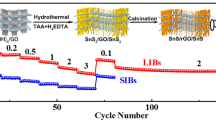
Abstract
The potential application of high-capacity Sn4P3 anode for potassium-ion batteries (PIBs) is hindered by the poor cycle stability mainly rooted from the huge volume changes upon cycling and low electronic conductivity. To address the above issues, sandwich-like structured Sn4P3/Ti3C2Tx was designed and synthesized as anode material for PIBs. As a result, Sn4P3/Ti3C2Tx presents superior cycle stability (retains a capacity of 103.2 mAh·g−1 even after 300 cycles at 1000 mA·g−1) and rate capability (delivers 60.7 mAh·g−1 at high current density of 2000 mA·g−1). The excellent electrochemical performance of sandwich-like structured Sn4P3/Ti3C2Tx is originated from the synergistic effect between Sn4P3 and Ti3C2Tx, where Ti3C2Tx acts as a conductive matrix to facilitate electron transfer and buffer the volume change of Sn4P3 particles upon cycling, while Sn4P3 serves as pillars to prevent the collapse and stacking of Ti3C2Tx sheets. Moreover, significant capacitive contribution is demonstrated as a major contributor to the excellent rate capability.
Graphical abstract

摘要
作为钾离子电池 (PIB)负极材料, Sn4P3具有较高的储钾容量。然而, 充放电过程中材料发生的巨大的体积变化和较低的电子电导率导致其循环稳定性较差, 阻碍了其实际应用。为了解决上述问题, 本文设计并制备了三明治结构的Sn4P3/Ti3C2Tx 复合材料, 并对其储钾性能进行了研究。研究结果显示, Sn4P3/Ti3C2Tx复合电极具有优越的循环稳定性能 (在 1000 mA·g−1电流密度下循环 300 次后仍保持103.2 mAh·g−1 的容量) 和倍率性能 (在 2000 mA·g−1 的高电流密度下容量可达到 60.7 mAh·g−1)。Sn4P3/Ti3C2Tx复合材料的优异的电化学性能来源于Sn4P3和Ti3C2Tx 之间的有效协同作用: Ti3C2Tx作为导电骨架, 促进了电子转移, 并缓冲了循环过程中Sn4P3颗粒的体积变化; Sn4P3作为支柱, 防止了Ti3C2Tx片的倒塌和堆积。此外, 显著的电容行为是Sn4P3/Ti3C2Tx倍率性能优异的主要原因之一。
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the spread of portable electronic devices and the boom in the new energy vehicle industry, there is an urgent need to develop energy storage devices with high performance and low cost [1, 2]. Due to the high energy density and good cycle stability, lithium-ion batteries (LIBs) have accounted for most of the consumer battery market. However, the limited resources of lithium ore and its uneven global distribution limit its use in large-scale energy storage applications [3]. Considering the high natural abundance of potassium (2.09 wt%, while 0.0017 wt% for Li) and the similar energy storage mechanism to that of LIBs, potassium-ion batteries (PIBs) have attracted much attention as a promising alternative to LIBs [4,5,6]. However, the larger K+ radius (0.138 nm) leads to slow reaction kinetics and larger volume changes of active material during cycling [7]. Up to now, the development of PIBs is still in its infancy and electrode materials with high capacity and good cycling stability are yet to be developed [8, 9].
Graphite is the most extensively used anode material with excellent electrical conductivity, and large layer spacing (3.4 nm) [10]. Unfortunately, the practical application of graphite anode material remains challenging mainly due to its low capacity. In this regards, alloy-based materials are potential alternatives due to their high capacity. Among the alloy-based materials, Sn4P3, which possesses a high theoretical capacity of 612 mAh·g−1 and synergistic benefits of Sn and P, is deemed as the most promising anode for PIBs [11,12,13]. However, the low electronic conductivity (30.7 S·cm−1) and huge volume changes during charge/discharge process lead to sluggish reaction kinetics and poor cycling stability [12, 14,15,16]. To tackle these limitations, an effective strategy is to combine Sn4P3 with highly conductive skeleton [16,17,18]. For instance, Zhang et al. [12] prepared Sn4P3/C composite via ball-milling technique and studied it as anode for PIBs, which delivers a reversible capacity of 384.8 mAh·g−1 at 50 mA·g−1. However, the Sn4P3-based composites prepared by the ball-milling method are poor in terms of cycle stability [14, 19, 20]. The capacity retention of Sn4P3/C composite is only ~ 6.6% after 120 cycles. Therefore, further efforts are still needed to achieve high capacity, long-term cycle stability as well as high-rate performance.
Ti3C2Tx, as a new member of two-dimensional layered material family, has shown unique advantages in the field of energy storage due to its layered structure, superior electrical conductivity (6500 S·cm−1), and low diffusion barrier [21,22,23,24,25]. Intensive study has been performed on the use of Ti3C2Tx as electrode materials for energy storage batteries, such as hybrid Ti3C2/NiCoP for sodium ion batteries [26], Ti3C2/Si composite for LIBs [27], and MoS2/MXene hybrids for PIBs [28]. Yet, incorporating MXene with high capacity Sn4P3 anode has rarely been tried.
Herein, we prepared a novel sandwich-like structured Sn4P3/Ti3C2Tx composite, which has rarely been reported before, via a solvothermal reaction followed by phosphorization process. Electrochemical study demonstrates that Sn4P3/Ti3C2Tx owns superior cycle stability and rate capability, which benefits from the synergistic effect between Sn4P3 and Ti3C2Tx. In Sn4P3/Ti3C2Tx composite, Ti3C2Tx acts as a conductive matrix to facilitate electron transfer and buffer the volume change of Sn4P3 particles during charge/discharge, while Sn4P3 serves as pillars to prevent the collapse and stacking of Ti3C2Tx sheets.
2 Experimental
Except for Ti3C2Tx powder was purchased from Jilin 11 Technology Co., Ltd, all other materials used in this experiment were purchased from Shanghai Macklin Biochemical Co., Ltd.
2.1 Synthesis of Sn4P3/Ti3C2Tx
In a typical preparation, SnCl2·2H2O and Ti3C2Tx powder with different mass ratios of 1:0.5, 1:1 and 1:2 were added into ethanol solution, followed by an ultrasonic treatment process for 1 h. Afterward, 20 ml NaOH solution with concentration of 0.2 mol·L−1 was added into the above mixture and stirred for 0.5 h. The solution was subsequently transferred into Teflon-lined stainless-steel autoclave and reacted at 180 °C for 10 h. The obtained SnO/Ti3C2Tx powder was then washed with deionized water and collected by freeze-drying. To get Sn4P3/Ti3C2Tx, appropriate NaH2PO2·H2O and SnO/Ti3C2Tx powder were annealed in tube furnace in Ar atmosphere with temperature of 280 °C for 0.5 h. Electrochemical results in Fig. S1 show that the Sn4P3/Ti3C2Tx sample with an initial SnCl2·2H2O to Ti3C2Tx mass ratio of 1:1 possesses the optimal performance. Therefore, it was selected for further research. As a control, bare Sn4P3 was prepared in the same process without adding Ti3C2Tx powder.
2.2 Material characterizations
The crystal structure, composition and morphology of the samples were investigated by X-ray diffraction (XRD, Rigaku, Ultima IV) with Cu Kα radiation, X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha), scanning electron microscope (SEM, Zeiss Sigma 300), and transmission electron microscope (TEM, JEOL JEM 2100F), respectively.
2.3 Electrochemical characterizations
The electrode was fabricated by coating the mixed slurry of 80 wt% of the as-prepared Sn4P3/Ti3C2Tx or bare Sn4P3, 10 wt% carbon black, and 10 wt% polyvinylidene fluoride (PVDF) binder in N-methyl pyrrolidone (NMP) onto the copper foil. The electrodes were then vacuum-dried at 85 °C for 12 h and cut into disks with loading of ~ 0.8 mg·cm−2. Electrochemical performance was measured with CR2032-type coin cells, where K foil works as counter/reference electrode and KPF6 in ethylene carbonate/diethyl carbonate works as electrolyte. Galvanostatic charge–discharge (GCD) was tested on a Land CT2001A battery testing system. Cyclic voltammograms (CV) and electrochemical impedance spectroscopy (EIS) tests were carried out at CHI660e electrochemistry workstation. CV was conducted in a voltage range of 0.01–2.00 V. EIS was tested over a frequency range from 10 mHz to 100 kHz by applying an alternating current (AC) signal of 5 mV.
3 Results and discussion
Figure 1 illustrates the synthesis process of Sn4P3/Ti3C2Tx composite. Firstly, SnO nanoparticles are loaded on the surface of Ti3C2Tx by a hydrothermal reaction. Afterward, in situ phosphorization reaction is conducted in a tube furnace and the Sn4P3/Ti3C2Tx composite material is finally obtained.
The crystal structures of the materials were investigated by XRD. As shown in Fig. 2, the defined peaks at ~ 6.46° could be assigned to (002) planes of titanium carbide, which agrees well with previous reports [29,30,31]. In the XRD pattern of final product of Sn4P3/Ti3C2Tx, the diffraction peaks locating at ~ 27.7°, 28.8°, 30.3, 31.4°, 44.5°, 45.7° and 56.5° are indexed as (104), (015), (0012), (107), (0114), (110) and (027) planes of rhombohedral Sn4P3 (JCPDS No. 73-1820) respectively [15, 32], while the extra peaks can be attributed to Ti3C2Tx, indicating the successful coupling of Sn4P3 and Ti3C2Tx.
Morphology of pristine Ti3C2Tx and the product of Sn4P3/Ti3C2Tx was investigated by SEM. Figure 3a shows the SEM image of Ti3C2Tx, in which a typical accordion-like and well-aligned layered structure is clearly observed. The layered structure still maintains even after a long time of hydrothermal reaction and high-temperature phosphating process (Fig. 3b), suggesting the excellent structural stability of Ti3C2Tx. Moreover, Sn4P3 nanoparticles are evenly distributed on the surface of Ti3C2Tx sheets, thus forming a sandwich-like structure (Fig. S2). In this structure, Sn4P3 nanoparticles can act as pillars to prevent the collapse and stacking of Ti3C2Tx sheets, while the Ti3C2Tx sheets could provide a good conductive matrix and unblocked channels for electron transmission and K+ transfer, respectively, thereby achieving synergistic effect. TEM images were collected to reveal the structural and morphological details of Ti3C2Tx and Sn4P3/Ti3C2Tx composite. As shown in Fig. 3c, obvious multilayer structure is observed for matrix. Figure 3d shows high-resolution transmission electron microscope (HRTEM) image and fast Fourier transformation (FFT) pattern of Sn4P3/Ti3C2Tx, clear fringes with lattice spacing of 0.320 and 0.204 nm are observed, corresponding to (104) and (0114) planes of Sn4P3, respectively. HRTEM images in Fig. S3 reveal the expansion of the interlayer spacing from Ti3C2Tx to Sn4P3/Ti3C2Tx, which is mainly caused by the insertion of cations between the layers during synthesis process. Elemental mappings in Fig. 3e suggest that Sn4P3 particles are homogeneously distributed on the matrix of Ti3C2Tx.
The chemical states of different elements in Sn4P3/Ti3C2Tx composite were analyzed by XPS. Figure 4a shows the full-scan spectrum of Sn4P3/Ti3C2Tx sample, demonstrating the existence of Ti, C, Sn, P, O and F. Figure 4b–f exhibits the high-resolution XPS spectra of Ti 2p, C 1s, Sn 3d, P 2p and O 1s, respectively. Ti 2p spectrum in Fig. 4b can be deconvoluted into four species of Ti2+, Ti3+, Ti–C and Ti–O, which is consistent with the previous literatures [33, 34]. Figure 4c displays four peaks at ~ 281.5, 284.7, 286.4 and 288.8 eV, which are assigned to the characteristic bonds of C–Ti, C–C, C–O and C=O, respectively. In terms of Sn 3d spectrum in Fig. 4d, the peak at ~ 487.1 eV is resulted from spin-orbital splitting photoelectrons of Sn, which is relevant to the Sn–P bond of Sn4P3 phase. While another peak situated at ~ 485.1 eV corresponds to Sn0 [35, 36]. As displayed in Fig. 4e, P 2p spectrum is divided into three peaks. Two peaks locating at ~ 129.6 and 130.3 eV are related to typical P 2p3/2 and P 2p1/3, corresponding to P–Sn bond of Sn4P3 phase. While the small peak at ~ 133.3 eV is attributed to P–O bond, indicating that the oxidized phosphate species remain on the surface of Sn4P3/Ti3C2Tx. For O 1s spectrum in Fig. 4f, the peaks at ~ 529.4, 531.3, 532.1 and 533.0 eV correspond to O–Ti, O=C, O–C and O–P, respectively. XPS results further illustrate that Sn4P3/Ti3C2Tx composite was synthesized successfully.
To evaluate potassium storage properties, the Sn4P3 and Sn4P3/Ti3C2Tx were investigated by using CR2032-type coin cells, in which potassium foils were used as counter/reference electrodes. CV was firstly carried out at a scan rate of 0.1 mV·s−1 with the potential window between 0.01 and 2.00 V. As shown in Fig. S4, a broad peak between 0.01 and 1.00 V is observed in the initial cathodic scan, which is derived from the potassiation reaction to form K-P and K-Sn phases as well as the formation of solid electrolyte interphase (SEI). In the following anodic scan, a weak peak centered at 0.8 V can be observed, corresponding to the de-potassiation process. The second and third CV curves overlap with each other, indicating the good reversibility of Sn4P3/Ti3C2Tx during charge and discharge.
Figure 5a shows the selected charge/discharge profiles at current density of 50 mA·g−1 between 0.01 and 2.00 V (vs. K/K+). In the first cycle, Sn4P3/Ti3C2Tx electrode delivers a discharge and charge capacity of 586.6 and 340.9 mAh·g−1, respectively, corresponding to an initial Coulombic efficiency of ~ 58%. The irreversible loss of the initial capacity is mainly resulted from the formation of SEI films on the surface of the electrode material [37]. In the subsequent cycles, Sn4P3/Ti3C2Tx gives stable capacity of ~ 304.8 mAh·g−1 and the Coulombic efficiency reaches up to > 98%. Compared with Sn4P3/Ti3C2Tx composite electrode, the capacity of Sn4P3 electrode is only ~ 228 mAh·g−1 under the same current density.
a Selected galvanostatic charge/discharge profiles of Sn4P3/Ti3C2Tx at current density of 50 mA·g−1; b rate capability and c cycle performance of Sn4P3 and Sn4P3/Ti3C2Tx electrodes at 500 mA·g−1; d Nyquist plots of Sn4P3 and Sn4P3/Ti3C2Tx electrodes before and after 50 cycles; e long-term cycling performance of Sn4P3/Ti3C2Tx electrode at 1 A·g−1 (working voltage of 0.01–2.00 V)
Rate capability of pure Sn4P3 and Sn4P3/Ti3C2Tx composite was measured at various current densities ranging from 0.05 to 2.00 A·g−1. As shown in Fig. 5b, Sn4P3/Ti3C2Tx composite delivers reversible capacities of 304.8, 270.7, 220.3, 167.4, 120.8 and 60.7 mAh·g −1 at current densities of 0.05, 0.10, 0.20, 0.50, 1.00 and 2.00 A·g−1, respectively, which is obviously superior to that of pure Sn4P3. In addition, most of the capacity is recovered (~ 292.5 mAh·g −1) when the current density returns to 0.05 A·g−1, demonstrating that the electrode is able to accommodate the massive current changes in application. It is worth mentioning that the rate capability of Sn4P3/Ti3C2Tx presented in our work surpasses Sn-based anodes reported previously (Fig. S5) [38,39,40], which is mainly due to that the accordion-like skeleton with good conductivity allows easy penetration of electrolyte and transfer of electrons.
Cycling performance of the bare Sn4P3 and Sn4P3/Ti3C2Tx was compared at 0.5 A·g−1 in Fig. 5c. Sn4P3/Ti3C2Tx electrode delivers a charge capacity of 173.1 mAh·g−1 for the first cycle, which is significantly higher than 64.5 mAh·g−1 obtained from Sn4P3 electrode. Moreover, the Sn4P3/Ti3C2Tx electrode retains a high capacity of 162.6 mAh·g−1 with capacity retention of ~ 94% after 50 cycles. By contrast, the capacity of bare Sn4P3 electrode decays quickly with cycling and only 48.2 mAh·g−1 is remained at 50th cycle. Nyquist plots were collected and exhibited in Fig. 5d. It can be clearly seen that the charge transfer resistance of Sn4P3/Ti3C2Tx decreases with cycling, due to the activation of electrode [14]. Whereas the Sn4P3 electrode shows larger impedance after 50 cycles, which is attributed to the pulverization and exfoliation of Sn4P3 particles (details will be presented by ex-situ SEM shown in Fig. 6). The smaller resistance well accounts for the excellent cycling stability and rate capability of Sn4P3/Ti3C2Tx composite. Long-term cycle performance of Sn4P3/Ti3C2Tx was further evaluated at a current density of 1.0 A·g−1. As displayed in Fig. 5e, Sn4P3/Ti3C2Tx electrode still delivers a specific capacity of 103.2 mAh·g−1 even after 300 cycles, which is ~ 81% of its initial reversible capacity.
To further explore the reason for the largely improved potassium storage performance of Sn4P3/Ti3C2Tx composite, morphology of the electrodes before and after cycling was studied by SEM. As shown in Fig. 6a, c, both Sn4P3 and Sn4P3/Ti3C2Tx electrodes present flat surface before cycling. While after 50 cycles, cracks are clearly observed in Sn4P3 electrode (Fig. 6b). In sharp contrast, the surface of Sn4P3/Ti3C2Tx electrode remains flat and intact, except for some fibers from glass fiber separator (Fig. 6d). These results reveal that the introduction of Ti3C2Tx could effectively alleviate the stress induced by volume change during charge/discharge, thus enabling the integrity of electrode and fast transfer of electrons, which contribute greatly to the electrochemical performance (Fig. 6e, f).
Reaction kinetics of Sn4P3/Ti3C2Tx electrode was analyzed by CV. As shown in Fig. 7a, the intensity of peaks increases with the scanning rate increasing from 0.2 to 1.0 mV·s−1. However, the CV curves always maintain a similar shape, which means that Sn4P3/Ti3C2Tx electrode owns good response ability under fast scanning rate. Diffusion and capacitive contribution are qualitatively determined by the relationship between current (i) and scan rate (v) according to the following equations:
where \(a\) and \(b\) are adjustable constants, and the value of b can be confirmed by the slope of lg\(i\)-lg\(v\) curves. When the value of \(b\) is close to 0.5 or 1.0, the reaction process is diffusion control or capacitance control, respectively. As depicted in Fig. 7b, the calculated b values of the anodic and cathodic peaks are 0.86 and 0.75 respectively, which implies that the electrochemical reaction process is controlled by both diffusion and capacitance behavior. The ratios of diffusion and capacitive contribution are obtained based on the following equation:
where \({k}_{1}v\) and \({k}_{2}{v}^{1/2}\) correspond to the contributions of the capacitive effect and diffusion-controlled process, respectively. As illustrated in Figs. 7c, d and S6, the ratio of capacitive contribution gradually increases with the increase of scanning rate. This demonstrates that the electrochemical reaction process of the Sn4P3/Ti3C2Tx electrode is mainly controlled by the capacitive behavior at high rate, which is beneficial for rate performance.
4 Conclusion
In summary, sandwich-like structured Sn4P3/Ti3C2Tx composite was synthesized via solvothermal reaction along with low-temperature phosphating process. The electrochemical properties of Sn4P3/Ti3C2Tx composite is evaluated as anode for PIBs. The introduction of the highly conductive Ti3C2Tx matrix not only provides channels for fast electron transfer, but also alleviates the volume change of Sn4P3 upon charge/discharge. Moreover, the loading of Sn4P3 nanoparticles serves as pillars to prevent Ti3C2Tx sheets from collapse or stack. Owing to the synergistic effect between the two components, Sn4P3/Ti3C2Tx exhibits significantly improved electrochemical performance than Sn4P3, which can be ranked as a high-performance anode material for PIBs.
References
Chu S, Majumdar A. Opportunities and challenges for a sustainable energy future. Nature. 2012;488(7411):294.
Dunn B, Kamath H, Tarascon JM. Electrical energy storage for the grid: a battery of choices. Science. 2011;334(6058):928.
Xia MT, Chen BJ, Gu F, Zu LH, Xu MZ, Feng YT, Wang ZJ, Zhang HJ, Zhang C, Yang JH. Ti3C2Tx MXene nanosheets as a robust and conductive tight on si anodes significantly enhance electrochemical lithium storage performance. ACS Nano. 2020;14(4):5111.
Xiong PX, Wu JX, Zhou MF, Xu YH. Bismuth-antimony alloy nanoparticle@porous carbon nanosheet composite anode for high-performance potassium-ion batteries. ACS Nano. 2020;14(1):1018.
Zhou JH, Guo SJ. Carbon-based anode materials for potassium-ion batteries: From material, mechanism to performance. SmartMat. 2021;2(2):176.
Liang JM, Zhang LJ, Xili DG, Kang J. Research progress on tin-based anode materials for sodium ion batteries. Rare Met. 2020;39(9):1005.
Zhou MF, Bai PX, Ji X, Yang JX, Wang CS, Xu YH. Electrolytes and interphases in potassium ion batteries. Adv Mater. 2021;33(7):e2003741.
Li X, Qi SH, Zhang WC, Feng YZ, Ma JM. Recent progress on FeS2 as anodes for metal-ion batteries. Rare Met. 2020;39(11):1239.
Qi SH, Deng JW, Zhang WC, Feng YZ, Ma JM. Recent advances in alloy-based anode materials for potassium ion batteries. Rare Met. 2020;39(9):970.
Ma JM, Li YT. Editorial for advanced energy storage and conversion materials and technologies. Rare Met. 2020;39(9):967.
Zhang WC, Pang WK, Sencadas V, Guo ZP. Understanding high-energy-density Sn4P3 anodes for potassium-ion batteries. Joule. 2018;2(8):1534.
Zhang WC, Mao JF, Li S, Chen ZX, Guo ZP. Phosphorus-based alloy materials for advanced potassium-ion battery anode. J Am Chem Soc. 2017;139(9):3316.
Lei KX, Wang J, Chen C, Li SY, Wang SW, Zheng SJ, Li FJ. Recent progresses on alloy-based anodes for potassium-ion batteries. Rare Met. 2020;39(9):989.
Li DP, Zhang YM, Sun Q, Zhang SN, Wang ZP, Liang Z, Si PC, Ci LJ. Hierarchically porous carbon supported Sn4P3 as a superior anode material for potassium-ion batteries. Energy Storage Mater. 2019;23:367.
Ran LB, Gentle I, Lin TE, Luo B, Mo N, Rana M, Li M, Wang LZ, Knibbe R. Sn4P3@porous carbon nanofiber as a self-supported anode for sodium-ion batteries. J Power Sources. 2020;461:228116.
Wang WH, Zhang JL, Yu DYW, Li Q. Improving the cycling stability of Sn4P3 anode for sodium-ion battery. J Power Sources. 2017;364:420.
Zhang JL, Li CL, Wang WH, Yu DYW. Facile synthesis of hollow Cu3P for sodium-ion batteries anode. Rare Met. 2021;40(12):3460.
Qian JF, Xiong Y, Cao YL, Ai XP, Yang HX. Synergistic Na-storage reactions in Sn4P3 as a high-capacity, cycle-stable anode of Na-ion batteries. Nano Lett. 2014;14(4):1865.
Liu SL, Zhang HZ, Xu LQ, Ma LB. Synthesis of hollow spherical tin phosphides (Sn4P3) and their high adsorptive and electrochemical performance. J Crystal Growth. 2016;438:31.
Zhang JL, Wang WH, Li BH. Effect of particle size on the sodium storage performance of Sn4P3. J Alloys and Compounds. 2019;771:204.
Meng RJ, Deng QY, Peng CX, Chen BJ, Liao KX, Li LJ, Yang ZY, Yang DL, Zheng L, Zhang C, Yang JH. Two-dimensional organic-inorganic heterostructures of in situ-grown layered COF on Ti3C2 MXene nanosheets for lithium-sulfur batteries. Nano Today. 2020;35:100991.
Meng RJ, Huang JM, Feng YT, Zu LH, Peng CX, Zheng LR, Zheng L, Chen ZB, Liu GL, Chen BJ, Mi YL, Yang JH. Black phosphorus quantum dot/Ti3C2 MXene nanosheet composites for efficient electrochemical lithium/sodium-ion storage. Adv Energy Mater. 2018;8(26):1801514.
Ming FW, Liang HF, Huang G, Bayhan Z, Alshareef HN. MXenes for rechargeable batteries beyond the lithium-ion. Adv Mater. 2021;33(1):2004039.
Kajiyama S, Szabova L, Sodeyama K, Iinuma H, Morita R, Gotoh K, Tateyama Y, Okubo M, Yamada A. Sodium-ion intercalation mechanism in MXene nanosheets. ACS Nano. 2016;10(3):3334.
Li K, Liang MY, Wang H, Wang XH, Huang YS, Coelho J, Pinilla S, Zhang YL, Qi FW, Nicolosi V, Xu YX. 3D MXene architectures for efficient energy storage and conversion. Adv Funct Mater. 2020;30(47):2000842.
Zhao DY, Zhao RZ, Dong SH, Miao XG, Zhang ZW, Wang CX, Yin LW. Alkali-induced 3D crinkled porous Ti3C2 MXene architectures coupled with NiCoP bimetallic phosphide nanoparticles as anodes for high-performance sodium-ion batteries. Energy Environ Sci. 2019;12(8):2422.
Hui XB, Zhao RZ, Zhang P, Li CX, Wang CX, Yin LW. Low-temperature reduction strategy synthesized Si/Ti3C2 MXene composite anodes for high-performance Li-ion batteries. Adv Energy Mater. 2019;9(33):1901065.
Li JH, Rui BL, Wei WX, Nie P, Chang LM, Le ZY, Liu MQ, Wang HR, Wang LM, Zhang XG. Nanosheets assembled layered MoS2/MXene as high performance anode materials for potassium ion batteries. J Power Sources. 2020;449:227481.
Zhao TK, Zhang JK, Du Z, Liu YH, Zhou GL, Wang JT. Dopamine-derived N-doped carbon decorated titanium carbide composite for enhanced supercapacitive performance. Electrochim Acta. 2017;254:308.
Pan ZH, Cao F, Hu X, Ji XH. A facile method for synthesizing CuS decorated Ti3C2 MXene with enhanced performance for asymmetric supercapacitors. J Mater Chem A. 2019;7(15):8984.
Guo X, Zhang WX, Zhang JQ, Zhou D, Tang X, Xu XF, Li BH, Liu H, Wang GX. Boosting sodium storage in two-dimensional phosphorene/Ti3C2Tx MXene nanoarchitectures with stable fluorinated interphase. ACS Nano. 2020;14(3):3651.
Pan E, Jin YH, Zhao CC, Jia M, Chang QQ, Jia MQ. Dopamine-derived N-doped carbon encapsulating hollow Sn4P3 microspheres as anode materials with superior sodium storage performance. J Alloys Compd. 2018;769:45.
Liu YT, Zhang P, Sun N, Anasori B, Zhu QZ, Liu H, Gogotsi Y, Xu B. Self-assembly of transition metal oxide nanostructures on MXene nanosheets for fast and stable lithium storage. Adv Mater. 2018;30(23):e1707334.
Li H, Chen R, Ali M, Lee H, Ko MJ. In situ grown MWCNTs/MXenes nanocomposites on carbon cloth for high-performance flexible supercapacitors. Adv Funct Mater. 2020;30(47):2002739.
Ran LB, Luo B, Gentle IR, Lin TE, Sun Q, Li M, Rana MM, Wang LZ, Knibbe R. Biomimetic Sn4P3 anchored on carbon nanotubes as an anode for high-performance sodium-ion batteries. ACS Nano. 2020;14(7):8826.
Liu Q, Ye JJ, Chen ZZ, Hao Q, Xu CX, Hou JG. Double conductivity-improved porous Sn/Sn4P3@carbon nanocomposite as high performance anode in lithium-ion batteries. J Colloid Interface Sci. 2019;537:588.
Fan L, Ma RF, Wang J, Yang HG, Lu BA. An ultrafast and highly stable potassium-organic battery. Adv Mater. 2018;30(51):e1805486.
Huang KS, Xing Z, Wang LC, Wu X, Zhao W, Qi XJ, Wang H, Ju ZC. Direct synthesis of 3D hierarchically porous carbon/Sn composites via in situ generated NaCl crystals as templates for potassium-ion batteries anode. J Mater Chem A. 2018;6(2):434.
Wang H, Xing Z, Hu ZK, Zhang Y, Hu Y, Sun YW, Ju ZC, Zhuang QC. Sn-based submicron-particles encapsulated in porous reduced graphene oxide network: advanced anodes for high-rate and long life potassium-ion batteries. Appl Mater Today. 2019;15:58.
Zhao XX, Wang WH, Hou Z, Wei GJ, Yu YK, Zhang J, Quan Z. SnP0.94 nanoplates/graphene oxide composite for novel potassium-ion battery anode. Chem Eng J. 2019;370:677.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 52100084) and Shenzhen Natural Science Fund (No. GXWD20201230155427003-20200824094017001).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, J., Li, CL., Chen, G. et al. Rational design of Sn4P3/Ti3C2Tx composite anode with enhanced performance for potassium-ion battery. Rare Met. 41, 2259–2267 (2022). https://doi.org/10.1007/s12598-021-01934-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-021-01934-7