Abstract
This study aimed to find an efficient way to recover Sb from the SbCl5 acid solution obtained by the chloride leaching process of the tin-depleted residue. Sb was recovered in the form of hydrated antimony pentoxide through the hydrolysis process. The effects of hydrolysis ratio and aging time on the Sb recovery process were studied, and the corresponding trends were established. The experimental results show that the amount of the antimony free ions increases with the hydrolysis ratio and aging time. This decreases the concentration quotient of the hydrolysis reaction and thus facilitates the reaction. Product crystallinity is affected by the solution supersaturation, which varies at different stages with aging time. As a result, the optimal conditions of recovering Sb correspond to the hydrolysis ratio of 1.5 and aging time of 7 days with the recovery rate of 97 %.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Antimony compounds, primarily antimony trioxide and pentoxide, are often used as flame retardants for the printed circuit boards. Anode slime is a by-product of the copper electrolytic recovery process from the waste printed circuit boards (WPCB) [1, 2]. Sb is traditionally leached from the anode slime to form an antimonial solution in hydrometallurgy treatment. Two solvent systems are utilized in antimony hydrometallurgy: alkaline sulfide and acidic chloride [3].
In the alkaline leaching process, the lixiviant is a mixture of sodium sulfide and sodium hydroxide. The electrodeposition of the antimony from the alkaline sulfide solution to the cathode metal was carried out via electrowinning in either diaphragm or non-diaphragm cells. The cathodic metal antimony product may attain over 99.5 % purity after washing [4].
In the acid leaching process, antimony is leached from the anode using hydrochloric acid, often in conjunction with ferric chloride. The acid solution can be treated by hydrolysis or electrolysis, or other methods to get antimony compound precipitation [5–9].
These studies concentrated mostly on the trivalent antimony, while the pentavalent antimony studies are rare. In this study, the tin-depleted residue was obtained after Au, Ag, Pb and Sn were recovered from the anode slime. Then, the chloride leaching process of the tin-depleted residue was carried out to obtain the SbCl5 acid solution. The influence of the hydrolysis and aging processes on the recovery of antimony from the SbCl5 acid solution was discussed, providing a valuable reference for the antimony recovery research community.
2 Experimental
2.1 Raw materials
Chemical composition analysis of the tin-depleted residue by the inductively coupled plasma (ICP) shows a relatively high amount of Sb (over 24 wt%), which deserves to be recovered. Chemical composition results are presented in Table 1. Antimony was first leached from the tin-depleted residue to obtain the SbCl5 acid solution. The concentration of antimony in the solution was 2.456 g·L−1, analyzed by ICP.
2.2 Experimental procedure
Deionized water was added to the 100 ml SbCl5 acid solution with agitation and kept for 0.5 h. The hydrolysis solution was filtered. The precipitate was treated with ammonia to produce a pure antimony oxide. After filtering and drying, the hydrolysate was weighed. The volume ratio of deionized water and the acid solution (hereinafter referred to as the hydrolysis ratio) was adjusted to 0.5, 1.0, 1.5 and 2.0, respectively.
The hydrolysis solution obtained by the above method with different hydrolysis ratios was aged for 0, 3, 5 and 7 days, respectively. Then, the mixed solution was filtered. After purifying, the obtained hydrolysate was dried and weighed as well.
2.3 Analysis methods
The content of Sb in the hydrolysate was analyzed by means of oxidation–reduction titration using cerium sulfate. Chemical composition of the hydrolysate was analyzed by means of X-ray fluorescence (XRF). X-ray diffraction (XRD) patterns were obtained with an X-ray diffractometer (Rigaku DMAX-RB) using Cu Kα radiation.
3 Results and discussion
3.1 Hydrolysis and aging time effects on antimony recovery rate
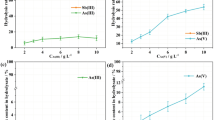
Figure 1 shows the recovery rate with different hydrolysis ratios after agitating for 0.5 h. It is found that the recovery rate tremendously increases from 41 % to 73 % with the hydrolysis ratio increasing from 0.5 to 1.5. When the hydrolysis ratio exceeds 1.5, the recovery rate becomes stable. Figure 2 shows the relationship between the recovery rate and the aging time at different hydrolysis ratios. The recovery rate increases with the aging time at different hydrolysis ratios. There is no significant difference between the recovery rates at hydrolysis ratios of 1.5 and 2.0. In this research, the recovery rate reaches 97 % with the optimal conditions (hydrolysis ratio of 1.5, aged for 7 days). Chemical composition of the hydrolysate is listed in Table 2. It is observed that the purity of product hydrated antimony pentoxide reaches 97.6 %. The product needs to be further purified.
The recovery rate is closely related to the pentavalent antimony hydrolytic process. Reaction (1) illustrates the hydrolysis reaction. In the Sb5+–H+–Cl− system, relationships among the three kinds of ions determine the hydrolysis reaction process. When the concentration quotient (J, defined as \( J = \frac{{[{\text{H}}^{ + } ]^{10} }}{{[{\text{Sb}}^{5 + } ]^{2} }} \)) is less than the equilibrium constant (K), the hydrolysis reaction runs. The reaction rate is affected by the driving force, which is determined by the difference between K and J values. Pentavalent antimony ions in the acid solution exist in the two forms: the free antimony ions, directly involved in the hydrolysis (Reaction (1)), and the Sb5+ complexes with Cl−, which cannot be directly involved in the reaction and become the majority (Reaction (2)) [10]. The free antimony is released as the reaction progresses to ensure that the hydrolysis reaction proceeds continuously and slowly.
Hydrolysis ratio represents the degree of dilution. With higher degree of dilution, the amount of free antimony increases and the hydrogen ion concentration [H+] decreases, leading to the decrease in the J value. The increasing difference enhances the driving force, thereby facilitating the reaction. Based on the above analysis, the recovery rate continues to increase with the hydrolysis ratio of <1.5. When the hydrolysis ratio exceeds 1.5, [H+] does not change much. There is no additional driving force for the hydrolysis, and the recovery rate tends to stabilize.
The aging process shares a similar mechanism. In the previous stage, free antinomy, which is directly involved in the hydrolysis, rarely exists. As the reaction progresses, free antimony is released because of the decrease of [Sb5+], and the reaction continues. Therefore, the recovery rate increases with the aging time.
3.2 Hydrolysis and aging time effects on crystallinity
Figure 3 shows the XRD patterns of hydrolysate with different hydrolysis ratios after agitating for 0.5 h. It is found that antimony is recycled in the form of hydrated antimony pentoxide, consistent with the former study [11]. The hydrolysate has an amorphous structure at the hydrolysis ratio of 0.5. It starts to crystallize and settles quickly in the solution when the hydrolysis ratio exceeds 0.5. The increase in hydrolysis ratio causes an increase in XRD intensity, which means that the crystallinity and the grain size of the hydrolysate increase.
The changes of crystallinity during the aging process at different hydrolysis ratios are quite similar. Therefore, the hydrolysis ratio of 1.5 is taken as an example. Figure 4 shows the XRD patterns of the hydrolysate at different aging time with the hydrolysis ratio of 1.5. It is found that the product crystallinity initially increases and then decreases. The best results are achieved when aged for 5 days.
The volume of the solution is the factor determining the crystallinity at different hydrolysis ratios after agitating for 0.5 h. For the hydrolysis ratio of 0.5, the small volume of solution leads to the supersaturation passing the critical limiting supersaturation (Fig. 5). According to the classical LaMer mechanism [12, 13], this condition can cause a large number of homogeneous nucleation, the nucleation rate is greater than the growth rate, and thus, an amorphous structure is obtained. When the hydrolysis ratio exceeds 0.5, the supersaturation is located in the unstable supersaturated zone and the crystal precipitate is obtained. The crystallinity and the grain size of the hydrolysate increase with the rate of hydrolysis reaction, according to the previous analysis.
Concentration quotient is the determining factor for the crystallinity in the aging process. Figure 5 shows the changes of supersaturation and grain morphology at different steps [14]. In Step I, J is much smaller than K, such that the supersaturation is located in the unstable supersaturated zone. Nucleation and subsequent growth occur as the reaction time prolongs. In Step II, according to above analysis, the reaction is restrained. The supersaturation decreases under the threshold concentration for nucleation and is located in the stable supersaturated zone. Aggregative growth and the Ostwald ripening [15] occur at this step to facilitate the crystalline structure integrity. Thus, the crystallinity increases. In Step III, the grain size cannot continue to increase because of the crystal defects, etc. The monomer cannot be consumed so that the supersaturation recovers to the unstable supersaturation zone and the nucleation and growth processes repeat. Therefore, the product crystallinity decreases to some extent.
4 Conclusion
Hydrolysis ratio and aging time affect the ion concentration in the Sb5+–H+–Cl− system, leading to different degrees of the hydrolysis reaction. The recovery rate reaches 97 % under the optimal conditions of hydrolysis ratio of 1.5 and aging time of 7 days. Supersaturation determines the product crystallinity. At the hydrolysis ratio of 0.5, the monomer concentration passes the critical limiting supersaturation and the product has an amorphous structure. When the hydrolysis ratio exceeds 0.5, supersaturation is located in the stable supersaturated zone and crystalline product is obtained. The increase in hydrolysis ratio causes higher degree of the hydrolysate crystallinity. During the aging process, the hydrolysate experiences nucleation and growth, Oswald ripening and aggregative growth, nucleation and growth stages. The product crystallinity first increases and then decreases.
References
Li B, Pan DA, Jiang YH. Recovery of copper and tin from stripping tin solution by electrodeposition. Rare Met. 2014;33(3):353.
Abdollahy M, Shafaei SZ. Optimized leaching conditions for selenium from SarCheshmeh copper anode slimes. Iran J Chem Chem Eng. 2004;23(2):101.
Anderson CG. Hydrometallurgically treating antimony-bearing industrial wastes. JOM. 2001;53(1):18.
Nordwick SM, Anderson CG. Advances in antimony electrowinning at the Sunshine mine. In: Proceedings of the Fourth International Symposium on Hydrometallurgy Fundamentals, Technology and Innovations, Salt Lake; 1993. 1107.
Xing WD, Fan XX, Dong HG. Kinetics of nickel and cobalt leaching from waste superalloys with sulfuric acid. Chin J Rare Met. 2014;38(4):674.
Macdonald MD, Stevens DA, Thibault JD. Process for producing antimony trioxide.US Patent; 5783166 A. 1998.
Motang T. New techniques for treating the Dachang jamesonite concentrate. J Cent South Inst Min Metall. 1981;198(4):18.
Nie ZR, Ma LW, Xi XL. “Complexation–precipitation” metal separation method system and its application in secondary resources. Rare Met. 2014;33(4):369.
Cao H, Chen J, Yuan H. Preparation of pure SbCl3 from lead anode slime bearing high antimony and low silver. Trans Nonferr Met Soc China. 2010;20(12):2397.
Neumann HM. Antimony (V) species in hydrochloric acid solution. J Am Chem Soc. 1954;76(10):2611.
Zheng G, Zhi B, Chen J. Hydrolysis of antimony pentachloride. Chin J Nonferr Met. 2006;16(9):1628.
Bullen CR, Mulvaney P. Nucleation and growth kinetics of CdSe nanocrystals in octadecene. Nano Lett. 2004;4(12):2303.
Seo H, Kim S. Development of NIR emitted CdTe quantum dots by concentration control method. Bull Korean Chem Soc. 2007;28(10):1637.
Wang F, Richards VN, Shields SP. Kinetics and mechanisms of aggregative nanocrystal growth. Chem Mater. 2013;26(1):5.
Richards VN, Shields SP, Buhro WE. Nucleation control in the aggregative growth of bismuth nanocrystals. Chem Mater. 2010;23(2):137.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (Nos. U1360202 and 51472030), the National Key Project of the Scientific and Technical Support Program of China (Nos. 2011BAE13B07, 2012BAC02B01 and 2011BAC10B02), the National Hi-Tech R&D Program of China (No. 2012AA063202), the Fundamental Research Funds for the Central Universities (No. FRF-TP-14-043A1), the China Postdoctoral Science Foundation Funded Project (No. 2014M560885), the Beijing Nova Program (No. Z141103001814006) and Sichuan Province Cyclic Economy Research Center.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meng, L., Zhang, SG., Pan, DA. et al. Antimony recovery from SbCl5 acid solution by hydrolysis and aging. Rare Met. 34, 436–439 (2015). https://doi.org/10.1007/s12598-015-0480-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-015-0480-y