Abstract
The entomopathogenic nematodes Steinernema weiseri, S. feltiae, S. carpocapsae and two strains of Heterorhabditis bacteriophora, isolated from Turkish soils, were evaluated against larvae of the Mediterranean fruit fly (medfly) Ceratitis capitata in plastic cups under laboratory conditions with sandy loam soil and 10% moisture level. At a rate of 100 infective juveniles (IJs)/cm2, the last instar larvae of C. capitata were susceptible to the entomopathogenic nematodes: the S. feltiae 09-31 strain recovered from Aydin provided 78% mortality, whereas S. weiseri and S. carpocapsae killed 50% and 56% of the larvae, respectively. Both strains of H. bacteriophora species caused less than 50% mortality. Except for S. feltiae, the majority of infected medflies died as prepupae or pupae within the puparia. More than 90% larval mortality was recorded at 200 and 400 IJs/cm2 for S. feltiae. None of the nematode isolates infected the medfly pupae within the puparia. In pot experiments containing soil, S. feltiae caused 96% and 97% mortality at 100 and 200 IJs/cm2, respectively. In pot experiments with grass present, more than 94% mortality was obtained in the presence of grass roots.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Entomopathogenic nematodes in the families Steinernematidae and Heterorhabditidae are available commercially in many parts of the world to control a number of different soil insect pests (Kaya and Gaugler 1993). In Turkey, the research with these nematodes is still in its early stages, but great strides are being made in studying their ecology, behavior, mass production, etc., with the goal of using them as biological control agents against soil insect pests (Hazir et al. 2003a). A great diversity of entomopathogenic nematodes occurs with a number of species being isolated from Turkish soils (Hazir et al. 2003b, c).
The biology of entomopathogenic nematodes makes them excellent candidates for controlling soil insect pests. Nematodes in both families are associated with mutualistic bacteria. In the genus Steinernema the associated bacteria are in the genus Xenorhabdus, whereas in the genus Heterorhabditis the associated bacteria are in the genus Photorhabdus. The bacterial cells are carried as symbionts in the intestinal tract of the only free-living stage of the nematode, the infective juvenile (IJ) (Kaya et al. 2006). IJs enter the insect host through natural orifices (mouth, anus, and spiracles) and subsequently penetrate into the hemocoel, where the bacterial cells are released, resulting in insect death within 48 h. Some nematode species can also penetrate directly into the insect’s hemocoel through the soft cuticle. In the insect cadaver, the bacteria serve as a food source and are required for nematode growth and reproduction. Once nutrients are exhausted in the insect cadaver, progeny nematodes develop into the IJ stage, carrying the bacterial symbiont, and exit the cadaver into the soil to search for a new host (Griffin et al. 2005).
A number of soil pests are potential candidates for biological control in Turkey using entomopathogenic nematodes (Hazir et al. 2003a). One of them is the Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann). It is a native to Africa and has invaded and become established in many parts of the world including Turkey (Fimani 1989; Liquido et al. 1991). It is a major pest capable of infesting more than 260 different species of fruits, vegetables, and nuts. The typical life cycle begins when the female medfly lays her eggs in the fruit, where they hatch in 1−3 days. The larvae feed for 1−2 weeks, going through three instars, and exit the fruit to pupate in the soil. After 1−2 weeks as pupae within the puparia in the soil, the adults emerge from the puparia to complete the life cycle (Fimani 1989). In Aydin, Turkey, there are at least six or seven generations of this pest per year, with the major infestations occurring in figs, persimmons and citrus (Anonymous 1995).
The medfly has a number of natural enemies including parasitoids and entomopathogens. The parasitoids can use their ovipositor to locate and parasitize medfly eggs and larvae within the fruit, but these medfly stages are generally protected from entomopathogens. During the period of time that the larvae leave the fruit and enter the soil, they are susceptible to infection by entomopathogens, especially fungi (Ekesi et al. 2007; Mochi et al. 2006) and nematodes (Bazman et al. 2008; Gazit et al. 2000; Lindegren and Vail 1986; Lindegren et al. 1990).
With entomopathogenic nematodes, laboratory studies showed that the Turkish isolate of Steinernema weiseri Mracek, Sturhan & Reid was effective against the medfly larvae, killing 100% of them when the larvae were held individually in wells with 50 IJs (Bazman et al. 2008). Gazit et al. (2000) tested 12 entomopathogenic nematode isolates against last instar C. capitata larvae and demonstrated that percentage mortality of C. capitata larvae caused by Steinernema riobrave Cabanillas, Poinar & Raulston (82.5%) and Heterorhabditis sp. (81.5%) were higher than that caused by Steinernema feltiae (Filipjev) (33.9%). In addition, Lindegren and Vail (1986) reported that C. capitata larvae were highly susceptible to Steinernema carpocapsae (Weiser) [= S. feltiae; during the 1980s, S. carpocapsae was re-named S. feltiae; see Poinar (1990)]. Lindegren et al. (1990) reported that in the field, a concentration of 5000 IJs/cm2 was more effective than 150 to 500 IJs/cm2 in reducing fly emergence from soil. They also suggested that investigations should include efficacy evaluations of alternate entomopathogenic nematode species or strains. Therefore, we evaluated the virulence of the four entomopathogenic nematode species (Steinernema weiseri, S. feltiae, S. carpocapsae and Heterorhabditis bacteriophora Poinar) isolated in Turkey against the larvae and pupae of the medfly in soil.
Materials and methods
Insect and nematode sources
Third-instar medfly larvae and pupae, reared on artificial medium, were obtained from a laboratory colony in Zirai Mucadele Institution of Bornova (Izmir, Turkey) and used in all experiments.
The nematodes were isolated from soils collected from the outskirts of the city of Aydin, which is an area with a high level of medfly infestations, and from Rize and Kirklareli Province in Turkey. The collected soils were baited with Galleria mellonella L. (Lepidoptera: Pyralidae) larvae, and those larvae showing typical signs of nematode infection (i.e., flaccid body, no putrid odor, tan to black and/or red cadavers) were set up individually on White traps (White 1927). The following nematode species were isolated and used in our study: Steinernema feltiae (isolate 09-31) from a vegetable garden in Aydin, S. carpocapsae from grassland in Rize, and S. weiseri from a pine forest in Aydin, and two isolates of Heterorhabditis bacteriophora (isolates 09-43 and 39-8) from peach orchard in Aydin and a grassland in Kirklareli, respectively. The nematodes were identified to species using 28S rRNA molecular technique by S. Patricia Stock (University of Arizona, Tucson) (personal correspondence).
All entomopathogenic nematode species were cultured for no more than 6 months in last instar G. mellonella larvae. The IJs that were harvested from White traps were kept at 10°C and used within 3 weeks (Kaya and Stock 1997).
Experimental design
Exp. 1: Susceptibility of medfly larvae to nematodes
Plastic cups (4 cm deep and 3.4 cm diam with a surface area of 9 cm2) were used for the experiments. Twenty grams of sterilized, air-dried sandy loam soil (56.4% sand, 30.7% silt, 12.8% clay) obtained from a peach orchard in Aydin where C. capitata infestation was very high, was placed into each cup and the soil moisture level was adjusted to 7% (w/w) by adding distilled water. Entomopathogenic nematodes were applied to the soil arena at a rate of 100 IJs/cm2 soil (Gazit et al. 2000). Final moisture content reached 10% after the nematodes were added in water suspension. The treated cups were kept at room temperature (23–24°C) for 1 h, after which ten third-instar medfly larvae were placed on the soil surface and the cups were capped with a lid. Control cups were prepared as above except that water only was added. There were ten replicates for each nematode isolate and the control with the experiment was conducted twice.
Five days after nematode treatment, the soil in each cup was sieved (1-mm pore diam) to obtain C. capitata larvae and pupae (Lindegren and Vail 1986). The number of larvae and puparia was recorded separately. To determine possible infectivity, reproduction and emergence of the nematodes, all dead larvae and all puparia were transferred individually to White traps. Puparia and larvae in the White traps were monitored daily for nematode or fly emergence. The number of adult flies that emerged from puparia was recorded. The larvae or puparia from which neither adult fly nor nematode emerged were dissected under a light microscope to verify nematode parasitism (Lindegren and Vail 1986).
Exp. 2: Susceptibility of medfly pupae
Twenty 2-day-old C. capitata puparia were placed in a 10-cm plastic petri dish that was lined with a piece of filter paper. A nematode suspension containing 500 IJs/300 µl in distilled water was added to each petri dish. For the control, the puparia in the petri dish were exposed to 300 µl distilled water. The dishes were placed into plastic bags to minimize desiccation and held at room temperature. One week later, the puparia were transferred individually onto White traps to assess nematode emergence for the next 20 days and the number of adult C. capitata emerged was recorded. The puparia from which no adults or nematodes emerged were dissected individually under the dissecting microscope (Lindegren and Vail 1986). There were ten replicates for each nematode species and strain and the experiment was conducted twice.
Exp. 3: Effect of entomopathogenic nematode density on larval mortality
Based on the results of the infectivity tests of the different nematode isolates on C. capitata in Experiment 1, the most virulent isolate was selected for further evaluation. Plastic cups were prepared as described in Experiment 1. Four nematode concentrations—0, 100, 200 or 400 IJs/cm2—were applied to the cups. One hour after treatment, ten third-instar medfly larvae were placed on the soil surface. Five days post treatment, each cup was checked and the number of dead larvae and all puparia was recorded. All dead larvae and puparia were placed individually onto White traps and the emergence of IJs from larvae and puparia or adult flies was recorded. The larvae and puparia without nematodes or adult fly emergence were dissected under the microscope to determine if the mortality was caused by the nematodes. Ten replicates were used for each concentration and the experiment was repeated twice.
Exp. 4: Treatment of medfly larvae in flower pots
Two experiments were conducted in plastic flower pots (13 cm deep and 12 cm diam with a surface area of 113 cm2) using the same nematode species as in Experiment 3. The first experiment was conducted with the same soil used in Experiment 1 and the second was conducted with field-collected grass placed into the pots. There were five replicates for each treatment concentration in each experiment, which was repeated three times.
In the first experiment, the flower pots were filled with 1300 g of soil, and the IJs suspended in water were applied at the rate of 0, 100 and 200 IJs/cm2 to the pots. The final moisture of the soil was adjusted to 10% (w/w). After 1 h acclimatization at room temperature, 50 last instar medfly larvae were added to the surface of the soil in each pot. The larvae were allowed to burrow naturally into the soil. The larvae which did not burrow into the soil in 5 min were replaced with new ones (Gazit et al. 2000). The pots were placed individually into screened cages (30 × 40 × 50 cm) (Lindegren and Vail 1986) and kept for 20 days at room temperature. The emerged medfly adults from each cage were collected and counted, and larval mortality was calculated by subtracting the number of emerged adults from the initial number of larvae added to each pot (Gazit et al. 2000; Lindegren et al. 1990).
In the second experiment, the flower pots as described above were filled with 600 g of soil as used in Experiment 1. Grass, Lolium perenne L. (20-25 cm tall) with its roots, was dug (at Adnan Menderes University) from a site not treated with chemical pesticides and free of entomopathogenic nematodes and was planted into the pots 1 week before treatment with nematodes. IJs suspended in distilled water were applied to the soil arena at a rate of 0 or 100 IJs/cm2. After 1 h acclimatization at room temperature, 50 last instar medfly larvae were added to the surface of the grass in each pot. Larvae not burrowing into the grass within 5 min were replaced with new ones. The pots were handled as described for the first pot experiment except that at 3-day intervals, 10 ml distilled water was added to each flower pot. The number of medfly adults in each cage was counted, and larval mortality was assessed as described for the first pot experiment.
Statistical analysis
One-way ANOVA was used to compare the mortality of C. capitata. Means were compared at the P = 0.05 level, and Tukey’s test was used to separate means (SPSS 1999). Arcsine transformation was carried out on mortality (%) before analyses.
Results
Exp. 1: Susceptibility of medfly larvae to nematodes
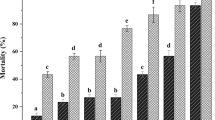
Third-instar C. capitata larvae were susceptible to all tested entomopathogenic nematode isolates (Fig. 1). However, mortality of C. capitata by S. feltiae (09-31) was significantly higher than H. bacteriophora, S. weiseri, and the control group, but not from S. carpocapsae. Except for H. bacteriophora (isolate 09-48), statistical differences were observed among all nematode isolates and the control group (F = 11.087; df = 5, 54; P < 0.001) (Fig. 1).
Mortality (%) of Ceratitis capitata larvae using different entomopathogenic nematode species isolated from Turkey, at a rate of 100 infected juveniles/cm2 soil surface. Black areas in the columns show pupal mortality and light areas show larval mortality when last instar larvae of C. capitata were exposed to entomopathogenic nematodes. (Means ± SE; columns with a common letter do not differ significantly at P = 0.05.) H.b.1 (Heterorhabditis bacteriophora-isolate 09-48), H.b.2 (H. bacteriophora-isolate 39-8), S.w. (Steinernema weiseri), S.c. (S. carpocapsae), S.f. (S. feltiae)
With all nematode isolates, mortality occurred as larvae or pupae within the puparia (Fig. 1). However, except for S. feltiae and H. bacteriophora (09-48), the majority of the medfly larvae exposed to the nematodes died from a nematode infection as prepupae or pupae within the puparia. For S. feltiae, 71% out of 78% medfly died as larvae and 7% died as pupae, whereas for H. bacteriophora (09-48) 20% out of 37% died as larvae and 17% died as pupae within the puparia. All nematode isolates reproduced in the medfly larvae and/or pupae as new IJs emerged from the larvae or puparia into the White traps.
Exp. 2: Susceptibility of medfly pupae
None of the nematode isolates infected the medfly pupae within the puparia. All puparia produced adult medflies within the 20-day holding period after nematode inoculation.
Exp. 3: Effect of entomopathogenic nematode density on mortality
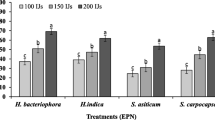
We selected S. feltiae for further studies because it performed slightly better than S. carpocapsae, has been more effective against a number of fly species, and has an intermediate foraging behavior.
Significant differences were observed between 100 IJs/cm2 and 200 and 400 IJs/cm2 as well as significant differences between nematode concentrations and the control group (F = 191.783; df = 3, 76; P < 0.001) (Fig. 2). We observed 78% mortality at 100 IJs/cm2 and >90% larval mortality at 200 and 400 IJs/cm2.
Exp. 4: Treatment of medfly larvae in flower pots
In the first flower pot experiment, no statistical difference was observed between 100 and 200 S. feltiae IJs/cm2, but significant differences were observed between the nematode treatment and the controls (F = 878.51; df = 2, 27; P < 0.001). S. feltiae caused 96 ± 2% and 97 ± 3% mortality at 100 and 200 IJs/cm2, respectively, whereas control mortality was 5.2 ± 0.3%.
In the second flower pot experiment, S. feltiae at the rate of 100 IJs/cm2 infected the medfly larvae in the presence of grass roots. Mortality was 94 ± 3% for the nematode treatment compared with 4.2 ± 0.3% for the control (F = 1118.36; df = 1, 18; P < 0.001).
Discussion
The third-instar larvae of C. capitata showed high susceptibility to some of the Turkish entomopathogenic nematode species with Steinernema species being more virulent than Heterorhabditis species (Exp. 1). Patterson Stark and Lacey (1999) also found that S. carpocapsae and S. feltiae were more effective than H. bacteriophora and H. marelatus Liu & Berry against larvae of the western cherry fruit fly, Rhagoletis indifferens Curran, which is very closely related to C. capitata. Most recently, Sirjani et al. (2009) demonstrated that S. feltiae was the most efficacious against third-instar larvae of the olive fruit fly, Bactrocera oleae (Rossi), compared to S. carpocapsae, S. riobrave, S. glaseri (Steiner), H. bacteriophora, and H. marelatus.
With the exception of S. feltiae, the majority of the third-instar larvae exposed to the nematodes became infected but continued to form puparia and died as prepupae or pupae. Our results confirm earlier work with the tephritids C. capitata, Bactrocera (Dacus) dorsalis (Hendel) and B. cucurbitae (Coquillett), exposed to S. carpocapsae (Lindegren and Vail 1986); R. indifferens exposed to S. feltiae and S. carpocapsae (Patterson Stark and Lacey 1999; Yee and Lacey 2003); and B. oleae exposed to six species of entomopathogenic nematodes including S. feltiae, where some larvae died after forming puparia. Yee and Lacey (2003) suggested that reduction of larval mortality might have been the result of larvae being close to pupation when exposed to the nematodes. With S. feltiae, our results showed that the majority of medfly larvae were killed before they could form puparia. These data suggest that our S. feltiae (09-31) Aydin isolate is highly virulent to medfly larvae and support the conclusion that this nematode species is adapted to dipterous larvae (Lewis et al. 2006).
When tested against C. capitata larvae, Gazit et al. (2000) demonstrated that S. riobrave (83% mortality) that they obtained from the USA out-performed S. feltiae (33.9% mortality) that was obtained from Germany. Since S. riobrave has not been isolated in Turkey to date, we were unable to test this species against the medfly. In fact, Bathon (1996) recommended that entomopathogenic nematode species should not be introduced into countries where they are not known to exist. Although the risk is very low for changes in the faunal composition of the import country, the cautious use of exotic entomopathogenic nematode species as biological control agents is recommended.
In our study we also confirmed the results obtained by Lindegren and Vail (1986) that S. carpocapsae cannot infect C. capitata pupae that occurred within the puparia. Similarly, Yee and Lacey (2003) demonstrated that S. feltiae and S. carpocapsae cannot infect R. indifferens pupae. Accordingly, these nematodes are unable to penetrate through the puparium which is the hard integument of the last instar larvae of some dipterous insects. On the other hand, infectivity of nematode species to hosts has been correlated with their foraging behavior and responses to host cues (Grewal et al. 1994; Griffin et al. 2005; Lewis 2002; Lewis et al. 1992). It is known that C. capitata larvae emerge from the fruit to pupate in the top few centimeters (0.9−1.7 cm) of the soil surface (Jackson et al. 1998). S. feltiae seeks its hosts at or just beneath the soil surface (Lewis et al. 2006) and is infectious to tephritid larval species (Patterson Stark and Lacey 1999; Sirjani et al. 2009; Yee and Lacey 2003).
In terms of infectivity, we have demonstrated that S. feltiae at 100 IJs/cm2 in cups with a surface area of 9 cm2 (Exp. 3) with ten medfly larvae caused 78% mortality. In contrast, S. feltiae at 100 IJs/cm2 in the large flower pots with a surface area of 113 cm2 (Exp. 4) with 50 larvae caused 96% mortality in pots without grass and 94% mortality in pots with grass. The reason for the higher mortality in the larger container can be explained, in part, because the medfly larvae were exposed to a higher density of S. feltiae (226 IJs/larva) than in the smaller cup (90 IJs/larva).
In a field study, S. feltiae and S. carpocapsae caused 79−85% mortality against R. indifferens larvae (Yee and Lacey 2003). Among the tested Turkish nematodes, promising results of S. feltiae (09-31) indicated that this isolate has a potential against C. capitata larvae and it should be tested under field conditions. However, we must be selective in how the nematodes are used in control programs. The medfly has six or seven generations per year in Aydin and overwinters as pupae. In early spring, the adults emerge and attack one of the earliest maturing fruits, which are peaches (Anonymous 1995). We propose to target the emerging medfly larvae from fruit around peach trees to reduce the first and second generation medfly adults, which will thereby reduce infestations in later maturing fruits such as figs, persimmons and citrus. With so many generations of the medfly, protecting the maturing fruits in late summer and autumn will be difficult using nematodes alone and a more integrated approach using other control tactics will be needed. In future studies, we will examine the best way to integrate S. feltiae in this integrated approach. One concept in our long range plan is to improve S. feltiae’s ability to recycle and persist in the soil environment.
References
Anonymous (1995). Zirai Mucadele Teknik Talimatlari (Plant Protection Technical Directors), T.C. Tarim ve Koy Isleri Bakanligi Koruma ve Kontrol Genel Mudurlugu. Ankara: Turkish Republic Ministry of Agriculture General Management of Protection and Control.
Bathon, H. (1996). Impact of entomopathogenic nematodes on non-target hosts. Biocontrol Science and Technology, 6, 421–434.
Bazman, I., Ozer, N., & Hazir, S. (2008). Bionomics of the entomopathogenic nematode, Steinernema weiseri (Rhabditida: Steinernematidae). Nematology, 10, 735–742.
Ekesi, S., Dimbi, S., & Maniania, N. K. (2007). The role of entomopathogenic fungi in the integrated management of fruit flies (Diptera: Tephritidae) with emphasis on species occurring in Africa. In Use of entomopathogenic fungi in biological pest management (pp. 239–274). Kerala, India: Research Signpost.
Fimani, P. (1989). Mediterranean Region. In A. S. Robinson, & G. Hooper (Eds.), Fruit flies: Their biology, natural enemies and control (pp. 39–50). Amsterdam, The Netherlands: Elsevier.
Gazit, Y., Rossler, Y., & Glazer, I. (2000). Evaluation of entomopathogenic nematodes for the control of Mediterranean fruit fly (Diptera: Tephritidae). Biocontrol Science and Technology, 10, 157–164.
Grewal, P. S., Lewis, E. E., Gaugler, R., & Campbell, J. F. (1994). Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes. Parasitology, 108, 207–215.
Griffin, C. T., Boemare, N. E., & Lewis, E. E. (2005). Biology and behaviour. In P. S. Grewal, R.-U. Ehlers, & D. Shapiro-Ilan (Eds.), Nematodes as biocontrol agents (pp. 47–59). Wallingford, UK: CABI.
Hazir, S., Kaya, H. K., Stock, S. P., & Keskin, N. (2003a). Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turkish Journal of Biology, 27, 181–202.
Hazir, S., Keskin, N., Stock, S. P., Kaya, H. K., & Ozcan, S. (2003b). Diversity and distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in Turkey. Biodiversity and Conservation, 12, 375–386.
Hazir, S., Stock, S. P., & Keskin, N. (2003c). A new entomopathogenic nematode species Steinernema anatoliense (Steinernematidae) from Turkey. Systematic Parasitology, 55, 211–220.
Jackson, C. G., Long, J. P., & Klungness, L. M. (1998). Depth of pupation in four species of fruit fly (Diptera: Tephritidae) in sand with and without moisture. Journal of Economic Entomology, 91, 138–142.
Kaya, H. K., & Gaugler, R. (1993). Entomopathogenic nematodes. Annual Review of Entomology, 38, 181–206.
Kaya, H. K., & Stock, S. P. (1997). Techniques in insect nematology. In L. A. Lacey (Ed.), Manual of techniques in insect pathology (pp. 281–324). London, UK: Academic.
Kaya, H. K., Aguillera, M. M., Alumai, A., Choo, H. Y., de la Torre, M., Fodor, A., et al. (2006). Status of entomopathogenic nematodes and their symbiotic bacteria from selected countries or regions of the world. Biological Control, 38, 134–155.
Lewis, E. (2002). Behavioural ecology. In R. Gaugler (Ed.), Entomopathogenic nematology (pp. 205–223). Wallingford, UK: CAB International.
Lewis, E., Gaugler, R., & Harrison, R. (1992). Entomopathogenic nematode host finding: response to host contact cues by cruise and ambush foragers. Parasitology, 105, 309–319.
Lewis, E., Campbell, J., Griffin, C., Kaya, H., & Peters, A. (2006). Behavioral ecology of entomopathogenic nematodes. Biological Control, 38, 66–79.
Lindegren, J. E., & Vail, P. V. (1986). Susceptibility of Mediterranean fruit fly, melon fly, and oriental fruit fly (Diptera: Tephritidae) to the entomogenous nematode Steinernema feltiae in laboratory tests. Environmental Entomology, 15, 465–468.
Lindegren, J. E., Wong, T. T., & McInnis, D. O. (1990). Response of Mediterranean fruit fly (Diptera: Tephritidae) to the entomogenous nematode Steinernema feltiae in field tests in Hawaii. Environmental Entomology, 19, 383–386.
Liquido, N. J., Shinoda, L. A., & Cunningham, R. T. (1991). Host plants of the Mediterranean fruit fly (Diptera: Tephritidae): An annotated world review. Miscellaneous Publication, Entomological Society of America. MPPEAL 77.
Mochi, D. A., Monteiro, A. C., De Bortoli, S. A., Dória, H. O. S., & Barbosa, J. C. (2006). Pathogenicity of Metarhizium anisopliae for Ceratitis capitata (Wied.) (Diptera: Tephritidae) in soil with different pesticides. Neotropical Entomology, 35, 382–389.
Patterson Stark, J. E., & Lacey, L. A. (1999). Susceptibility of western cherry fruit fly (Diptera: Tephritidae) to five species of entomopathogenic nematodes in laboratory studies. Journal of Invertebrate Pathology, 74, 206–208.
Poinar Jr., G. O. (1990). Taxonomy and biology of Steinernematidae and Heterorhabditidae. In R. Gaugler, & H. K. Kaya (Eds.), Entomopathogenic nematodes in biological control (pp. 23–61). Boca Raton, FL, USA: CRC.
Sirjani, F. O., Lewis, E. E., & Kaya, H. K. (2009). Evaluation of entomopathogenic nematodes against the olive fruit fly, Bactrocera oleae (Diptera: Tephritidae). Biological Control (in press).
SPSS. (1999). SPSS for Windows, release 10.0.1. Chicago, IL, USA: SPSS.
White, G. F. (1927). A method for obtaining infective nematode larvae from culture. Science, 66, 302–303.
Yee, W., & Lacey, L. A. (2003). Stage-specific mortality of Rhagoletis indifferens (Diptera: Tephritidae) exposed to three species of Steinernema nematodes. Biological Control, 27, 349–356.
Acknowledgments
We thank Dr. Ibrahim Cakmak for his excellent assistance with the statistical analyses, and the Plant Protection Institute of Bornova in Izmir for providing the medfly larvae.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karagoz, M., Gulcu, B., Hazir, C. et al. Biological control potential of Turkish entomopathogenic nematodes against the Mediterranean fruit fly Ceratitis capitata . Phytoparasitica 37, 153–159 (2009). https://doi.org/10.1007/s12600-008-0020-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12600-008-0020-5