Abstract
The structure stability and electronic and optical properties of a series of Au@ZnS core–shell nanocomposites with different sizes were investigated theoretically by first-principle calculation based on density functional theory (DFT). A series of Aun@(ZnS)42 structures with different n values from 6 to 16 were optimized and obtained. Based on the core–shell interaction energy and second-order difference of total energy of these structures, it is found that Au13@(ZnS)42 turns out to be the most stable structure. Based on the model of Au13@(ZnS)42, the density of state and charge density difference were studied and provided a deeper understanding of the electronic structures of Au@ZnS. On the other hand, absorption coefficient and dielectric function were investigated to study the optical properties. It is found that an optical absorption peak appears in visible-light region, indicating that the photo-catalytic can be improved prominently due to the optical redshift to visible-light region when forming core–shell structure from ZnS bulk. And the redshift phenomenon accords well with experiment. Furthermore, the electronic structure further confirms the existence of redshift of optical absorption spectrum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Core–shell nanoparticles have received tremendous attentions in materials science and engineering due to the wide application in different areas such as catalysis [1,2,3], electronics [4, 5], biomedical [6, 7] and enhancing photoluminescence [8] with the advantages of avoiding the inner core exposure to solvent and maximizing the interaction between the core and shell. By controlling chemical constitution, size and morphology, the adjustable properties of core–shell structure can be utilized to a large range of different fields. In particular, core–shell nanoclusters comprised of noble metal core and semiconductor shell as building blocks have emerged as a quiet promising system in recent years [9, 10] because of their peculiar structures and remarkable physicochemical properties compared with individual component nanomaterial.
Among all kinds of semiconductors, ZnS as one of the most popular photo-catalysts plays an important role in photonics that have been extensively utilized in emission devices, cathodoluminescence, light-emitting diodes and sensors [11,12,13]. However, the visible-light photocatalyst application has been restricted by the low visible-light absorption of ZnS. To overcome this problem, one of the most promising way is to involve noble metals with complementary properties to ZnS structure. For instance, it has been reported that the introduction of Au clusters into ZnS structure benefits the photo-catalytic activity by extending the optical absorption edge to the visible-light region [14]. In the past decade, the Au–ZnS nanocomposites with different morphologies have been discussed extensively.
For example, Chen et al. [15] have prepared core-satellite ZnS–Au nano-assemblies, in which each of the ZnS nanosphere was surrounded by a few Au nanoparticles. It exhibited high photo-catalytic efficiency and thus was applied for degradation of cationic dye (TH) under ultraviolet–visible (UV) light. Zhang et al. [16] have synthesized Au nanoparticles loaded on ZnS nanostructures. They found that Au nano particles obviously enhanced photo-catalytic hydrogen production rate of ZnS structure. Geng et al. [17] have synthesized Au–ZnS hybrid nanostructure. The formation mechanism and optical properties of the nanocomposites with different Au doping concentrations were also discussed. Sagheer and Madkour [18] found that a significant increase in the photo-catalytic activity takes place upon the loading of Au nanoparticles on ZnS. And the optoelectronic properties of the nanoparticles in terms of band structure were determined.
However, for the above Au–ZnS nanocomposites, Au nanoparticles exposed to the surrounding environment are likely to suffer from corrosion and thus reduce catalytic activity. Core–shell composite can resist corrosion by insulating the metallic core from its surroundings environment.
For example, Mrinmoy et al. [14] have proposed a simple, novel and quick preparation of Au@ZnS core–shell nanostructure. They have investigated the effect of Au core concentration on electrical properties and optical behavior. The visible photo-catalytic activity was observed to get improved with the increase in concentration of Au core in Au@ZnS nanostructure. In summary, when putting Au cluster as the inner core of ZnS outer shell, Au@ZnS core–shell nanoparticles will exhibit excellent photoelectron property and enhanced catalytic performance compared with individual component material.
Nevertheless, there are not many researches about Au@ZnS nanocomposite experimentally and theoretically, and the synthesis of Au@ZnS nanoparticles is still a challenge, although there have been a few successful preparations. Especially, as we can see, most works pay more attention to the experimental process and description of the phenomenon. The theoretical study about the physical mechanism and the explanation of experiment results is still scarce, which has become a major roadblock for the investigation of core–shell structure. In this work, the structural stability and functional properties of a series of Aun@(ZnS)42 (n = 6–16) nanocomposites were investigated by density functional theory (DFT) which include finding the most stable nanostructure and studying their electronic and optical properties.
2 Computational methods
In this study, first-principles calculation based on DFT was performed to investigate Ag@ZnS core–shell nanostructure, as implemented in Vienna ab initio simulation package (VASP). The generalized gradient approximation (GGA) methods as described by Perdew, Burke and Ernzerhof (PBE) [19] was chosen to describe the exchange correlation function. A plane wave basis was set with a cutoff energy of 480 eV, and only the gamma point sampling of the Brillouin zone was adopted for the core–shell nanostructure calculations. A cubic box with side length of 2 nm was used to contain the particle in all directions to reduce the interactions between periodic images. All atoms in model systems were fully relaxed to obtain optimized structures with the convergence criteria for energy and maximum force of 1.0 × 10−5 eV and 0.05 eV·nm−1, respectively. The structural relaxation terminated until the criteria were satisfied. The electronic and optical properties were calculated based on the optimized Ag@ZnS nanostructures.
3 Results and discussion
3.1 Structural properties of Aun@(ZnS)42 (n = 6–16) hetero-nanostructure
3.1.1 Structural properties of Aun@(ZnS)42 (n = 6–16)
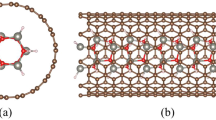
The multiplicity and indeterminacy of core–shell hetero-structure involve huge difficulties in the theoretical investigation of core–shell structure. With the lack of a proper model and the increasing number of atoms, the optimization of obtaining a stable structure in DFT calculation usually fails. Here, a series structures of Aun@(ZnS)42 with different numbers of Au atoms (n) were built. Inspired by the work on Au@ZnO [20] and TM@ZnO (TM = Fe, Co, Ni) [21], Au@ZnS structure was constructed since ZnO and ZnS share the similar wurtzite structure in bulk materials. Then, the structure was optimized by using conjugate gradient algorithm until the criteria of the convergence tolerance of energy and maximum force were reached. The obtained converged structure of Aun@(ZnS)42 for n from 6 to 16 is shown in Fig. 1. It can be seen that for these stable core–shell structure, the shapes of the outer shell remain almost unchanged with just slight differences.
To investigate the relative stability among these structures with different sizes of inner core, as shown in Fig. 2, the core–shell interaction energy (Ecs) and the second-order difference of total energy (Δ2E) are displayed, respectively. The core–shell interaction energy (Ecs) is defined as:
where N = n + 42 is the total atom number of Aun@(ZnS)42 and En, Ecore and Eshell are the total energy of Aun@(ZnS)42, Aun inner core and (ZnS)42 outer shell, respectively. The second-order differences of total energies (Δ2E) are calculated as:
where En is the total energy and n is the number of Au atoms.
It can be noted that the second-order differences of total energies and core–shell interaction energy are sensitive to the structure stability of nanoparticles. As shown in Fig. 2, the core–shell interaction energy reaches a minimum and Δ2E is maximal at Au13@(ZnS)42, indicating that Au13@(ZnS)42 is the most stable structure. It is reported that Au13 cluster is particularly stable which is well-known “magic numbers” [22]. That is also why Au13@(ZnS)42 core–shell composite with 13-Au acting as the inner core exhibits enhanced stability compared with other Aun clusters.
The calculated average bond lengths are also demonstrated in Table 1 and compared with bulk ZnS and bulk Au. The results show that the bond lengths of Zn–Zn, Zn–S and Au–Au all decrease compared with that of bulk materials, which means that the outer shell and inner core tend to contract and become denser to form a stable united structure.
3.1.2 Structural properties of Au cluster
In addition, structural properties of Au cluster in shell from the isolated one are also discussed. In Fig. 3, the optimized structures of Au13 isolated cluster and Au13 core separated from outer shell are shown, respectively. As can be clearly seen that when putting Au13 cluster in ZnS outer shell, optimized Au13 core shows some deformation compared with the isolated cluster, which is likely attributed to strong interfacial electronic interaction between Au inner core and ZnS outer shell.
3.1.3 Interface properties of Au13@(ZnS)42
For core–shell nanostructure, it is of great importance to investigate the interface between Au cluster and ZnS shell, which is closely associated with functions and properties of nanostructure. Au–S and Au–Zn nearest distance at interface for Au13@(ZnS)42 are shown in Table 2. And charge transfer between core and shell for per Au is also presented. From Table 2, it can be concluded that, except for a few cases, the smaller the Au–S distance or Au–Zn distance is, the more the charge transfer occurs between Au core and ZnS shell. For example, for Au13@(ZnS)42 nanostructure, Au2 atom has more charge transfer of 0.159 than the average value of 0.117, with corresponding smaller Au–S distance of 0.2676 nm. Another example, Au11 atom has more charge transfer of 0.190, which is corresponding to smaller Au–Zn distance of 0.2611 nm.
In addition, from core–shell nanostructure in Fig. 1, it can be seen that some S atoms move toward the surface, and part of Zn atoms toward internal, which will lead to the atom recombination on the shell. Therefore, Au@ZnS core–shell structure can be approximately regarded as one side for Zn termination and the other side for O termination at the interface of core–shell, which is similar to other work about Ag@ZnO nanostructure [25]. On the other hand, some Au, Zn and S atoms blend together at the interface, indicating that the inter-attraction between Au cluster and ZnS shell further enhances the relativistic effect (RE) and promotes bonds formation at the interface.
3.2 Electronic structure properties of Aun@(ZnS)42 (n = 6–16) hetero-nanostructure
3.2.1 Electronic structure properties of Au13@(ZnS)42 (n = 6–16) nanostructure
In order to get deep insights into the electronic structure, the spin-polarized electronic densities of states (DOS) of the stable structure Au13@(ZnS)42 and bulk-ZnS structures were all calculated for comparison. Figure 4 shows the total and partial DOS of Au13@(ZnS)42 composite and ZnS bulk structures. The total DOS (TDOS) near Fermi level is displayed as the inset. It is easy to see that there is a great difference in DOS curves between Au13@(ZnS)42 and ZnS bulk structure. From the total DOS curve of Au13@(ZnS)42, it can be found that it is mainly composed of two components. One of them locates at low-energy level region, and S-s, S-p, Zn-d and Au-d orbitals have a large contribution to the DOS. The other one is near the Fermi level, which is mainly originated from S-s, S-p and Zn-s, Au-d orbitals. For bulk ZnS structure, the DOS located at low-energy level mainly comes from the S-s, S-p and Zn-d orbitals, and the energy region near the Fermi level is mainly built up from the S-p state.
As can be seen from the inset of TDOS of wurtzite ZnS bulk structure, band gap between valence band and conduction band is about 2.3 eV, which is similar to other calculation works, such as 2.232 eV [26]. From the inset of Au13@(ZnS)42 TDOS, it can be noted that there also exists energy gap at Fermi level for the Au13@(ZnS)42 structure, indicating a semiconductor behavior for the core–shell configurations. But, the energy gap between the valence band maximum (VBM) and the conduction band minimum (CBM) of the Au13@(ZnS)42 is very small, which may be attributed to the occurrence of gap states near the Fermi level. The partial density of state (PDOS) for Au13@(ZnS)42 shows that gap states mainly result from S-p, Au-d orbitals, implying a lot of electrons interactions between Au-d and the S-p orbitals near the Fermi level. In other words, when doping Au cluster as inner core of ZnS outer shell, the core–shell interaction results in the occurrence of the hybridization between Au atom and its neighboring S atom. Besides, Au-d and S-p hybridization may partly help to explain why the nanostructure stability enhances when Au-cluster serving as the inner core in ZnS outer shell.
The above conclusion can be confirmed by the calculated charge density difference, as presented in Fig. 5a and b. It can be clearly seen that a large number of charges are lost from the Au atoms of inner core, and the interface region between inner core and out shell is occupied by a higher bonding charge. That is to say, there exists the obvious electron transfer between the Au atom and neighbor S atom. The above indicates strong electrons interaction between inner core and outer shell region, which leads to the formation of Au–S bonds.
To obtain a better understanding for electronic structure properties at interface, the 2D charge density difference of Au13@(ZnS)42 nanocluster is shown in Fig. 6 and charge transfer data of the typical atom have also been marked out. It can be further confirmed that there are a lot of electrons accumulated at interface and lots of electrons transfer from Au atoms and ZnS shell.
As we all know, because the Fermi energy level of Au is higher than that of ZnS structure, the electrons transfer from valence band of Au cluster to ZnS conduction band will occur. Therefore, these new occupied states near the Fermi energy level supply a bridge for electrons excitation. And the electron excitation may lead to a significant redshift of the absorption spectrum, that is, a new optical absorption in the visible-light region. The redshift phenomenon is also found in other core–shell nanoclusters, theoretically and experimentally [20, 27,28,29]. The optical property of Au@ZnS nanocluster is very complex. Therefore, in the next section, the optical properties of the Au13@(ZnS)42 core–shell nanostructure will be discussed in detail.
3.2.2 Electronic structure properties of Au cluster
In order to understand the difference of electronic structure properties of Au cluster in shell with the isolated one, the total density of state (TDOS) and partial density of state (PDOS) of Au13 isolated cluster and Au13 inner core are displayed in Fig. 7. As can be seen that, when putting Au13 cluster in the ZnS outer shell, the integrity of DOS of Au13 inner core becomes weak, which indicates that some electrons are lost and transfer from Au cluster to ZnS outer shell. The conclusion is consistent with the above discussion in electronic structure properties of Au13@(ZnS)42 core–shell nanostructure.
In addition, it can also be seen that the DOS curves of Au13 inner core become smoother and some peaks disappear compared with isolated cluster, which is mainly due to strong electrons interaction between Au core and ZnS outer shell. Just as DOS of Au13@ZnS42 shown in Fig. 4, when Au13 serving as inner core of ZnS outer shell, some peaks disappear compared to ZnS bulk.
3.3 Optical properties of Au13@(ZnS)42 and bulk ZnS structure
3.3.1 Absorption coefficient analysis
The optical properties of Au13@(ZnS)42 nanocluster can be systematically analyzed by means of absorption coefficient, which are presented in Fig. 8, together with ZnS bulk structure for comparison. As can be seen, for Au13@(ZnS)42 core–shell composite, there is mainly one absorption peak located in the visible-light spectrum range of about 560 nm, which is similar to the experimental detection absorption peaks occurred at 530 [17], 537 [14], and 518 nm [18]. The deviation compared with experimental result may originate from the undervalued energy gap calculated by DFT + GGA method. As shown in Fig. 8, for ZnS bulk configuration, the absorption spectrum has hardly absorption peaks in the visible-light range, suggesting that for the ZnS bulk structure, visible light is hard to be absorbed, and thus visible-light photo-catalyst gets limited. Therefrom, it can be concluded that the absorption peak of Au13@(ZnS)42 nanostructure is distinctly broadened and exhibits an obvious redshift compared to that of ZnS bulk, which suggests enhancement of visible absorption efficiency to some degree. The occurrence of redshift phenomenon may result from the strong charge transfer occurring at the interface between d-character of Au atom and p-character of surrounding S atoms. Besides, the redshift is in accord with the above discussion in DOS plots and charge density difference map.
Consequently, the visible-light absorption capability gets improved when Au cluster serving as the core of empty caged ZnS shell structure, and thus, the efficiency of photo-catalytic reaction is raised by extending the optical absorption spectra to the visible-light range.
3.3.2 Imaginary part and real part of dielectric function analysis
In addition, the frequency-dependent dielectric function was also investigated and analyzed to identify the optical properties of Au13@(ZnS)42 composite cluster. For bulk ZnS wurtzite-type structure, the imaginary part, ε2(w), where w denotes the frequency, (or real part, ε1(w)) of the dielectric function includes two parts, the one part is ε2(w)∥(or ε1(w)∥), with the polarization vector parallel to the z direction, and the other one is ε2(w)⊥(or ε1(w)⊥) which is determined by the average of the spectra for polarizations perpendicular to z direction. Figure 9a and b presents ε2(w) and ε1(w) of dielectric function of ZnS wurtzite structure, which is consistent with the other calculated work [30]. One clearly sees that the line shape of two components for ε2(w) or ε1(w) is almost the same in the whole energy range, in order to facilitate the comparison with Au@(ZnS)42 structure, and the averaged dielectric function with the polarization direction along the x, y and z axes, that is, ε2(w) = (ε2x + ε2y + ε2z)/3 or ε1(w) = (ε1x + ε1y + ε1z)/3) is also used, as shown in Fig. 9c and d.
Figure 9c and d displays the imaginary part and real part of dielectric function of Au13@(ZnS)42 and ZnS bulk material, respectively. It is known from the ε2(w) curve that the all ε2(w) peaks of Au13@(ZnS)42 weaken compared with that ZnS bulk structure in the high energy region, but there is a strong peak located in the visible-light energy region of about 2.4 eV, indicating that the Au13@(ZnS)42 nanocomposite absorbs lots of visible light. For the ε1(w) curve, the ε1(w) intensity of Au@(ZnS)42 is also weaker than that of ZnS bulk structure, and there is also an obvious peak in the lower energy region for Au13@(ZnS)42. It is worth to note that bulk ZnS structure exhibits dielectric property in low-energy range but behaves like a metal in the high energy range of about 7–18 eV. However, the ε1(w) value of the Au@ZnS core–shell nanostructure is almost all positive and dielectric in the whole energy range. Therefore, it can be concluded that the dielectric property of Au13@(ZnS)42 nanomaterial is better than that of ZnS bulk.
4 Conclusion
In summary, although the theoretical investigation of core–shell hetero-nanostructure is quite complicated in first principle calculation, a series of optimized structures for Aun@(ZnS)42 core–shell nanoparticles with different n values from 6 to 16 were built. The core–shell interaction energy and second-order difference of total energy indicate a most stable structure of Au13@(ZnS)42. Furthermore, the electronic structure properties of core–shell nanostructure were studied by means of the calculated DOS and charge density difference, and the results show that there are lots of electron transfers from Au atoms to ZnS shell. On the other hand, the optical properties of Au13@(ZnS)42 were also investigated based on the absorption coefficient and dielectric function; it is found that there exists a redshift of optical absorption to visible-light region from ZnS bulk to Au@ZnS core–shell structure, implying an enhancement of the photo-catalytic at visible-light region. And it is also found that the dielectric property of Au13@(ZnS)42 nanomaterial is better than that of ZnS bulk.
References
Gan L, Heggen M, Rudi S, Strasser P. Core–shell compositional fine structures of dealloyed PtxNi1−x nanoparticles and their impact on oxygen reduction catalysis. Nano Lett. 2012;12(10):5423.
Jackson A, Strickler A, Higgins D, Jaramillo TF. Engineering Ru@Pt core–shell catalysts for enhanced electrochemical oxygen reduction mass activity and stability. Nanomaterials. 2018;8(1):38.
Li ZY, Zeng R, Jiang LJ. Pt-based core–shell catalyst for proton exchange membrane fuel cell. Chin J Rare Met. 2017;41(8):925.
Wang JJ, Sun L, Mpoukouvalas K, Lienkamp K, Lieberwirth I, Fassbender B, Bonaccurso E, Brunklaus G, Muehlebach A, Beierlein T, Tilch R, Butt HJ, Wegner G. Construction of redispersible polypyrrole core–shell nanoparticles for application in polymer electronics. Adv Mater. 2009;21(10–11):1137.
Lee CH, Ma Y, Jang KI, Banks A, Pan T, Feng X, Kim JS, Kang D, Raj MS, McGrane BL, Morey B, Wang X, Ghaffari R, Huang Y, Rogers JA. Soft core/shell packages for stretchable electronics. Adv Funct Mater. 2015;25(24):3698.
Jovanovic AV, Flint JA, Varshney M, Morey TE, Dennis DM, Duran RS. Surface modification of silica core–shell nanocapsules: biomedical implications. Biomacromolecules. 2006;7(3):945.
Atabaev T, Lee JH, Lee JJ, Han DW, Hwang YH, Kim HK, Hong NH. Mesoporous silica with fibrous morphology: a multifunctional core–shell platform for biomedical applications. Nanotechnology. 2013;24(34):345603.
Park J, Kim S-W. CuInS2/ZnS core/shell quantum dots by cation exchange and their blue-shifted photoluminescence. J Mater Chem. 2011;21(11):3745.
Zhang N, Liu SQ, Fu XZ, Xu YJ. Synthesis of M@TiO2 (M = Au, Pd, Pt) core–shell nanocomposites with tunable photoreactivity. J Phys Chem C. 2011;115(18):9136.
Hirakawa T, Kamat PV. Charge separation and catalytic activity of Ag@TiO2 core–shell composite clusters under UV–irradiation. J Am Chem Soc. 2005;127(11):3928.
Chen ZG, Zou J, Wang DW, Yin LC, Liu G, Liu QF, Sun CH, Yao XD, Li F, Yuan XL, Sekiguchi T, Lu GQ, Cheng HM. Field emission and cathodoluminescence of ZnS hexagonal pyramids of zinc blende structured single crystals. Adv Funct Mater. 2009;19(3):484.
Kim S, Kim T, Kang M, Kwak SK, Yoo TW, Park LS, Yang I, Hwang S, Lee JE, Kim SK, Kim SW. Highly luminescent InP/GaP/ZnS nanocrystals and their application to white light-emitting diodes. J. Am Chem Soc. 2012;134(8):3804.
Wang XF, Xie Z, Huang HT, Liu Z, Chen D, Shen GZ. Gas sensors, thermistor and photodetector based on ZnS nanowires. J Mater Chem. 2012;22(14):6845.
Misra M, Gupta RK, Paul AK, Singla M. Influence of gold core concentration on visible photocatalytic activity of gold-zinc sulfide core–shell nanoparticle. J Power Sources. 2015;294(30):580.
Chen WT, Hsu YJ. L-cysteine-assisted growth of core-satellite ZnS–Au nanoassemblies with high photocatalytic efficiency. Langmuir. 2010;26(8):5918.
Zhang J, Wang Y, Zhang J, Lin Z, Huang F, Yu J. Enhanced photocatalytic hydrogen production activities of Au-loaded ZnS flowers. ACS Appl Mater Interfaces. 2013;5(3):1031.
Geng J. One-pot fast synthesis of spherical ZnS/Au nanocomposites and their optical properties. J Mater Sci. 2013;48(2):636.
Sagheer FA, Madkour M. Au/ZnS and Ag/ZnS nanoheterostructures as regenerated nanophotocatalysts for photocatalytic degradation of organic dyes. Opt Mater Express. 2017;7(1):158.
Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys Rev Lett. 1996;77(18):3865.
Hu YW, Huo JR, Wang XX, Wang RM. First-principles investigation on Aun@(ZnO)42 (n = 6–16) core–shell nanoparticles: structure stability and catalytic activity. J Phys Condens Matter. 2017;29(43):435701.
Hu YW, Ji CT, Wang XX, Huo JR, Liu Q, Song YP. The structural, magnetic and optical properties of TMn@(ZnO)42(TM = Fe, Co and Ni) heteronanostructure. Sci Rep. 2017;7(1):16485.
Shichibu Y, Suzuki K, Konishi K. Facile synthesis and optical properties of magic-number Au13 clusters. Nanoscale. 2012;4(14):4125.
Azpiroz JM, Infante I, Lopez X, Ugalde JM, De Angelis F. A first-principles study of II–VI (II = Zn; VI = O, S, Se, Te) semiconductor nanostructures. J Mater Chem. 2012;22(40):21453.
Assadollahzadeh B, Schwerdtfeger P. A systematic search for minimum structures of small gold clusters Aun (n = 2–20) and their electronic properties. J Chem Phys. 2009;131(6):064306.
Cheng HX, Wang XX, Hu YW, Song HQ, Huo JR, Li L, Qian P. Ag@ZnO Core–Shell nanoparticles study by first principle: the structural, magnetic and optical properties. J Solid State Chem. 2016;244:181.
Karazhanov S, Ravindran P, Kjekshus A, Fjellvåg H, Grossner U, Svensson BG. Coulomb correlation effects in zinc monochalcogenides. J Appl Phys. 2006;100(4):043709.
Cheng HX, Wang XX, Hu YW, Huo JR, Li L, Qian P, Wang RM. Theoretical investigation of geometries, stabilities, electronic and optical properties for advanced Agn@(ZnO)42 (n = 6–18) hetero-nanostructure. AIP Adv. 2016;6(7):075023.
Aguirre ME, Rodriguez HB, Roman ES, Feldhoff A, Grela MA. Ag@ZnO core–shell nanoparticles formed by the timely reduction of Ag+ ions and zinc acetate hydrolysis in N,N-dimethylformamide: mechanism of growth and photocatalytic properties. J Phys Chem C. 2011;115(50):24967.
Liu HR, Shao GX, Zhao JF, Zhang ZX, Zhang Y, Liang J, Liu XG, Jia HS, Xu BS. Worm-like Ag/ZnO core–shell heterostructural composites: fabrication, characterization, and photocatalysis. J Phys Chem C. 2012;116(30):16182.
Karazhanov S, Ravindran P, Kjekshus A, Fjellvåg H, Svensson BG. Electronic structure and optical properties of ZnX (X = O, S, Se, Te): a density functional study. Phys Rev B. 2007;75(15):155104.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (Nos. 2016YFB0700500 and 2018YFB0704300).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, HY., Liu, Q., Wang, XX. et al. First-principles calculation of Aun@(ZnS)42 (n = 6–16) hetero-nanostructure system. Rare Met. 39, 1165–1173 (2020). https://doi.org/10.1007/s12598-019-01298-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-019-01298-z