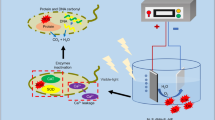
Abstract
Antibiotic misuse has resulted in the emergence of superbugs, warranting new antibacterial methods. Surface amorphisation oxygen vacancy-rich porous Sn3Ox nanosheets in situ grown on Ni foam are successfully designed via a simple, one-step hydrothermal method, resulting in enhanced photoelectrochemical (PEC) bacterial inactivation. In this system, the porous structure enriches its surface with oxygen vacancies, which can extend the absorption spectrum into the near-infrared region, while oxygen vacancies can enhance the separation of electron–hole pairs. Most importantly, the sheet-like porous structure enhances surface active sites and increase the contact area between bacteria and electrodes. Therefore, the reactive oxygen species produced during the PEC process can directly act on the surface of bacteria and is 100% effectively against drug-resistant Gram-positive and Gram-negative bacteria in water within 30 min. This study acts as a foundation for the development of novel photoelectrocatalyst electrodes for efficient water purification.
Graphical Abstract

摘要
细菌感染每年困扰着数百万人,已成为一个全球严峻的公共卫生问题。由于抗生素的滥用,出现了具有多重耐药性的“超级细菌”。因此,迫切需要开发杀菌的新试剂和新手段。光电催化抗菌是将光催化和电催化协同联用的新型化学方法,是一种绿色灭菌技术,具有杀菌效果好、无二次污染、稳定性高和广谱抗菌等特点。本工作中,通过简单的一步水热法在泡沫镍上原位生长了表面非晶化富氧空位的多孔 Sn3Ox 纳米片,具有增强的光电化学灭菌性能。在该系统中,Sn3Ox 多孔结构在其表面富集了氧空位,可以将吸收光谱从可见光扩展到近红外区域,而且氧空位也可以增强电子-空穴对的分离。最重要的是,片状多孔结构增加了表面活性位点,与细菌与电极之间的接触面积,光电化学灭菌过程中产生的活性氧自由基可以直接作用于细菌表面。在30 min内,能够100%的消灭水中的耐药菌。该研究为开发用于高效水净化的新型光电催化系统提供了指导
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Global antimicrobial drug abuse currently is an extremely serious issue, with "superbug" infections killing at least 700,000 people annually [1, 2]. The number of deaths is expected to increase to 10 million per year by 2050 [3, 4]. Various disinfection techniques, such as ultraviolet (UV) irradiation [5], ozonation [6], and chlorination [7], have been widely used to inactivate drug-resistant bacteria. However, those disinfection techniques consume large amounts of chemicals, producing harmful by-products, and are energy-intensive, contributing to climate change [8,9,10,11]. Furthermore, since most drug-resistant bacteria live in water, their inactivation is challenging [12, 13]. It is critical to develop new antibacterial agents that can effectively kill drug-resistant bacteria in water without causing antibiotic resistance, ensuring safe drinking water.
Photocatalytic purification has recently garnered significant attention because of its environmental friendliness, safety, excellent stability, and high reusability [14,15,16,17,18]. Reactive oxygen species (ROS), which can be generated on a photocatalyst exposed to light, can effectively kill bacteria [19,20,21,22]. Most importantly, solar light is the most abundant free renewable energy source that can be used as the light source for the photocatalytic system [23, 24]. A large number of photocatalysis, including TiO2, have been discovered, and present an economical, effective, and environmentally friendly oxidation process for purification [25,26,27,28]. Furthermore, these inorganic nanomaterial antibacterial agents would not cause bacteria to develop drug resistance [29,30,31]. Because of the large band gap (approximately 3.20 eV), conventional TiO2 can only absorb ultraviolet (UV) light and is therefore inactive in the visible spectral range, with bactericidal efficiency far from optimal for real-world applications [32,33,34]. Furthermore, nanomaterials are difficult to recycle and are bound to cause secondary pollution when used for water purification [35,36,37].
The photoelectric approach successfully bridges the gap in current photocatalysis and provides a multitude of potential advantages: (1) Quick electron transport via direct contact between the substrate electrode and photocatalytic active material [38, 39]. (2) A large number of photocatalytically active sites to assure high photoelectric conversion efficiency [40, 41]. (3) The photoelectric catalytic electrode is simple to remove from the solution, produces no secondary pollution, and can be reused [42, 43].
Recent studies have demonstrated photoelectrocatalytic application in the environment and energy fields [44,45,46]. Zhang and colleagues [47] successfully grew MoS2/MoOx on the Ti film electrodes, demonstrating excellent bacterial inactivation activity (the bacteria inactivation efficiency of Escherichia coli (E. coli) reached 99.9999% in 2 h). Ye and colleagues reported the development of a three-dimensional (3D) lupine-like TiO2/Sn3O4 heterostructure photoanode with a high water-splitting performance [48]. According to previous research, a Sn3O4/Ni foam photoanode was synthesised via enhanced photoelectrocatalytic degradation of polyacrylamide. Sn3O4 is an oxide with a layered structure of mixed valence Sn2+ and Sn4+ and a band gap of approximately 2.8 eV [49]. It has been established that Sn3O4 can harvest visible light and use it to catalyse the organic degradation of various compounds [50]. Thus, Sn3O4 may exhibit a higher potential for photoelectrocatalytic bacterial inactivation than TiO2.
In this study, surface amorphisation oxygen vacancy-rich porous Sn3Ox nanosheets were grown on Ni foam using a simple, one-step hydrothermal method, resulting in enhanced photoelectrochemical (PEC) bacterial inactivation properties. This method is simple, convenient, and safe when compared to the traditional H2 post-processing method for generating oxygen vacancies. With the enhancement of the PEC performance, the photo anode killed bacteria with an antibacterial efficiency of up to 100% in 30 min.
2 Results and discussion
Firstly, the Sn3O4 nanosheets were grown in situ on Ni foam surfaces using a hydrothermal method similar to our previous research [51]. During the nucleation of Sn3O4, ascorbic acid was added to obtain an oxygen vacancy-rich Sn3O4. Ascorbic acid with reducing properties an adsorb oxygen atoms and increases the oxygen vacancy concentration of the material. To facilitate the distinction, we referred to the Sn3O4 that was mixed with ascorbic acid during the preparation process as Sn3Ox. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to examine the morphologies and structural characteristics of the samples. Figure S1a shows a clear 3D network structure of the Ni foam, with the branches interlacing to form pores of varying sizes. The Ni foam has a smooth surface (Fig. S1b). 3D foam-like network structure is still present growing Sn3O4 on the surface (Fig. 1a). The high-resolution SEM image (Fig. 1b), however, shows that Ni foam is uniformly covered by Sn3O4 nanosheets with diameters ranging from 500 to 800 nm. Furthermore, as shown in Fig. 1c, the thickness of Sn3Ox nanosheets is approximately 30–40 nm. The thickness of Sn3Ox layer coating on the Ni foam is approximately 3.8 μm (Fig. 1d). Sn3Ox was separately synthesized and exhibits a spherical structure composed of nanosheets agglomerated into micro flowers (Fig. S2). TEM results indicate that both the Sn3O4 (Fig. S3) and Sn3Ox (Fig. 1e) have a nanosheet structure, which is consistent with SEM. High-resolution TEM (HRTEM) image (Fig. 1f) shows a crystalline lattice characteristic of Sn3Ox. The lattice fringe spacing measured is approximately 0.35 nm, corresponding to the (101) plane of the triclinic structure of Sn3Ox. Sn3Ox structure becomes porous owing to ascorbic acid regulation. The pores are approximately 2 nm in size. Furthermore, an amorphous layer is formed on the surface of Sn3Ox.
Based on the X-ray diffraction (XRD) results (Fig. 2a), the 2θ peak diffraction peak is identified at 44.5°, 51.8° and 76.4° corresponding to Ni (JCPDS No. 04-0850) (111), (200) and (220), respectively. The 2θ peak diffraction at 27.1°, 31.7°, 32.3°, 37.1°, 50.0°, 60.9° and 63.5° are consistent with Sn3O4 (JCPDS No. 16-0737) (111), (\({\overline{2}10}\)), (\({\overline{1}21}\)), (130), (\({\overline{3}01}\)), (042) and (312), respectively. These findings strongly reveal that Sn3O4/Ni foam is successfully prepared, as no other impurity peaks are observed. The structure of the samples was investigated using Raman spectroscopy (Fig. 2b). Raman peaks of Sn3O4 at 70, 82, 136, 165 and 235 cm–1 can be seen in Sn3O4/Ni and Sn3Ox/Ni foam photoanodes, indicating that Sn3O4 nanosheets have been successfully loaded onto the surface of Ni foam. Figure 2c shows the optical capabilities of the samples. Sn3O4 nanosheets spectrum is typical of wide bandgap oxide semiconductors, with an intense absorption band and steep cut-off at 440 nm. Sn3O4/Ni and Sn3Ox/Ni foams absorb more light in the visible to near-infrared (NIR) spectral region than pure Sn3O4 nanosheets.
The surface chemical compositions and composing elements of these catalysts were then investigated using X-ray photoelectron spectroscopy (XPS). The broad-scan spectrum of Sn3Ox/Ni foam photoanode is shown in Fig. S4; Sn 3d, Ni 2p and O 1s peaks are observed, confirming that the sample elements contain Sn, Ni and O. Sn 3d signals are decomposed into two characteristic peaks, Sn 3d3/2 and Sn 3d5/2, with components at 495.55 and 486.70 eV corresponding to Sn2+, respectively, and components near 494.55 and 486.05 eV corresponding to Sn4+, respectively (Fig. 2d).
These findings confirm the presence of Sn2+ and Sn4+ in Sn3Ox/Ni foam. The characteristic peaks in Ni 1s spectra of Sn3Ox/Ni foam are attributed to Ni salt, Ni 2p2/3 and Ni 2p1/2 at 861.08, 855.65 and 873.83 eV, respectively (Fig. 2e). Furthermore, O 1s XPS profile could be fitted to two characteristic peaks. The 530.2 eV peak is caused by oxygen atoms bound to the metal, while the 531.4 eV peak is caused by defect sites with low oxygen coordination, indicating the presence of oxygen vacancies.
To evaluate PEC performance of Sn3Ox/Ni foam photoanode, it is used to accelerate the processing of E. coli in water under light irradiation with a 0.8 V bias. When compared to Ni and Sn3O4/Ni foam photoanodes, the Sn3Ox/Ni foam photoanode shows the highest E. coli inactivation performance (Fig. 3a). Figure 3b shows the direct E. coli inactivation properties by Sn3Ox/Ni foam photoanode under different conditions. In the control group, the bacterial population remains unchanged after 40 min of exposed to Sn3Ox/Ni foam photoanode. It has been demonstrated that Sn3Ox/Ni foam photoanode is nontoxic to bacteria. However, under light irradiation or 0.8 V bias, approximately 1.7 and 2.2 log of E. coli was inactivated in 40 min. In contrast, E. coli is completely inactivated after 30 min of PEC treatment at 0.8 V bias and light irradiation. Reducing the bias potential of 0.2–0.8 V significantly increases the bacterial inactivation efficiency, indicating that the photocatalytic ability is positively related to the applied external bias (Fig. 3c).
E. coli inactivation under different conditions: a comparison of Ni foam, Sn3O4/Ni foam and Sn3Ox/Ni foam; b comparison of light, 0.8 V bias and light + 0.8 V bias; c bias potential varying from 0.2 to 0.8 V, where *p < 0.05, **p < 0.01, ***p < 0.001 indicate significant differences compared to control group; d PEC inactivation of different bacteria (E. coli, Chlr E. coli, S. aureus and MRSA); e corresponding photographs of bacterial colonies; f SEM images of Chlr E. coli before and after PEC oxidation for 30 min, and corresponding fluorescence microscopic images (where C is terminal concentration of bacteria and C0 is e concentration at t = 0 of experiments)
Furthermore, to demonstrate the broad-spectrum bactericidal effect, we tested Gram-negative bacteria, Gram-positive bacteria, and drug-resistant bacteria (chloramphenicol-resistant E. coli (Chlr E. coli) and methicillin-resistant Staphylococcus aureus (MRSA)), as shown in Fig. 3d, e. These findings demonstrate the universality of PEC oxidation in the treatment of various bacteria.
SEM was used to study the morphology of Chlr E. coli before and after treatment to decipher the antibacterial behaviour. As shown in Fig. 3f, before treatment, Chlr E. coli presented an intact cellular structure with a typical rod-like shape. Chlr E. coli is severely misshapen and fractured after PEC oxidation, indicating that the generated ROS sabotage the bacterial cell wall. This result is also confirmed in the bacterial live/dead assay, distinguishing the live bacteria (stained in green) and dead bacteria (stained in red). As shown in Fig. 3f, initially, almost none of Chlr E. coli are killed. Following PEC oxidation, all bacteria turned red, indicating mortality.
The photoelectric properties were tested to clarify the sterilization mechanism. In the dark, Sn3O4/Ni and Sn3Ox/Ni foam photoanodes display extremely weak current densities, as shown in Fig. 4a. When the photoanodes are illuminated with light, an anodic photocurrent occurs, which increases as the bias potential increases. The flow of the photogenerated electrons through the outer circuit causes the photocurrent. As shown in Fig. 4b, Sn3Ox/Ni foam photoanode demonstrates a significantly higher photocurrent than the Sn3O4/Ni foam photoanode, attributed to the improved charge separation and interfacial charge transfer in the photoanode. Furthermore, when the bias is varied (0.2, 0.4, 0.6 and 0.8 V), Sn3Ox/Ni foam photoanode exhibits fast and reversible photocurrent responses for each on and off cycle and the photocurrent density increases as the bias voltages increase (Fig. S5).
a Current–voltage curves in dark and under light irradiation of Sn3O4/Ni foam and Sn3Ox/Ni foam photoanodes; b photocurrent density ON–OFF curves of Sn3O4/Ni foam and Sn3Ox/Ni foam photoanodes; c EIS Nyquist plots under dark and light irradiation of Sn3O4/Ni foam and Sn3Ox/Ni foam (impedance is a complex number, Z′ and Z″ represent real and imaginary parts, respectively); d EPR spectra of Sn3O4 and Sn3Ox nanosheets
According to the electrochemical impedance spectroscopy Nyquist analysis, the charge transfer resistance of electrodes, which is inversely proportional to the rate of charge transfer in the PEC process, can be calculated using the diameter of the fitted semi-circle [52, 53]. The obtained Nyquist plots of Sn3O4/Ni and Sn3Ox/Ni foam photoanodes are shown in Fig. 4c. Nyquist plots of Sn3O4/Ni and Sn3Ox/Ni foam photoanodes show semicircles. The low ohmic resistance of the as-fabricated photoanode suggests a fast charge transfer. Furthermore, the impedance is reduced further under light illumination, demonstrating that illumination promotes faster carrier transport on the surface. When compared to Sn3O4/Ni foam, the impedance of Sn3Ox/Ni foam is reduced, indicating that the separation and transfer of photogenerated charges from Sn3Ox/Ni foam yields the most efficient and fastest charge transfer performance that would improve PEC activities.
Furthermore, electron paramagnetic resonance (EPR) spectroscopy was carried out at 25 °C to demonstrate the formation of oxygen vacancies following ascorbic acid reduction. As shown in Fig. 4d, a single EPR signal associated with oxygen vacancies was observed in both Sn3O4 and Sn3Ox. However, the signal of oxygen vacancies in Sn3Ox is significantly higher than in Sn3O4, contributing to enhanced light absorption and the separation of photogenerated carriers.
Based on the above experimental results, we propose a mechanism for PEC charge transfer and bacterial inactivation that occur on Sn3Ox/Ni foam photoanode, as shown in Fig. 5. Sn3Ox initially absorbs visible light to generate electron and hole pairs. The available wavelength range can be extended to the NIR region by forming a porous structure rich in oxygen vacancies. Under light irradiation, electron–hole pairs are formed, and the electron holes remain separated at the applied voltage and do not rapidly compound. On the photoanode, the generated holes from Sn3Ox can react with H2O to produce ·OH, which has the strongest oxidising ability of any ROS. Furthermore, ·O2– is produced on the cathode by electron reduction of O2. Sn3Ox/Ni foam has a porous structure, and the rough surfaces of the photoanode increase the contact area with bacteria. Further, the ROS produced directly acts on the bacteria, resulting in an excellent bactericidal effect.
3 Conclusion
To summarise, surface amorphisation oxygen vacancy-rich porous Sn3Ox nanosheets in situ grown on Ni foam were successfully designed via a simple, one-step hydrothermal method, resulting in enhanced PEC activity for bacterial inactivation. PEC characterisation demonstrated an enhancement in photoelectric catalytic performance owing to a broadened absorption spectrum and effective separation of the electron–hole pairs. This outstanding photoelectrocatalyst demonstrated a 100% antibacterial effect in water against drug-resistant Gram-positive and Gram-negative bacteria. This surface amorphisation oxygen vacancy-rich porous Sn3Ox nanosheet strategy can inspire the design and fabrication of other PEC systems for efficient photoelectrocatalytic bacterial inactivation.
References
Alharthi S, Alavi SE, Moyle PM, Ziora ZM. Sortase A (SrtA) inhibitors as an alternative treatment for superbug infections. Drug Discov Today. 2021;26(9):2164. https://doi.org/10.1016/j.drudis.2021.03.019.
Van Boeckel TP, Pires J, Silvester R, Zhao C, Song J, Criscuolo NG, Gilbert M, Bonhoeffer S, Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365(6549):1944. https://doi.org/10.1126/science.aaw1944.
De Kraker MEA, Stewardson AJ, Harbarth S. Will 10 million people die a year due to antimicrobial resistance by 2050. PLoS Med. 2016;13(11):1002184. https://doi.org/10.1371/journal.pmed.1002184.
Li XH, Fan H, Zi H, Hu HK, Li BH, Huang J, Luo PC, Zeng XT. Global and regional burden of bacterial antimicrobial resistance in urinary tract infections in 2019. J Clin Med. 2022;11(10):2817. https://doi.org/10.3390/jcm11102817.
Luo XR, Zhang BP, Lu YH, Mei Y, Shen L. Advances in application of ultraviolet irradiation for biofilm control in water and wastewater infrastructure. J Hazard Mater. 2022;421(5): 126682. https://doi.org/10.1016/j.jhazmat.2021.126682.
Issaka E, Amu-Darko JNO, Yakubu S, Fapohunda FO, Ali N, Bilal M. Advanced catalytic ozonation for degradation of pharmaceutical pollutants-a review. Chemosphere. 2022;289: 133208. https://doi.org/10.1016/j.chemosphere.2021.133208.
Xing ZX, Cheng GJ, Yang H, Xue XX, Jiang PG. Mechanism and application of the ore with chlorination treatment: a review. Miner Eng. 2020;154:106404. https://doi.org/10.1016/j.mineng.2020.106404.
Conejo AN, Birat JP, Dutta A. A review of the current environmental challenges of the steel industry and its value chain. J Environ Manage. 2020;259:109782. https://doi.org/10.1016/j.jenvman.2019.109782.
Liu LY, Ji HG, Lu XF, Wang T, Zhi S, Pei F, Quan DL. Mitigation of greenhouse gases released from mining activities: a review. Int J Miner Metall Mater. 2021;28:513. https://doi.org/10.1007/s12613-020-2155-4.
Tauseef SM, Abbasi T, Abbasi SA. Energy recovery from wastewaters with high-rate anaerobic digesters. Renew Sustain Energy Rev. 2013;19:704. https://doi.org/10.1016/j.rser.2012.11.056.
Lv R, Liang YQ, Li ZY, Zhu SL, Cui ZD, Wu SL. Flower-like CuS/graphene oxide with photothermal and enhanced photocatalytic effect for rapid bacteria-killing using visible light. Rare Met. 2022;41(2):639. https://doi.org/10.1007/s12598-021-01759-4.
Waso M, Reyneke B, Havenga B, Khan S, Khan W. Insights into Bdellovibrio spp. mechanisms of action and potential applications. World J Microbiol Biotechnol. 2021;37:85. https://doi.org/10.1007/s11274-021-03054-x.
Wang LW, Gao FE, Wang AZ, Chen XY, Li H, Zhang X, Zheng H, Ji R, Li B, Yu X, Liu J, Gu ZJ, Chen FL, Chen CY. Defect-rich adhesive molybdenum disulfide/rGO vertical heterostructures with enhanced nanozyme activity for smart bacterial killing application. Adv Mater. 2020;32(48):2005423. https://doi.org/10.1002/adma.202005423.
Wang ZM, Shen ZY, Li YM, Zuo JL. Preparation and photoelectrocatalytic performance of Ru loaded TiO2 nanotubes. Chin J Rare Met. 2020;44(6):609. https://doi.org/10.13373/j.cnki.cjrm.XY18120018.
Wang LW, Zhang X, Yu X, Gao FE, Shen ZY, Zhang XL, Ge SG, Liu J, Gu ZJ, Chen CY. An all-organic semiconductor C3N4/PDINH heterostructure with advanced antibacterial photocatalytic therapy activity. Adv Mater. 2019;31(33):1901965. https://doi.org/10.1002/adma.201901965.
Ferraz NP, Nogueira AE, Marcos FCF, Machado VA, Rocca RR, Assaf EM, Asencios YJO. CeO2–Nb2O5 photocatalysts for degradation of organic pollutants in water. Rare Met. 2020;39(3):230. https://doi.org/10.1007/s12598-019-01282-7.
Huang G, Xu ZH, Luo TT, Yan ZX, Zhang M. Fluorescent light enhanced graphitic carbon nitride/ceria with ultralow-content platinum catalyst for oxidative decomposition of formaldehyde at ambient temperature. Rare Met. 2021;40(11):3135. https://doi.org/10.1007/s12598-021-01756-7.
Zhao Y, Liu J, Han M, Yang G, Ma L, Wang Y. Two comparable Ba-MOFs with similar linkers for enhanced CO2 capture and separation by introducing N-rich groups. Rare Met. 2021;40(2):499. https://doi.org/10.1007/s12598-020-01597-w.
Yang RQ, Song GX, Wang LW, Yang ZW, Zhang J, Zhang X, Wang S, Ding LH, Ren N, Wang AZ, Yu X. Full solar-spectrum-driven antibacterial therapy over hierarchical Sn3O4/PDINH with enhanced photocatalytic activity. Small. 2021;17(39):2102744. https://doi.org/10.1002/smll.202102744.
Zhou Z, Li B, Liu X, Li Z, Zhu S, Liang Y, Cui Z, Wu S. Recent progress in photocatalytic antibacterial. ACS Appl Bio Mater. 2021;4:3909. https://doi.org/10.1021/acsabm.0c01335.
Li PP, Wu HX, Dong A. Ag/AgX nanostructures serving as antibacterial agents: achievements and challenges. Rare Met. 2022;41(2):519. https://doi.org/10.1007/s12598-021-01822-0.
Zeng JY, Li ZM, Jiang H, Wang XM. Progress on photocatalytic semiconductor hybrids for bacterial inactivation. Mater Horiz. 2021;8:2964. https://doi.org/10.1039/D1MH00773D.
Yu X, Jin X, Chen XY, Wang AZ, Zhang JM, Zhang J, Zhao ZH, Gao MM, Razzari L, Liu H. A microorganism bred TiO2/Au/TiO2 heterostructure for whispering gallery mode resonance assisted plasmonic photocatalysis. ACS Nano. 2020;14(10):13876. https://doi.org/10.1021/acsnano.0c06278.
Li JF, Li ZY, Liu XM, Li CY, Zheng YF, Yeung KWK, Cui ZD, Liang YQ, Zhu SL, Hu WB, Qi YJ, Zhang TJ, Wang XB, Wu SL. Interfacial engineering of Bi2S3/Ti3C2Tx MXene based on work function for rapid photo-excited bacteria-killing. Nat Commun. 2021;12:1. https://doi.org/10.1038/s41467-021-21435-6.
Guo H, Li J, Zou XR, Wang HS, Kang A, Zhou H, Li MJ, Zhao XY. Fabrication of GO-TiO2/(Ca, Y)F-2:Tm, Yb composites with high-efficiency optical driving photocatalytic activity for degradation of organic dyes and bacteriostasis. Rare Met. 2022;41(2):650. https://doi.org/10.1007/s12598-021-01831-z.
Li JY, Ma AQ, Li HF, Dong YH, Gao YQ. Tunable micromorphology and photocatalytic properties of monoclinic BiVO4 prepared by bionic template method. Chin J Rare Met. 2020;44(9):912. https://doi.org/10.13373/j.cnki.cjrm.xy19060008.
Zhang P, Yu L, Lou XW. Construction of heterostructured Fe2O3-TiO2 microdumbbells for photoelectrochemical water oxidation. Angew Chem Int Ed. 2018;57:15076. https://doi.org/10.1002/anie.201808104.
Ji YC, Yang RQ, Wang LW, Song GX, Wang AZ, Lv YW, Gao MM, Zhang J, Yu X. Visible light active and noble metal free Nb4N5/TiO2 nanobelt surface heterostructure for plasmonic enhanced solar water splitting. Chem Eng J. 2020;402: 126226. https://doi.org/10.1016/j.cej.2020.126226.
Sun JY, Wen JH, Wu GZ, Zhang Z, Chen X, Wang GC, Liu MY. Harmonizing the electronic structures on BiOI with active oxygen vacancies toward facet-dependent antibacterial photodynamic therapy. Adv Func Mater. 2020;30(42):2004108. https://doi.org/10.1002/adfm.202004108.
Vorobyova V, Vasyliev G, Uschapovskiy D, Lyudmyla K, Skiba M. Green synthesis, characterization of silver nanoparticals for biomedical application and environmental remediation. J Microbiol Methods. 2022;193:106384. https://doi.org/10.1016/j.mimet.2021.106384.
Fu JN, Zhu WD, Liu XM, Liang CY, Zheng YF, Li ZY, Liang YQ, Zheng D, Zhu SL, Cui ZD, Wu SL. Self-activating anti-infection implant. Nature Commun. 2021;12:1. https://doi.org/10.1038/s41467-021-27217-4.
Feng YJ, Wang Y, Wang KW, Ma JP, Duan YY, Liu J, Lu X, Zhang B, Wang GY, Zhou XY. Ultra-fine Cu clusters decorated hydrangea-like titanium dioxide for photocatalytic hydrogen production. Rare Met. 2022;41(2):385. https://doi.org/10.1007/s12598-021-01815-z.
Yu X, Zhao ZH, Sun DH, Ren N, Ding LH, Yang RQ, Ji YC, Li LL, Liu H. TiO2/TiN core/shell nanobelts for efficient solar hydrogen generation. Chem Commun. 2018;54(47):6056. https://doi.org/10.1039/C8CC02651C.
Wang WC, Zhu S, Cao YN, Tao Y, Li X, Pan DL, Phillips DL, Zhang DQ, Chen M, Li GS, Li HX. Edge-enriched ultrathin MoS2 embedded yolk-shell TiO2 with boosted charge transfer for superior photocatalytic H2 evolution. Adv Func Mater. 2019;29(36):1901958. https://doi.org/10.1002/adfm.201901958.
Chen WJ, Li SY, Wang J, Sun K, Si YB. Metal and metal-oxide nanozymes: bioenzymatic characteristics, catalytic mechanism, and eco-environmental applications. Nanoscale. 2019;11:15783. https://doi.org/10.1039/C9NR04771A.
Li XM, Wu DH, Hua T, Lan XQ, Han SP, Cheng JH, Du KS, Hu YY, Chen YC. Micro/macrostructure and multicomponent design of catalysts by MOF-derived strategy: opportunities for the application of nanomaterials-based advanced oxidation processes in wastewater treatment. Sci Total Environ. 2022;804: 150096. https://doi.org/10.1016/j.scitotenv.2021.150096.
Soares SF, Fernandes T, Trindade T, Daniel-da-Silva AL. Recent advances on magnetic biosorbents and their applications for water treatment. Environ Chem Lett. 2020;18:151. https://doi.org/10.1007/s10311-019-00931-8.
Wang RQ, Wang FK, Zhang X, Feng X, Zhao CD, Bu KJ, Zhang Z, Zhai TY, Huang FQ. Improved polarization in the Sr6Cd2Sb6O7Se10 oxyselenide through design of lateral sublattices for efficient photoelectric conversion. Angew Chem Int Ed. 2022;61(33):e202206816. https://doi.org/10.1002/ange.202206816.
Zheng XL, Wu DX, Liu YH, Li J, Yang YJ, Huang W, Liu WF, Shen YJ, Tian XL. Photocatalytic reduction of water to hydrogen by CuPbSbS3 nanoflakes. Mater Today Energy. 2022;25: 100956. https://doi.org/10.1016/j.mtener.2022.100956.
Liu ZR, Wang LW, Yu X, Zhang J, Yang RQ, Zhang XD, Ji YC, Wu MQ, Deng L, Li LL, Wang ZL. Piezoelectric-effect-enhanced full-spectrum photoelectrocatalysis in p–n heterojunction. Adv Func Mater. 2019;29(41):1807279. https://doi.org/10.1002/adfm.201807279.
Yang RQ, Ji YC, Wang LW, Song GX, Wang AZ, Ding LH, Ren N, Lv YW, Zhang J, Yu X. Crystalline Ni-doped Sn3O4 nanosheets for photocatalytic H2 production. ACS Appl Nano Mater. 2020;3:9268. https://doi.org/10.1021/acsanm.0c01886.
Cho YU, Lee JY, Jeong UJ, Park S, Lim SL, Kim KY, Jang JW, Park JH, Kim HW, Shin H, Jeon H, Jung YM, Cho IJ, Yu KJ. Ultra-low cost, facile fabrication of transparent neural electrode array for electrocorticography with photoelectric artifact-free optogenetics. Adv Funct Mater. 2022;32(10):2105568. https://doi.org/10.1002/adfm.202105568.
Zheng XL, Yang YJ, Liu YH, Deng PL, Li J, Liu WF, Rao P, Jia CM, Huang W, Du YL, Shen YJ, Tian XL. Fundamentals and photocatalytic hydrogen evolution applications of quaternary chalcogenide semiconductor: Cu2ZnSnS4. Rare Met. 2022;41(7):2153. https://doi.org/10.1007/s12598-021-01955-2.
Wan ST, Li HT, Ma ZH, Zhang HC, Zheng YZ. 2D/2D heterostructured MoS2/PtSe2 promoting charge separation in FTO thin film for efficient and stable photocatalytic hydrogen evolution. Rare Met. 2022;41(5):1735. https://doi.org/10.1007/s12598-021-01954-3.
Zhang M, Xuan XX, Wang WL, Ma CY, Lin ZQ. Anode photovoltage compensation-enabled synergistic CO2 photoelectrocatalytic reduction on a flower-like graphene-decorated Cu foam cathode. Adv Funct Mater. 2020;30(52):2005983. https://doi.org/10.1002/adfm.202005983.
Yang RQ, Ji YC, Li Q, Zhao ZH, Zhang RT, Liang LL, Liu F, Chen YK, Han SW, Yu X, Liu H. Ultrafine Si nanowires/Sn3O4 nanosheets 3D hierarchical heterostructured array as a photoanode with high-efficient photoelectrocatalytic performance. Appl Catal B. 2019;256:117798. https://doi.org/10.1016/j.apcatb.2019.117798.
Zhang G, Zhang ZH, Xia DH, Qu Y, Wang WQ. Solar driven self-sustainable photoelectrochemical bacteria inactivation in scale-up reactor utilizing larg-scale fabricable Ti/MoS2/MoOx photoanode. J Hazard Mater. 2020;392: 122292. https://doi.org/10.1016/j.jhazmat.2020.122292.
Zhu LP, Lu H, Hao D, Wang LL, Wu ZH, Wang LJ, Li P, Ye JH. Three-dimensional lupinus-like TiO2 nanorod@Sn3O4 nanosheet hierarchical heterostructured arrays as photoanode for enhanced photoelectrochemical performance. ACS Appl Mater Interfaces. 2017;9:38537. https://doi.org/10.1021/acsami.7b11872.
Balgude S, Sethi Y, Kale B, Amalnerkar D, Adhyapak P. Sn3O4 microballs as highly efficient photocatalyst for hydrogen generation and degradation of phenol under solar light irradiation. Mater Chem Phys. 2019;221:493. https://doi.org/10.1016/j.matchemphys.2018.08.032.
Li CM, Yu SY, Dong HJ, Liu CB, Wu HJ, Che HN, Chen G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl Catal B. 2018;238:284. https://doi.org/10.1016/j.apcatb.2018.07.049.
Yang RQ, Ji YC, Zhang J, Zhang RT, Liu F, Chen YK, Liang LL, Han SW, Yu X, Liu H. Efficiently degradation of polyacrylamide pollution using a full spectrum Sn3O4 nanosheet/Ni foam heterostructure photoelectrocatalyst. Catal Today. 2019;335:520. https://doi.org/10.1016/j.cattod.2019.02.019.
Yang RQ, Liang N, Chen XY, Wang LW, Song GX, Ji YC, Ren N, Lv YW, Zhang J, Yu X. Sn/Sn3O4−x heterostructure rich in oxygen vacancies with enhanced visible light photocatalytic oxidation performance. Int J Miner Metall Mater. 2021;28(1):150. https://doi.org/10.1007/s12613-020-2131-z.
Yu X, Zhao ZH, Zhang J, Guo WB, Qiu JC, Li DS, Li Z, Mou XN, Li LL, Li AX. Rutile nanorod/anatase nanowire junction array as both sensor and power supplier for high-performance, self-powered, wireless UV photodetector. Small. 2016;12:2759. https://doi.org/10.1002/smll.201503388.
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (Nos. 51732007 and 52272212), the Natural Science Foundation of Shandong Province (No. ZR2022JQ20).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, LW., Liu, L., You, Z. et al. Surface amorphization oxygen vacancy-rich porous Sn3Ox nanosheets for boosted photoelectrocatalytic bacterial inactivation. Rare Met. 42, 1508–1515 (2023). https://doi.org/10.1007/s12598-022-02208-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02208-6