial formulation (Dipel) containing B. thuringiensis subspecies kurstaki with a density of active toxin 3.2%, other inert material 96.8% with the potency of 16,000 i.u., was provided by BioSciences Corporation (Libertyville, IL, USA). One gram of powder was dissolved in 2 ml of sterile distilled water and gently streaked on the nutrient agar media (5 g peptone, 5 g NaCl, 1.5 g beef extract, 1.5 g yeast extract, 15 g agar and 1,000 ml distilled H2O) added with suitable antibiotic. Then, the spores and crystals were collected by centrifugation at 16,000 rpm for 15 min at 4°C temperature for the extraction of Bt toxin (Crecchio & Stotzky 2001; Hernández et al. 2005). The pellet was washed three times with cold 1 M NaCl and re-suspended in 1 M NaCl. Estimation of spore-crystal concentration was carried out in 1:100 dilutions by measuring the optical density at 600 nm (Hernández et al. 2005) and the samples were stored in the refrigerator until used.
Fungal isolation and conidial preparation
Metarhizium anisopliae isolate was originally isolated from the soil sample collected from harvested tomato fields in the Rawalpindi district (Pakistan). The fungus was isolated using the Galleria bait method (Zimmermann 1986) with third or fourth larval instars of the wax moth Galleria mellonella L. (Lepidoptera: Pyralidae). The larvae before baiting were immersed in water at 56°C for 15 s in order to minimize their ability to produce silk webbing in the soil (Woodring & Kaya 1988). The soil sample was sieved through 5 mm mesh and 60 g (Rodrigues et al. 2005) of soil was poured in the plastic cups (6 cm high, 4.5 cm diam). Ten larvae of G. mellonella were placed in cups sealed with the perforated lids and incubated at 25°C. The cups were shaken and inverted daily for the first 5 days to ensure the movement of the larvae in the soil. After 15–20 days the dead cadavers were shifted to other cups and surface sterilized with 0.05% sodium hypochlorite. The cups were provided with the moist filter paper and incubated at 25°C until the appearance of external growth of fungi. The fungi were identified morphologically by preparing the slides and the fungus was sub-cultured on Sabouraud Dextrose Agar (32.5 g SDA; 7.5 g of Bacto Agar; 5 g yeast in 1 l distilled water) for mass production and incubated at 25°C, 75% r.h. with 16 h illumination per day. After 14 days of incubation, the plates were kept under the aluminum foil roasting pan on the bench top for 1 week for drying. The fungal conidia were harvested by scraping the plates using a sterilized (70% ethanol) scalpel. The fungal conidia were dissolved in 0.05% Tween-80 solution and filtered through muslin cloth to remove the mycelial debris. The desired concentrations were recorded by dilution plate count method (Marannino et al. 2006), estimating the colony forming units.
Mortality and pupation of H. armigera larval instars
Two concentrations of B. thuringiensis (0.5 and 1 μg g−1), one of M. anisopliae (1.3 × 106 conidia ml−1) and chlorantraniliprole (0.01 ppm) individually and 0.5 μg g−1 of B. thuringiensis + M. anisopliae, 1 μg g−1 of B. thuringiensis + M. anisopliae, 0.5 μg g−1 of B. thuringiensis + chlorantraniliprole and 1 μg g−1 of B. thuringiensis + chlorantraniliprole were applied to assess the mortality and pupation of different larval (L2–L5) instars of H. armigera. The larvae in vials without any treatment served as control. B. thuringiensis and chlorantraniliprole were applied by mixing in artificial diets. So, five batches of artificial diets were prepared: two batches having two different concentrations of B. thuringiensis (0.5 and 1 μg g−1), one batch of chlorantraniliprole (0.01 ppm), one batch for 0.5 μg g−1 of B. thuringiensis + chlorantraniliprole and one batch for 1 μg g−1 of B. thuringiensis + chlorantraniliprole. The treatments were thoroughly mixed in an electric shaker for 30 s in 1 l jug to distribute them evenly in the artificial diets. Then the pre-starved (24 h) larval instars of each population were put separately in the plastic vials (base radius 2.8 cm × height 7 cm) and allowed to feed separately on each treated batch of artificial diet (1 cm3 piece) for 48 h. Then the fed larvae were removed and immersed individually for 10 s into fungal solution. The treated larvae were allowed to crawl freely in an empty petri dish to remove an excess of fungal suspension and were put in plastic vials containing an artificial diet until the larvae died or pupated. The bioassays were conducted at 25 ± 2°C, 75% r.h. and L16:D8 h photoperiod. Each treatment consisted of 20 larvae for every population and the bioassays were repeated three times independently to avoid the phenomenon of pseudo-replication. The data for mortality were recorded after every 24 h and the last count was recorded after 12 days for all the populations and larval instars (L2–L5). After removing the dead individuals, the remaining larvae were kept until pupation. The larvae were prodded with a blunt needle and those unable to move in a coordinated manner were considered as dead (Ma et al. 2008).
Statistical analysis
The data were transformed with arcsine square root to check the homogeneity and the normality of error variances before analysis. The data were analyzed by analysis of variance with Minitab 13.2 (Minitab, 2002 Software Inc., Northampton, MA, USA) with significance detected at P = 0.05. Means for mortality and pupation were separated and compared with Tukey’s Kramer test (HSD) (Sokal & Rohlf 1995). The type of interaction between different treatments was determined by equation CTF = (Oc-Oe)/Oe × 100, where CTF is the co-toxicity factor, Oc is the observed percentage mortality resulted from the combined application, and Oe the expected percentage mortality, that is, the total percentage produced by each of the treatments used in the combination (Mansour et al. 1966). The interactions were categorized into three groups: a positive factor of 20 or more meaning synergism, a negative factor of 20 or more meaning antagonism, and any intermediate value (i.e., between −20 and +20) was considered additive (Mansour et al. 1966; Wakil et al. 2012).
Results
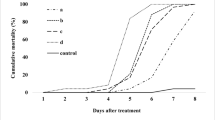
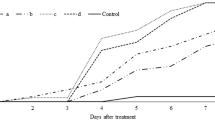
There were significant differences in mortality in all the tested populations when treated with the combined or individual concentrations of M. anisopliae, B. thuringiensis and chlorantraniliprole. The main effects were (localities: F 5,647 = 171.44, P ≤ 0.01; larval instars: F 3,647 = 1372.73, P ≤ 0.01; treatments: F 8,647 = 2648.04, P ≤ 0.01) and their associated interactions (localities x larval instars: F 15,647 = 4.62, P ≤ 0.01; localities × treatments: F 40,647 = 9.29, P ≤ 0.01; larval instars x treatments: F 24,647 = 72.47, P ≤ 0.01). The synergistic effects on the mortality of H. armigera larval instars were exhibited by the combined applications of low dose of B. thuringiensis with M. anisopliae and high dose of B. thuringiensis with chlorantraniliprole. The additive interaction was evident when a low dose of B. thuringiensis and chlorantraniliprole was combined; however, a high dose of B. thuringiensis showed antagonistic interaction with M. anisopliae (Table 1). The highest mortality (100%) of 2nd instar larvae of H. armigera was observed in Rawalpindi (F 8,26 = 173, P ≤ 0.01), Sargodha (F 8 ,26 = 270, P ≤ 0.01) and Lahore (F 8,26 = 178, P ≤ 0.01) populations; the lowest pupation recorded was in Rawalpindi (F 8,26 = 176, P ≤ 0.01) followed by Sargodha (F 8,26 = 308, P ≤ 0.01) and Lahore (F 8,26 = 223, P ≤ 0.01) by applying the combination of B. thuringiensis (1 μg g−1) with chlorantraniliprole; however, the lowest mortality (F 8,26 = 152, P ≤ 0.01) was observed in Gujranwala, with (F 8,26 = 132, P ≤ 0.01) pupation.
The combined treatments of chlorantraniliprole with a high dose of B. thuringiensis showed higher mortality (100%) of the 3rd instar larvae from Rawalpindi (F 8,26 = 391, P ≤ 0.01) and Sargodha (F 8,26 = 565, P ≤ 0.01) with minimum pupation (Rawalpindi: F 8,26 = 291, P ≤ 0.01; Sargodha: F 8,26 = 480, P ≤ 0.01), compared with control treatment (Table 2). The lowest mortality (F 8,26 = 77.4, P ≤ 0.01) and pupation (F 8,26 = 115, P ≤ 0.01) was observed in Gujranwala. The larval mortality was significantly increased in combined rather than individual treatments. The interaction between the combined treatments was synergistic and additive; however, the antagonistic interaction was noted with combined application of 1 μg g−1 of B. thuringiensis + M. anisoplaie showing −21.67 co-toxicity factor.
The mortality of 4th instar larvae of H. armigera was again higher, with less pupation, when exposed to the combined treatments of B. thuringiensis (1 μg g−1) with chlorantraniliprole (Table 3). The larval mortality was significantly increased in the population from Rawalpindi (F 8,26 = 101, P ≤ 0.01) and with pupation (F 8,26 = 94.2, P ≤ 0.01). The Gujranwala population was less susceptible (F 8,26 = 41.2, P ≤ 0.01), followed by Sheikhupura (F 8,26 = 80.4, P ≤ 0.01), Faisalabad (F 8,26 = 110, P ≤ 0.01) and Lahore (F 8,26 = 121, P ≤ 0.01) and the same trend was exhibited from L2–L5 of H. armigera. The synergistic and additive interaction was observed in all combined treatments except high dose of B. thuringiensis with M. anisopliae treatment was antagonistic in all the tested populations. Among individual treatments the chlorantraniliprole showed significantly more mortality in all populations tested with maximum 35.51% and 59.12% pupation in Rawalpindi; and least in Gujranwala with 24.67% mortality and 69.96% pupation.
The synergistic and additive effect was noted in combined treatments against 5th instar larvae of H. armigera among all the populations tested and mortality was higher in the combined than individual treatments (Table 4). The antagonistic interaction was noted in the high dose of B. thuringiensis with M. anisopliae treatment. The high dose of B. thuringiensis with chlorantraniliprole application against various populations showed the decreasing mortality trend (Rawalpindi: F 8,26 = 55.9, P ≤ 0.01; Sargodha: F 8,26 = 44.2, P ≤ 0.01; Lahore: F 8,26 = 59.1, P ≤ 0.01; Faisalabad: F 8,26 = 55, P ≤ 0.01; Sheikhupura: F 8,26 = 59.5, P ≤ 0.01; Gujranwala: F 8,26 = 30.6, P ≤ 0.01); however, the pupation tendency was in ascending order (Rawalpindi: F 8,26 = 113, P ≤ 0.01; Sargodha: F 8,26 = 55.7, P ≤ 0.01; Lahore: F 8,26 = 71, P ≤ 0.01; Faisalabad: F 8,26 = 55.1, P ≤ 0.01; Sheikhupura: F 8,26 = 66, P ≤ 0.01; Gujranwala: F 8,26 = 49.4, P ≤ 0.01).
Discussion
The present studies were conducted to determine the influence of individual and combined applications of M. anisopliae, B. thuringiensis and chlorantraniliprole against different larval instars of field populations of H. armigera. The entomopathogenic fungi have great potential to control lepidopterous insect pests (Vega-Aquino et al. 2010), confirming the present study in which M. anisopliae showed satisfactory results against different larval instars. Laboratory bioassays demonstrating the effectiveness of M. anisopliae against the various larval instars of H. armigera (Nguyen et al. 2007) gave further confirmation of the present findings. Several isolates of M. anisopliae have also shown high levels of virulence against the various forest pests (Remadevi et al. 2010); similarly, 90% mortality of both Agriotes obscurus L. (Coleoptera: Elateridae) and the unidentified species of Limonius was induced (Kabaluk et al. 2001) by the use of M. anisopliae under laboratory conditions. The mortality of larval instars showed a declining trend in all the populations from first to fifth instar supported by Inglis et al. (2001) that different developmental stages of insects vary in their susceptibility to infection by entomopathogenic fungi. This could be due to the increase of melanin contents in the cuticle and mid gut of the insects which prevents the penetration of the fungal germ tube (Wilson et al. 2001). According to Hafez et al. (1997), early larval instars of the potato tuber moth Phthorimaea operculella (Z.) (Lepidoptera: Gelechiidae) were more susceptible to B. bassiana than older larval stages. On contrary, Vandenberg et al. (1998) found that 3rd and 4th instars of the diamondback moth Plutella xylostella L. (Lepidoptera: Plutellidae) were more susceptible to entomopathogenic fungi than 2nd instars.
In the current study, efficacy of B. thuringiensis toxin decreased with the growth of H. armigera larvae; this is confirmed by Herbert & Harper (1985), who noted a decline in the insecticidal activity of Bt against Helicoverpa zea Boddie (Lepidoptera: Noctudiae) with the growth development of larvae. Similarly, Zehnder & Gelernter (1989) recorded 40–98% mortality of 2nd instars compared with 52% mortality of 3rd instars of the Colorado potato beetle after 96 h with the application of B. thuringiensis var. san diego (M-ONE). In another study, B. thuringiensis at high and low labeled concentrations (1.17 and 7.0 l ha−1) provided fair to excellent control against Colorado potato beetle (Lacey et al. 1999). Likewise, Zehnder et al. (1992) and Ghidiu & Zehnder (1993) suggested that the appropriate time for the application of B. thuringiensis should coincide with the hatching of eggs of Colorado potato beetle and also in the presence of early larval instars.
Chlorantraniliprole gave fair control of all larval instars of H. armigera in all the tested populations, but the second instar larvae showed the higher susceptibility in the present study. Cordova et al. (2006), Lahm et al. (2007) and Temple et al. (2009) reported the efficacy of chlorantraniliprole against lepidopteran insect pests at very low concentrations which was further confirmed by Wakil et al. (2012) by assessments against H. armigera, with promising mortality. The chlorantraniliprole showed a high level of mortality against Cry1Ac susceptible and resistant strains of H. armigera (Cao et al. 2010), as it increases the esterase and glutathione-S-transferase activities in both strains. Moreover, chlorantraniliprole has the unique mode of action which attacks on the ryanodine receptors in muscle cells resulting in unregulated release of Ca+2 and the death of insects (Temple et al. 2009).
The results indicate clearly that the mortality was higher when B. thuringiensis was combined with M. anisopliae and chlorantraniliprole. These findings are in accordance with Lacey et al. (1999), who reported the lowest number of adults of Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae) in the plots treated with the combination of entomopathogenic fungi and B. thuringiensis, while the highest number was recorded in control plots. Similarly, Wraight & Ramos (2005) noted the significant reduction in the larval population of Colorado potato beetle in combined treatments of B. bassiana and B. thuringiensis compared with their individual applications. The mortality of Ostrinia nubilalis Hübner (Lepidoptera: Pyralidae) was increased when B. bassiana and B. thuringiensis were applied in combination (Lewis et al. 1996). The additive interaction is also noted in the present study similar to these findings; in laboratory bioassays Meissle et al. (2009) found additive interaction in Bt maize and M. anisopliae against Diabrotica virgifera virgifera LeConte (Coleoptera: Chrysomelidae) and this might be due to sublethal damage induced by B. thuringiensis toxin that enhanced (Lawo et al. 2008) the effectiveness of M. anisopliae. Furthermore, Gao et al. (2012) also confirmed Bt–B. bassiana synergism as interruption of larval feeding by Bt intoxication may lead to starvation stress and cause detrimental effects on host physiology and immune response. The possible reasons for synergistic interaction between entomopathogenic fungi and B. thuringiensis could be due to starvation, because bacteria may arrest the nutrition of insects (Kryukov et al. 2009) and the fungal spores ultimately kill the weakened larvae. The inter-molt period also increased due to starvation and this was the suspected reason for increased susceptibility of the larvae of Colorado potato beetle (Furlong & Groden 2003); also the increased susceptibility of Asian longhorned beetle to Metarhizium brunneum Petch (Hypocreales: Clavicipitaceae) was due to the reduced feeding of the insect (Russell et al. 2010). On the other hand, in the present study the antagonistic effect of the high dose rate (1 μg g−1) of B. thuringiensis in combination with M. anisopliae against all the larval instars of H. armigera was observed. Ma et al. (2008) reported the antagonistic effect of B. bassiana and sublethal concentrations of Cry1Ac of B. thuringiensis against Asiatic corn borer applied at the rate of 3.2 or 13 μg g−1 and 1.8 × 105 and 106 conidia ml−1. The antagonistic interaction in this study could be due to the feeding-deterrent effect of a high dose B. thuringiensis toxin, which reduces the consumption rate (Lawo et al. 2008) of the larvae; additionally, at a higher dose rate the toxin inhibits the conidial germination (Toledo et al. 2011). In another study, Costa et al. (2001) reported no synergistic interaction against the fourth instar larvae of Colorado potato beetle (L. decemlineata) that survived after the treatment of B. thuringiensis and entomopathogenic fungi.
This is the first report in which the effectiveness of M. anisopliae, B. thuringiensis and chlorantraniliprole were tested against different larval instars of H. armigera populations originating from different geographical locations in Punjab province (Pakistan). In the light of our findings, the population from Gujranwala appeared to be more resistant to M. anisopliae, B. thuringiensis and chlorantraniliprole compared with the remaining populations collected from other localities. The variable response exhibited by the field populations of H. armigera to different treatments in the current study could be attributed to the genetic variation and the indiscriminate and repeated excessive spray schedule of insecticides on the crops grown in these particular localities.
References
Ahmad, M., Arif, M. I., & Zahoor, A. (2001). Resistance to carbamate insecticides in Helicoverpa armigera (Lepidoptera: Noctudiae) in Pakistan. Crop Protection, 20, 427–432.
Ahmad, M., Arif, M. I., & Zahoor, A. (2003). Susceptibility of Helicoverpa armigera (Lepidoptera: Noctuidae) to new chemistries in Pakistan. Crop Protection, 22, 539–544.
Bravo, A., Gomez, I., Conde, J., Munoz-Garay, C., Sanchez, J., Miranda, R., et al. (2004). Oligomerization triggers binding of Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to Cry1Ac δ-endotoxin of Bacillus thuringiensis in the cotton bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae). Journal of Economic Entomology, 96, 1290–1299.
Cao, G., Lu, Q., Zhang, L., Guo, F., Liang, G., Wu, K., et al. (2010). Toxicity of chlorantraniliprole to Cry1Ac-susceptible and resistant strains of Helicoverpa armigera. Pesticide Biochemistry and Physiology, 98, 99–103.
Carner, G. R., & Yearian, W. C. (1989). Development and use of microbial agents for control of Heliothis spp. in the USA. In E. G. King, & R. D. Jackson (Eds.), Proceedings of the workshop on biological control of Heliothis: increasing the effectiveness of natural enemies (1985, New Delhi, India) (pp. 467–481). Far Eastern Regional Research Office, US Department of Agriculture.
Cherry, A. J., Rabindra, R. J., Parnell, M. A., Geetha, N., Kennedy, J. S., & Grzywacz, D. (2000). Field evaluation of Helicoverpa armigera nucleopolyhedrovirus formulations for control of the chickpea pod-borer, H. armigera (Hubn.), on chickpea (Cicer arietinum var. Shoba) in southern India. Crop Protection, 19, 51–60.
Cordova, D., Benner, E. A., Sacher, M. D., Rauh, J. J., Sopa, J. S., Lahm, G. P., et al. (2006). Anthranilic diamides: a new class of insecticides with a novel mode of action, ryanodine receptor activation. Pesticide Biochemistry and Physiology, 84, 196–214.
Cordova, D., Benner, E. A., Sacher, M. D., Rauh, J. J., Sopa, J. S., Lahm, G. P., et al. (2007). Elucidation of the mode of action of Rynaxypyr®, a selective ryanodine receptor activator. In H. Ohkawa, H. Miyagawa, & P. W. Lee (Eds.), Pesticide chemistry, crop protection, public health, and environmental safety (pp. 121–126). Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA.
Costa, S. D., Barbercheck, M. E., & Kennedy, G. G. (2001). Mortality of Colorado potato beetle (Leptinotarsa decemlineata) after sublethal stress with the CRYIIIA delta-endotoxin of Bacillus thuringiensis and subsequent exposure to Beauveria bassiana. Journal of Invertebrate Pathology, 77, 173–179.
Crecchio, C., & Stotzky, G. (2001). Biodegradation and insecticidal activity of the toxin from Bacillus thuringiensis subsp. kurstaki bound on complexes of montmorillonite-humic acids-Al hydroxypolymers. Soil Biology and Biochemistry, 33, 573–581.
Entwistle, P. F., Cory, J. S., Bailey, M., & Higgs, S. (Eds.). (1993). Bacillus thuringiensis, an environmental biopesticide: theory and practice. New York, NY: Wiley.
Furlong, M. J., & Groden, E. (2003). Starvation induced stress and the susceptibility of the Colorado potato beetle, Leptinotarsa decemlineata, to infection by Beauveria bassiana. Journal of Invertebrate Pathology, 83, 127–138.
Gao, Y., Oppert, B., Lord, J. C., Liu, C., & Lei, Z. (2012). Bacillus thuringiensis Cry3Aa toxin increases the susceptibility of Crioceris quatuordecimpunctata to Beauveria bassiana infection. Journal of Invertebrate Pathology, 109, 260–263.
Ghidiu, G. M., & Zehnder, G. W. (1993). Timing of the initial spray application of Bacillus thuringiensis for control of the Colorado potato beetle (Coleoptera: Chrysomelidae) in potatoes. Biological Control, 3, 348–352.
Gunning, R. V., Moores, G. D., & Devonshire, A. L. (1998). Insensitive acetycholinesterase causes resistance to organophosphates in Australian Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Pesticide Science, 54, 319–320.
Hafez, M., Zaki, F. N., Moursy, A., & Sabbour, M. (1997). Biological effects of the entomopathogenic fungus, Beauveria bassiana on the potato tuber moth Phthorimaea operculella (Zeller). Journal of Pesticide Science, 70, 158–159.
Herbert, D. A., & Harper, J. D. (1985). Bioassay of δ-exotoxin of Bacillus thuringiensis against Heliothis zea larvae. Journal of Invertebrate Pathology, 46, 247–250.
Hernández, C. S., Andrew, R., Bel, Y., & Ferre, J. (2005). Isolation and toxicity of Bacillus thuringiensis from potato-growing areas in Bolivia. Journal of Invertebrate Pathology, 88, 8–16.
Inglis, G. D., Goettel, M. S., Butt, T. M., & Strasser, H. (2001). Use of hyphomycetous fungi for managing insect pests. In T. M. Butt, C. Jackson, & N. Magan (Eds.), Fungi as biocontrol agents. Progress, problems and potential (pp. 23–69). Wallingford, UK: CABI Publishing.
Kabaluk, T., Goettel, M., Vernon, B., & Noronha, C. (2001). Evaluation of Metarhizium anisopliae as a biological control for wireworms. Organic Agriculture Center of Canada.http://www.organicagcentre.ca/ResearchDatabase/res_biol_ctrl_wireworms.asp; accessed on 20.12.2010.
Kryukov, V. Y., Khodyrev, V. P., Yaroslavtseva, O. N., Kamenova, A. S., Duisembekov, B. A., & Glupov, V. V. (2009). Synergistic action of entomopathogenic hyphomycetes and the bacteria Bacillus thuringiensis ssp. morrisoni in the infection of Colorado potato beetle Leptinotarsa decemlineata. Prikladnaya Biokhimiya i Mikrobiologiya, 45, 571–576.
Lacey, L. A., Horton, D. R., Chauvin, R. L., & Stocker, J. M. (1999). Comparative efficacy of Beauveria bassiana, Bacillus thuringiensis, and aldicarb for control of Colorado potato beetle in an irrigated desert agroecosystem and their effects on biodiversity. Entomologia Experimentalis et Applicata, 93, 189–200.
Lahm, G. P., Stevenson, T. M., Selby, T. P., Freudenberger, J. H., Cordova, D., Flexner, L., et al. (2007). Rynaxypyr™: a new insecticidal anthranilic diamide that acts as a potent and selective ryanodine receptor activator. Bioorganic & Medicinal Chemistry Letters, 17, 6274–6279.
Lawo, N. C., Mahon, R. J., Milner, R. J., Sarmah, B. K., Higgins, T. J. V., & Romeis, J. (2008). Effectiveness of Bacillus thuringiensis-transgenic chickpeas and the entomopathogenic fungus Metarhizium anisopliae in controlling Helicoverpa armigera (Lepidoptera: Noctuidae). Applied and Environmental Microbiology, 74, 4381–4389.
Lewis, L. C., Berry, E. C., Obrycki, J. J., & Bing, L. A. (1996). Aptness of insecticides (Bacillus thuringiensis and carbofuran) with endophytic Beauveria bassiana in suppressing larval populations of European corn borer. Agriculture, Ecosystems and Environment, 57, 27–34.
Ma, X., Liu, X., Ning, X., Zhang, B., Han, F., Guan, X., et al. (2008). Effects of Bacillus thuringiensis toxin Cry1Ac and Beauveria bassiana on Asiatic corn borer (Lepidoptera: Crambidae). Journal of Invertebrate Pathology, 99, 123–128.
Mansour, N. A., Eldefrawi, M. E., Toppozada, A., & Zeid, M. (1966). Toxicological studies on the Egyptian Cotton Leafworm, Prodenia litura VI potentiation and antagonism of carbamate insecticide. Journal of Economic Entomology, 59, 307–311.
Marannino, P., Santiago-Álvarez, C., de Lillo, E., & Quesada-Moraga, E. (2006). A new bioassay method reveals pathogenicity of Metarhizium anisopliae and Beauveria bassiana against early stages of Capnodis tenebrionis (Coleoptera; Buprestidae). Journal of Invertebrate Pathology, 93, 210–213.
Martin, T., Ochou, G. O., Hala, N. F., Wassal, J., & Waissayre, M. (2000). Pyrethroid resistance in cotton bollworm Helicoverpa armigera in West Africa. Pest Management Science, 56, 549–554.
Marzban, R., He, Q., Liu, X., & Zhang, Q. (2009). Effects of Bacillus thuringiensis toxin Cry1Ac and cytoplasmic polyhedrosis virus of Helicoverpa armigera (Hübner) (HaCPV) on cotton bollworm (Lepidoptera: Noctuidae). Journal of Invertebrate Pathology, 101, 71–76.
Meissle, M., Pilz, C., & Romeis, J. (2009). Susceptibility of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) to the entomopathogenic fungus Metarhizium anisopliae when feeding on Bacillus thuringiensis Cry3Bb1-expressing maize. Applied and Environmental Microbiology, 75, 3937–3943.
Nguyen, T. H. N., Borgemeister, C., Poehling, H., & Zimmermann, G. (2007). Laboratory investigations on the potential of entomopathogenic fungi for biocontrol of Helicoverpa armigera (Lepidoptera: Noctuidae) larvae and pupae. Biocontrol Science and Technology, 17, 853–864.
Purwar, J. P., & Sachan, G. C. (2006). Synergistic effect of entomogenous fungi on some insecticides against Bihar hairy caterpillar Spilarctia oblique (Lepidoptera: Arctiidae). Microbiological Research, 161, 38–42.
Qaim, M., Pray, C. E., & Zilberman, D. (2008). Economic and social considerations in the adoption of Bt crops. In J. Romeis, A. M. Shelton, & G. G. Kennedy (Eds.), Integration of insect-resistant genetically modified crops within IPM programs (pp. 329–356). Dordrecht, the Netherlands: Springer.
Remadevi, O. K., Sasidharan, T. O., Bhattacharya, J., Vossbrinck, C. R., & Rajan, P. D. (2010). Some pathological effects and transmission potential of a microsporidian isolate (Nosema sp.) from the teak defoliator Hyblaea puera (Lepidoptera: Hyblaeidae). International Journal of Tropical Insect Science, 30, 138–144.
Rodrigues, R. H., Bechara, I. J., Messias, C. L., & Piedrabuena, A. E. (2005). Effectiveness of Metarhizium anisopliae against immature stages of Anastrepha fraterculus fruitfly (Diptera: Tephritidae). Brazilian Journal of Microbiology, 36, 94–99.
Russell, C. W., Ugine, T. A., & Hajek, A. E. (2010). Interactions between imidacloprid and Metarhizium brunneum on adult Asian longhorned beetles (Anoplophora glabripennis (Motschulsky)) (Coleoptera: Cerambycidae). Journal of Invertebrate Pathology, 105, 305–311.
Sokal, R., & Rohlf, F. J. (1995). Biometry (3rd ed.). New York, NY: W. H. Freeman and Company.
Temple, J. H., Pommireddy, P. L., Cook, D. R., Marçon, P., & Leonard, B. R. (2009). Susceptibility of selected Lepidopteran pests to rynaxypyr®, a novel insecticide. The Journal of Cotton Science, 13, 23–31.
Toledo, A. V., Alippi, A. M., & de Remes Lenicov, A. M. M. (2011). Growth inhibition of Beauveria bassiana by bacteria isolated from the cuticular surface of the corn leafhopper, Dalbulus maidis and the planthopper, Delphacodes kuscheli, two important vectors of maize pathogens. Journal of Insect Science, 11, 29. available online: insectscience.org/11.29.
Vandenberg, J. D., Jackson, M. A., & Lacey, L. A. (1998). Relative efficacy of blastospores and aerial conidia of Paecilomyces fumosoroseus against the Russian wheat aphid. Journal of Invertebrate Pathology, 72, 181–183.
Vega-Aquino, P., Sanchez-Peña, S., & Blanco, C. A. (2010). Activity of oil-formulated conidia of the fungal entomopathogens Nomuraea rileyi and Isaria tenuipes against lepidopterous larvae. Journal of Invertebrate Pathology, 103, 145–149.
Wakil, W., Ashfaq, M., Ghazanfar, M. U., Afzal, M., & Riasat, T. (2009a). Integrated pest management of Helicoverpa armigera in chickpea in rainfed areas of Punjab, Pakistan. Phytoparasitica, 37, 415–420.
Wakil, W., Ashfaq, M., Kwon, Y. J., & Ghazanfar, M. U. (2009b). Trends in integrated pest management strategies for the control of Helicoverpa armigera (Hübner) caterpillars on chickpea (Cicer arietinum L.). Entomological Research, 39, 84–88.
Wakil, W., Ghazanfar, M. U., Kwon, Y. J., Qayyum, M. A., & Nasir, F. (2010). Distribution of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae) in tomato fields and its relationship to weather factors. Entomological Research, 40, 290–297.
Wakil, W., Ghazanfar, M. U., Nasir, F., Qayyum, M. A., & Tahir, M. (2012). Insecticidal efficacy of Azadirachta indica, Nucleopolyhedrovirus and chlorantraniliprole singly or combined against field populations of Helicoverpa armigera Hübner (Lepidoptera: Noctuidae). Chilean Journal of Agricultural Research, 72, 53–61.
Wakil, W., Ghazanfar, M. U., Sahi, S. T., Kwon, Y. J., & Qayyum, M. A. (2011). Effect of modified meridic diet on the development and growth of tomato fruitworm Helicoverpa armigera (Lepidoptera: Noctuidae). Entomological Research, 41, 88–94.
Wilson, K., Cotter, S. C., Reeson, A. F., & Pell, J. K. (2001). Melanism and disease resistance in insects. Ecology Letters, 4, 637–649.
Woodring, J. L., & Kaya, H. K. (1988). Steinernematid and heterorhabditid nematodes: a handbook of biology and techniques. Fayetteville, AR USA: Arkansas Agricultural Experiment Station.
Wraight, S. P., & Ramos, M. E. (2005). Synergistic interaction between Beauveria bassiana and Bacillus thuringiensis tenebrionis-based biopesticides applied against field populations of Colorado potato beetle larvae. Journal of Invertebrate Pathology, 90, 139–150.
Zehnder, G. W., & Gelernter, W. D. (1989). Activity of the M-One formulation of a new strain of Bacillus thuringiensis against the Colorado potato beetle (Coleoptera: Chrysomelidae): relationship between susceptibility and insect life stage. Journal of Economic Entomology, 82, 756–761.
Zehnder, G. W., Ghidiu, G. M., & Speese, J. (1992). Use of the occurrence of peak Colorado potato beetle (Coleoptera: Chrysomelidae) egg hatch for timing of Bacillus thuringiensis spray applications in potatoes. Journal of Economic Entomology, 85, 281–288.
Zimmermann, G. (1986). The Galleria bait method for detection of entomopathogenic fungi in soil. Journal of Applied Entomology, 102, 213–215.
Zimmermann, G. (2007). Review on safety of the entomopathogenic fungus Metarhizium anisopliae. Biocontrol Science and Technology, 17, 879–920.
Acknowledgments
This study is financially supported by projects from the Higher Education Commission (HEC), Islamabad, Pakistan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wakil, W., Ghazanfar, M.U., Riasat, T. et al. Effects of interactions among Metarhizium anisopliae, Bacillus thuringiensis and chlorantraniliprole on the mortality and pupation of six geographically distinct Helicoverpa armigera field populations. Phytoparasitica 41, 221–234 (2013). https://doi.org/10.1007/s12600-012-0282-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-012-0282-9