Abstract
The pine wood nematode Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle is the causal agent of pine wilt disease. We evaluated the efficacy of emamectin benzoate (EB) for preventing wilt disease in the field and its effect on the vector Monochamus galloprovincialis (Olivier) (Coleoptera: Cerambycidae). Four experimental plots were delimited in a maritime pine (Pinus pinaster Aiton) forest in Portugal. Trunk-injection trials with EB included three dose-rates: 0.032 g a.i. cm−1 diameter at breast height—DBH, n = 75 trees; 0.064 g a.i. cm−1 DBH, n = 75 trees; and 0.128 g a.i. cm−1 DBH, n = 50 trees; along with an untreated control plot (n = 75 trees). EB was successfully injected and translocated in pines at an effective concentration. None of the treated trees died after a period of 26 months, contrasting with a 33% mortality of non-treated pines. Analysis of residues successfully detected EB in branches of treated pines, with the quantity increasing relative to the injection dose rate, and was found to have a clear effect on the longevity and feeding activity of adult M. galloprovincialis feeding on branches. EB was efficient in preventing wilt disease and bark beetle attacks in the terrain, and its application by trunk injection is a new option for wilt disease management programs in Portugal and in Europe.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pinewood nematode Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle is the causal agent of pine wilt disease (PWD) and a major sanitary problem of conifer species, in particular in the genus Pinus. Although it is native and innocuous in North America, this nematode was first reported to cause tree mortality in Japan at the beginning of the 20th Century, and has since spread to other Asian countries such as China, Taiwan and Korea (Evans et al. 1996; Kishi 1995). In 1999 the pinewood nematode was detected for the first time in Portugal and other places in Europe, and despite concerted actions of government agencies the disease continued to spread, and new wilt foci have recently been detected in central Portugal (Anon. 2008).
Short-distance dispersal of the nematode is done by the adults of the genus Monochamus (Coleoptera: Cerambycidae), with Monochamus galloprovincialis (Olivier) being the only vector species in Portugal (Sousa et al. 2001). Nematode transmission to living conifer trees occurs when the recently emerged adults carry out maturation feeding in the branches (Naves et al. 2007). Once introduced into a susceptible host, the nematodes feed and destroy the xylem tissues and begin to reproduce, interrupting the water and resin flow and eventually killing the tree (Fukuda 1997; Linit 1988).
The current management of pine wilt disease in Portugal is based on sanitation, depending on location, felling and destruction of wilt-affected and symptomatic pines during the winter months. In addition, flying insect vectors are captured by traps baited with chemical attractants during the summer months (Rodrigues & Sousa 2009). Both strategies are labor-intensive and expensive, and have limited success in highly infested areas. The progressive spread of pine wilt disease in Portugal and elsewhere in Europe underlines the necessity to develop and promote new preventive measures. Chemical preventive methods such as the treatment of live trees with nematicide/insecticide compounds have been tested with success in Japan (Kazuya et al. 1999; Takai et al. 2003a) and the United States (James et al. 2006), having the advantage of being target-specific and environmentally friendly (Takai et al. 2003b). No such studies have been conducted in Portugal until now, and there is no chemical product registered for use in the forest against the pine wood nematode or its Monochamus vector.
Emamectin benzoate (EB) is a semi-synthetic and second generation avermectin-derived insecticide found to have the strongest nematicide activity against B. xylophilus among several chemical substances (Takai et al. 2000), and therefore can be considered to be a strong candidate for use as a preventive trunk injection agent against pine wilt disease. Furthermore, this chemical has been found to have strong insecticidal effects on bark and wood-boring beetles (Grosman & Upton 2006; Grosman et al. 2009), and could prove useful in controlling M. galloprovincialis.
The objectives of our study were to evaluate the efficacy of three dose rates of emamectin benzoate applied by trunk-injection on reducing the incidence of pine wilt disease and bark beetle attacks on the terrain, and to determine its effect on the longevity of adult M. galloprovincialis insects feeding on treated branches under laboratory conditions.
Materials and methods
Pine treatments with EB
Field trials were conducted at Herdade da Comporta, 100 km south of Lisbon, in a forest of approximately 20-year-old maritime pines (Pinus pinaster Aiton) where high tree mortality had occurred in the previous years, caused by both pine wilt disease and bark beetle attacks (Ips sexdentatus (Borner) and Orthotomicus erosus (Wollaston). Four homogenous plots of 0.5 ha each were delimited, comprising approximately 170 healthy pine trees in each plot. Before tree injections all dead and wilted pines were felled and removed from the plots.
The insecticide treatments with emamectin benzoate (formulated as 4% SL, A16297A, Syngenta Corporation) included three dose rates: a “low-dose” (0.032 g a.i. cm−1 diameter at breast height—DBH, n = 75 trees), a “mid-dose” (0.064 g a.i. cm−1 DBH, n = 75 trees) and a “high-dose” (0.128 g a.i. cm−1 DBH, n = 50 trees), along with an untreated control plot (n = 75 trees). Each plot was assigned to one of the treatments with injected pines randomly selected throughout the plot and leaving several non-treated trees in between. Plots were distanced 50 m from each other.
Trunk-injections of the low and mid-dose rates were made using the Arborjet Quickjet micro-infusion system (Arborjet, Inc.), while in the high-dose rate the Arborjet pressurized version (press “Tree I.V.”, with 4 bar/60 psi) was used. The injection holes (0.95 cm diameter and 10–12 cm deep) were drilled pointing slightly downwards at the base of the pines (20 cm above ground), with an autonomous drilling machine. A plastic plug was inserted into the opening of each hole, inserting the needle of the device through the septum and forcing the liquid under pressure into the sapwood of the tree.
The number of injection holes on each tree varied according to the dose-rate and the DBH, in order to overcome the resin pressure and obtain a homogenous distribution of the formulations in the larger trees (Takai et al. 2003b): for the low-dose rate, two holes for 22 cm DBH or less, three holes over 23 cm and four holes over 33 cm; for the mid-dose rate, three holes for 16 cm or less, four holes over 17 cm, five holes over 22 cm and six holes over 2 cm. A total of 8 ml was inoculated into each hole, with two consecutive injections of 4 ml. For the high-dose rate, using the Arborjet pressurized device a fixed number of four holes per tree was required to insert the necessary amount of EB (Table 1).
Trunk-injections were made over three consecutive days (24–26) of March 2009, before the emergence peak of M. galloprovincialis in July (Naves et al. 2008), because according to Takai et al. (2003b) treatments should be done at least 3 months before the emergence of the vectors and the subsequent nematode introductions by maturation feeding.
The sanitary condition of the pines was assessed periodically from 2009 to 2011 by visually recording the discoloration and wilting of the tree canopies, as symptomless trees infested with the PWN have never been recorded in Portugal (Sousa et al. 2011). All wilting and dead trees were felled and analyzed for the presence of bark- and wood-boring beetles and the PWN. Wood samples were collected at a height of 1.5 m from the wilted and dead trees and taken to the INRB laboratories to extract nematodes by the modified tray method, in which wood is soaked in water for 48 h and nematodes subsequently recovered with a 38 μm wire mesh.
Quantitative analysis of emamectin benzoate in pine tissues
Four trees in each treatment were randomly selected and two branches from each pine were cut for the quantitative analysis of EB in the tissues. As the Monochamus vector feeds mainly on young shoots, 1–3-year-old branches were selected for analyzing the presence of EB.
Tissue samples were obtained by collecting wood shavings from the branches using a pillar drill (1.5 mm diam × 20 mm length) with four holes in each branch. Five grams of wood shavings were collected from each branch and soaked in 20 ml acetonitrile, kept on a roller bed for 6 h. Samples were centrifuged and 1 ml of the supernatant was put into LC-MS vials for sampling. Additionally, a 2 ml acetonitrile surface wash of the branches treated with the highest dose rate was performed to collect any surface residues on the bark.
Analysis was carried out using a Thermo UHPLC Accela pump coupled to a Vantage TSQ (Thermo Electron Corporation, Marietta, OH, USA) gradient equipped with an Ion Max Source operating in positive ion mode. Elution of the analyte was achieved using an Acquity UPLC column (BEH C18 1.7 μm, 2.1 × 50 μm) (Waters Acquity, Manchester, UK), with a gradient of acetonitrile/water containing 0.2% formic acid, with an end time of 6 min.
Standard curves for Emamectin B1a and Emamectin B1b (the two components of EB found in a 90:10 ratio) were constructed using reference calibration solutions. Possible compounding effects were taken into account by spiking untreated bark samples with the reference compounds. Total compounds in bark washings and bark samples were determined from the standard curve. The EB content of the sample solution was obtained by the sum of the amounts of EB1a and EB1b calculated from the LC-MS peak areas, with EB1a—parent mass 886.5, product mass 157.5–158.5, retention time 2.65; and EB1b – parent mass 872.5, product mass 157.5–158.5 and retention time 2.58.
Adult branch-feeding bioassay
To obtain insects for the experiments, dead P. pinaster trees were felled in Comporta in January 2009 and divided into small bolts which were transported to the INRB laboratories at Oeiras. The M. galloprovincialis larvae were collected from the wood and kept individually in petri dishes under controlled conditions of temperature and humidity (25°C and 70% r.h.).
In early July 2009, two branches of the lower canopy were cut from ten trees randomly selected from each treatment. In the laboratory, the branches were divided into 10-cm sections and kept isolated and refrigerated (2–5°C) for a few days prior to use.
Newly-emerged (one or two days old) adult M. galloprovincialis were sexed, weighed and kept individually at room temperature (23°C ± 2°C) in plastic jars (600 ml volume) sealed at the top with a cork stopper to allow air circulation. The 120 insects were randomly assigned to one of the four treatments (including the untreated trees), with 15 males and 15 females per treatment. Each insect was given one maritime pine branch (≈10 cm in length, 2–3 cm in diameter) to feed on, which was replaced weekly with a similar branch from the same pine tree. Beetles were checked daily for mortality.
The amount of weekly feeding on the pine branches by the adult beetles was measured by recording the surface-feed areas (where the bark had been chewed) into a transparent paper sheet, and afterwards into a square graph paper with a 1-mm grid, allowing the measurement of the grids affected by the insect’s feeding activity. Measurements of feeding areas continued until the death of the insects.
Data analysis
Analysis of variance (ANOVA) with an α-level of 0.05 was used to compare parameters, followed by post hoc Fisher’s Least Significant Difference test (LSD) to compare significant means. Values are presented as means ± SD. Analyses were carried out using the software Statistica 6 (StatSoft Inc.).
Results
Pine treatments with EB
None of the treated trees died during a period of more than 2 years. The situation differed in the control plot: by May 2001 a total of 25 trees (corresponding to 33%) had died. The PWN was detected in 76% of those dead trees, with attacks of the bark-beetle O. erosus detected in all dead pines (Table 2).
Quantitative analysis of emamectin benzoate in pine tissues
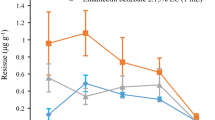
Emamectin benzoate was successfully detected in all sampled branches, with values ranging from 0.479 μg per 5 g wood sample (low dose rate) to 11.997 μg per 5 g wood sample (high dose rate), with the quantity of EB increasing in relation to the treatment rates. Similar amounts of EB1a and EB1b were found in the branches (Fig. 1).
Concerning the surface residues, minimal amounts of EB were detected on the bark surface of the trees treated with the highest rate, with a mean value of 0.00317 ± 0.001695 μg per 5 g wood sample, and a range between 0.000239 μg and 0.007006 μg.
Adult branch-feeding bioassay
The EB dose rates significantly affected the longevity of the adult insects under laboratory conditions (F = 12.7520, df = 3, P = 0.00001). Trees injected with higher doses were more toxic to M. galloprovincialis, irrespective of the sex (F = 2.9730, df = 1, P = 0.08742). Emamectin benzoate treatments decreased adult longevity by 26% in the low dose, 44% in the mid dose and 67% in the high dose, compared with the control-treatment insects (Table 3).
Irrespective of treatments, smaller insects were found to die faster than larger ones (F = 7.4181, df = 1, P = 0.007438, mean longevity of 14.7 ± 11.5 days for beetles with 153.5 ± 33.9 mg, and 20.6 ± 14.5 days for beetles with 279.4 ± 64.2 mg).
Besides longevity, the EB treatments significantly affected the feeding activity of the adult insects (F = 11.9130, df = 3, P = 0.00001), causing a 17% decrease in the mean feeding area for the low dose, a 47% decrease for the mid dose and a 54% decrease for the high dose, compared with untreated pines. Male feeding area was significantly higher than female (F = 5.6373, df = 1, P = 0.018222; means of 60.1 ± 3.9 cm2 day−1 and 47.4 ± 3.5 cm2day−1, respectively).
Discussion
Emamectin benzoate was successfully injected and diffused in adult maritime pines at an effective concentration. The presence of EB in the trunk and branches prevented pines from being killed by B. xylophilus and other biotic agents, and the three doses tested exceeded the IC95 threshold of 0.031 μg g−1 required to inhibit nematode propagation in young pine shoots (Takai et al. 2003b). Although EB1a and EB1b are generally found in a 90:10% ratio (Farer et al. 1999), in our study they were recovered from the pine branches in similar amounts, which could derive from a shorter retention time of EB1b (with a higher solubility in water resulting in higher mobility and better distribution to the branches), a higher metabolic stability of EB1b or a less efficient extraction method of EB1a.
Under laboratory conditions the three dose rates were toxic to M. galloprovincialis, resulting in a dose–response for the mid dose and above which affected the insect’s feeding appetence and longevity. This is important as beetles with lower longevity would die before beginning to transmit B. xylophilus into the host, because nematode transmission initiates only 2 weeks after the vector’s emergence (Naves et al. 2007). Furthermore, the lower longevity also affects the insect’s reproductive potential as females only start laying eggs 20 days after emergence (Naves et al. 2006).
Irrespective of dose, bigger insects lived longer than smaller ones, as they may have required a higher concentration of EB to die and/or a longer exposure for the product to be effective. Nevertheless, differential mortality affecting the smaller beetles was also reported for healthy M. galloprovincialis under laboratory conditions (Naves et al. 2006), and may therefore be an intrinsic characteristic of this species or an outcome of other factors such as a general low vigor of smaller insects.
Overall, our results suggest that EB injected by trunk injection can protect trees in the field and diminish the M. galloprovincialis populations in the field and B. xylophilus infection in the forests. As trunk injection of EB is considered an environmentally friendly measure (Takai et al. 2003b), it is currently a new option to be used in Portugal and elsewhere in Europe to protect high-value urban trees, recreational and ornamental forests, trees near high-risk areas such as commercial ports and sawmills, or to create buffer zones with chemically protected pines near recent foci of wilt disease. Future studies should evaluate the EB persistence in the terrain over the years, although in Japan a similar liquid formulation gave pines 3 years of protection against pine wilt disease (Takai et al. 2003a, b).
References
Anon. (2008). Portaria no. 305-A/2008 de 21 de Abril. Diário da República, Portugal.1a série No. 78.
Evans, H. F., McNamara, D. G., Braasch, H., Chadoeuf, J., & Magnusson, C. (1996). Pest risk analysis (PRA) for the territories of the European Union (as PRA area) on Bursaphelenchus xylophilus and its vectors in the genus Monochamus. EPPO Bulletin, 26, 199–249.
Farer, L. J., Hayes, J., Rosen, J., & Knight, P. (1999). Determination of emamectin benzoate in medicated fish feed. Journal of AOAC International, 82, 1281–1287.
Fukuda, K. (1997). Physiological process of the symptom development and resistance mechanism in pine wilt disease. Journal of Forest Research, 2, 171–181.
Grosman, D. M., & Upton, W. W. (2006). Efficacy of systemic insecticides for protection of Loblolly pine against southern pine engraver beetles (Coleoptera: Curculionidae: Scolytinae) and wood borers (Coleoptera: Cerambycidae). Journal of Economic Entomology, 99, 94–101.
Grosman, D. M., Clarke, S. R., & Upton, W. W. (2009). Efficacy of two systemic insecticides injected into Loblolly pine for protection against southern pine bark beetles (Coleoptera: Curculionidae). Journal of Economic Entomology, 102, 1062–1069.
James, R., Tisserat, N., & Todd, T. (2006). Prevention of pine wilt of scots pine (Pinus sylvestris) with systemic abamectin injections. Arboriculture & Urban Forestry, 32, 195–201.
Kazuya, T., Soejima, T., Suzuki, T., & Kawazu, K. (1999). Studies on development of a novel trunk-injection agent against the pine wilt disease. Pesticide Management Science, 56, 10.
Kishi, Y. (1995). The pine wood nematode and the Japanese pine sawyer. Tokyo, Japan: Thomas Company Ltd.
Linit, M. (1988). Nematode-vector relationships in the pine wilt disease system. Journal of Nematology, 20, 227–235.
Naves, P., Camacho, S., Sousa, E., & Quartau, J. (2007). Transmission of the pine wood nematode Bursaphelenchus xylophilus through feeding activity of Monochamus galloprovincialis (Coleoptera; Cerambycidae). Journal of Applied Entomology, 131, 21–25.
Naves, P., Sousa, E., & Quartau, J. (2006). Reproductive traits of Monochamus galloprovincialis (Coleoptera; Cerambycidae) under laboratory conditions. Bulletin of Entomological Research, 96, 289–294.
Naves, P., Sousa, E., & Rodrigues, J. M. (2008). Biology of Monochamus galloprovincialis (Coleoptera, Cerambycidae) in the Pine Wilt Disease affected zone, Southern Portugal. Silva Lusitana, 16, 132–147.
Rodrigues, J. M., & Sousa, E. (2009). Portuguese national action plan for pinewood nematode control: strategy, actions and results. p. 23. Proceedings of the International Symposium on Pine Wilt Disease (Nanjing, China).
Sousa, E., Bravo, M. A., Pires, J., Naves, P., Penas, A. C., Bonifácio, L., et al. (2001). Bursaphelenchus xylophilus (Nematoda; Aphelenchoididae) associated with Monochamus galloprovincialis (Coleoptera; Cerambycidae) in Portugal. Nematology, 3, 89–91.
Sousa, E., Rodrigues, J., Bonifácio, L., Naves, P., & Rodrigues, A. (2011). Management and control of the Pine Wood Nematode, Bursaphelenchus xylophilus, in Portugal. In F. Boeri & J. Chung (Eds.), Nematodes: Morphology, functions and management strategies. New York, NY: Nova.
Takai, K., Soejima, T., Suzuki, T., & Kawazu, K. (2000). Emamectin benzoate as a candidate for a trunk-injection agent against the pine wood nematode, Bursaphelenchus xylophilus. Pest Management Science, 56, 937–941.
Takai, K., Suzuki, T., & Kawazu, K. (2003a). Development and preventative effect against pine wilt disease of a novel liquid formulation of emamectin benzoate. Pest Management Science, 59, 365–370.
Takai, K., Suzuki, T., & Kawazu, K. (2003b). Distribution and persistence of emamectin benzoate at efficacious concentrations in pine tissues after injection of a liquid formulation. Pest Management Science, 60, 42–48.
Acknowledgments
We would like to thank the INRB, I.P. colleagues Dr. Luís Bonifácio and Mr. Adérito Matos for assistance in the field, and Dr. Lurdes Inácio and Margarida Fontes for nematode identification. The Herdade da Comporta kindly allowed the experiments to be conducted on their property. Thanks also to Dr. Peter Wyss, Mr. Rui Delgado and Mr. Victor Anderau of Syngenta Crop Protection AG for technical assistance and support during the field and lab experiments and to Drs. Rob Lind, Alison Fraser and Miriam Daniels of Syngenta Biosciences at Jealotts Hill (UK) who conducted the EB analysis on the pine branches. The work was partially funded by the Portuguese National Forestry Authority (AFN) and Fundo Florestal Permanente through the national project “O nemátode-da-madeira-do-pinheiro (NMP), Bursaphelenchus xylophilus - Área de trabalho 4: Controlo do insect vector”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sousa, E., Naves, P. & Vieira, M. Prevention of pine wilt disease induced by Bursaphelenchus xylophilus and Monochamus galloprovincialis by trunk injection of emamectin benzoate. Phytoparasitica 41, 143–148 (2013). https://doi.org/10.1007/s12600-012-0272-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-012-0272-y