Abstract
Uniform lanthanide orthophosphate (LnPO4, Ln = La and Ce) nanorods were successfully synthesized by a simple ultrasonic irradiation method using lanthanide nitrate salt (Ln(NO3)3·6H2O, Ln = La and Ce) and sodium phosphate (Na3PO4) in aqueous solutions with the pH of 1–3. The products were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), high-resolution transmission electron microscopy (HRTEM), Fourier transform infrared spectrometer (FT-IR), and UV–visible (UV-Vis) spectroscopy. In this research, the products are nanorods of monoclinic LaPO4 and hexagonal CePO4 structures and the vibration modes of PO4 3−, including the strong peaks at 227 nm for LaPO4, and at 225 and 278 nm for CePO4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
According to the LnPO4, Ln = La,…, and Gd of the periodic table of the elements, lanthanide phosphate compounds, the important family members of the rare-earth compounds, have increasing attention and application as luminescent materials, moisture sensors, heat-resistant materials, hosts for radioactive nuclear wastes, photocatalytic materials, and energy fuels. They have their particular 4f–5d and 4f–4f electronic transitions which are different from other elements [1–5]. Among them, LnPO4 (Ln = La and Ce) are materials of this group which can be applied for luminescent lamps as highly efficient emitters of green light. Even below the temperature of 1,200 K, monoclinic CePO4 remains as a stable compound which makes it as useful high-temperature material and as a number of applications. There are two polymorphs, which are designated as monoclinic and hexagonal CePO4 structures. The monoclinic CePO4 can be applied as heat-resistant and ceramic materials, while the hexagonal CePO4 can be functioned for tribology applications [2, 6–8]. LaPO4 and its solid solutions are able to be employed in luminescent lamps as highly-efficient emitters of green light. Monoclinic monazite LaPO4 is one of the most stable materials even at high temperature. It is applied in ceramic composites (CMCS) and in optoelectronic domains [7, 9].
There are many synthetic methods to produce 1D LaPO4 and CePO4 nanostructures such as microemulsion [2, 10], solid-state reaction [4], hydrothermal [3, 8, 9], and slow-cooling [11]. These methods require the use of high temperatures, special conditions, tedious procedures, catalytic materials, and templates. Thus, the development of practical methods for producing a large numbers of 1D nanostructure at low cost is still a great challenge for future study. Chemical methods seem to provide an alternative and intriguing strategy for producing 1D nanomaterials with respect to material diversity, cost, versatility, synthetic tenability, and potential for large scale production. In the past few decades, nanomaterials with controlled shapes and sizes were synthesized and applied in many areas such as catalysis, optics, electronics, ceramics, and magnetics. Different shapes usually display different surfaces which cause different active planes to be exposure. Their sizes, surfaces, and particle interactions have the influence on some unique properties and performances of nanomaterials. Thus, it is important to look for the controllable synthesis of nanomaterials [12]. However, a sonochemical method was regarded as an effective route for the production of high quality anisotropic nanomaterials. The sonochemical process was proved to be an available technique to obtain novel materials and to produce nanomaterials with unique morphology and unusual properties. During sonication, propagation of pressure wave is intense enough to make the formation, growth, and implosive collapse of bubbles in a liquid medium. During acoustic cavitation, these bubbles generate localized hot spots, which have an extremely high temperature (>5,000 K), pressure (>20 MPa), and cooling rate (1 × 1010 K·s−1). Thus, the sonication process provides an ideal route for the preparation of nanomaterials. The advantages of this method include a rapid reaction rate, controllable reaction condition, and the ability to form materials with uniform shape, narrow size distribution, and high purity [13–15]. There are many reports on the synthesis of nanomaterials by sonochemical method. Zhang et al. [16] synthesized polycrystalline CeO2 nanoparticles with size of 4 nm which were coated on CNTs by a simple ultrasonication method under ambient air. Pan et al [17] investigated the CO oxidation properties of CeO2 nanorods, nanowires, nanotubes, and nanocubes. They found that CeO2 nanotubes had the best performance CO oxidation due to the large BET surface area and the novel inner surface. Zhang et al. [18] studied various reaction parameters, such as the content of polyethylene glycol (PEG), molecular weight of PEG, and concentration of KOH, pH value, and sonication time on the as-synthesized polycrystalline CeO2 nanorods by ultrasonication using PEG as a structure-directing agent at room temperature. The content of PEG, molecular weight of PEG, and sonication time was proved to be the crucial factors determining the formation of one-dimensional CeO2 nanorods.
This paper presents the preparation of lanthanide orthophosphate (LnPO4, Ln = La and Ce) nanorods via ultrasonic irradiation without any surfactant or template. Phases, morphologies, and optical properties of these nanomaterials were investigated and discussed.
2 Experimental
In the typical preparation of 1D LnPO4 (Ln = La and Ce) nanorods, 0.005 mol Ln(NO3)3·6H2O (Ln = La and Ce) and Na3PO4 were each dissolved in 50 ml distilled water under continuous stirring. Then, the two solutions were mixed, and the pH was adjusted to be 1, 2, and 3 by a concentrated HNO3 solution. Subsequently, the mixtures were processed under ultrasonic irradiation (35 kHz) for 3–6 h. At the conclusion of the process, white green precipitates were synthesized, collected, washed for several times with distilled water and absolute alcohol, and dried at 70 °C for 12 h.
The X-ray powder diffraction (XRD) patterns of all samples were performed on a Philips X’Pert MPD X-ray diffractometer with Cu Kα radiation under a voltage and current of 45 kV and 35 mA, respectively. Fourier transform infrared (FTIR) spectra were recorded on a Perkin Elmer RX spectrophotometer with KBr as a diluting agent and operating in the range of 400–4,000 cm−1 with a resolution of 4 cm−1. The specific surface area of product was determined by nitrogen adsorption Brunauer–Emett–Teller (BET) method. The BET measurements were performed on a Quantachrome Autosorb-1 MP instrument. Field-emission scanning electron microscopic (FE-SEM) and transmission electron microscopic (TEM) images were taken using a JEOL JSM-6445F and JEM-2010 operated at a beam energy of 15.0 and 200 kV using LaB6 as an electron gun. UV–visible (UV–Vis) spectroscopy was characterized by a Lambda 25 Perkin Elmer spectrometer at room temperature.
3 Results and discussion
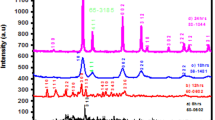
Figure 1 shows the XRD patterns of the as-prepared LnPO4 (Ln = La and Ce) products at pH 1 under ultrasonic irradiation for 5 h. The diffraction peaks can be readily indexed to be monoclinic phase of LaPO4 and hexagonal CePO4 in consistent with the JCPDS No.32-0493 for LaPO4 and 75-1880 for CePO4 [19]. Peaks of other phases such as La2O3 and CeO2 are not detected in these XRD patterns, indicating that the products are of high purity and crystallinity. Their lattice parameters can be calculated from plane spacing equations in Eq. (1) for the monoclinic structure and Eq. (2) for hexagonal structure as shown below [20]:
where a, b, and c are the lattice unit cells, h, k, and l are the lattice indexes, d is the plane spacing, and β is the angle between the a and c axes (103.6°). Their lattice parameters are a = 0.67470 nm, b = 0.70026 nm, and c = 0.64344 nm for the monoclinic LaPO4 structure and a = b = 0.69604 nm and c = 0.64511 nm for the hexagonal CePO4 structure, in good agreement with the corresponding standards.
The LaPO4 and CePO4 vibrations belonged to tetrahedral PO4 3−, by group theory, are A1(R) + E(R) + 2F2(IR + R), where ν1(A1) (symmetric stretching vibration frequency) and ν2(E) (symmetric bending mode) are Raman active; ν3(F2) (asymmetric stretching frequency) and ν4(F2) (asymmetric bending frequency) have Raman (R) and IR double activity [11]. The symmetry of PO4 3− in the CePO4 crystals decreases from T d to C 1, and the non-IR active modes become IR active. The broad absorption band at 3,460 cm−1 in Fig. 2a can be assigned to the vibration of O–H of the water molecules adsorbed on surface of the samples. The other bands are assigned to the vibration of phosphate (PO4 3−) groups in wavenumber of 400–1,400 cm−1 as shown in Fig. 2b. They can be classified into two groups with bands at 400–700 cm−1 and 850–1,100 cm−1. The bands at 1,064 cm−1 are ascribed to the asymmetric stretching vibration of the PO4 3− groups, and the bands centered at 609 and 536 cm−1 belong to the O=P–O and O–P–O bending vibrations of PO4 3− known as the ν4 mode. The four medium-intensity peaks from 400 to 700 cm−1 are due to the bending modes of P–O links in PO4 3− distorted tetrahedrons. Their stretching modes appear as clusters of very strong peaks between 850 and 1,110 cm−1 at 955, 993, 1,058, and 1,091 cm−1, due to stretching vibrations of PO4 3− in the ν3 region [9, 11, 21, 22].
Figure 3 shows the difference of length and diameter from the morphological evolution of LnPO4 (Ln = La and Ce) samples prepared at different pH values. Figure 3a–c shows SEM images of the LaPO4 products obtained at pH 3–1. In this research, LaPO4 at pH 3 is a mixture of nanoparticles with sizes below 50 nm and nanorods with 20 nm in diameter and 100–300 nm in length. By SEM observations, the yield of nanoparticles and nanorods is close to 30 % and 70 %. With the pH of the solution decreasing to 2 and 1, the percent of LaPO4 nanoparticles deceases to be less than 10 % at pH 2 and is not detected at pH 1. Only uniform LaPO4 nanorods with 20–25 nm in diameter and 300–600 nm in length are detected at pH 1, and their surfaces are smooth. Figure 3d–f reveals SEM images of CePO4 samples produced by the ultrasonic irradiation method at pH 3–1. The dependence of the morphological evolution for CePO4 samples on pH is the same as for the LaPO4 nanorods. The morphologies of CePO4 at pH 3 are a mixture of 85 % uniform aggregates of parallel aligned nanorods with a diameter of 30–35 nm and length of 500 nm–1 μm and 15 % of agglomerated nanoparticle islands with the size of 200–300 nm. But for CePO4 at pH 2 and 1, agglomerated nanoparticle islands are not detected, showing only CePO4 nanorods. It should be noted that CePO4 at pH 1 is longer than that at pH 2. The length of CePO4 nanorods is 800 nm–1 μm at pH 2 and 1–2 μm at pH 1. In addition, the surface area of LaPO4 and CePO4 nanorods is 63.32 and 35.02 m2·g−1.
From the SEM results, the morphologies of LnPO4 (Ln = La and Ce) nanorods are influenced by the pH of the solution precursor due to the effect of the chemical potential in the same way as previous reports [9, 23, 24]. For example, Zhang and Guan [9] investigated the effects of pH on the morphologies of LaPO4 nanocrystals and found that the 1D LaPO4 nanowires could only be prepared in a pH range of 0.5–1.5. The chemical potential of LaPO4 crystals is higher, which supports the growth of elongated LaPO4 nanocrystals and increases in the rate of hydrolysis and higher acidic conditions. In this research, the chemical potential of the anisotropic 1D LnPO4 (Ln = La and Ce) nanorods is thermodynamically dependent on the acidity of the reaction system. Faster ionic migration usually promotes a reversible pathway between the liquid and solid phases, which promotes ions to occupy at the right positions of the crystal lattices. In conclusion, a high chemical potential of LnPO4 (Ln = La and Ce) crystals in the solutions is another major driving force for the growth of the elongated anisotropic LnPO4 (Ln = La and Ce) nanorods [23].
In addition, the effect of reaction time on the formation of LnPO4 (Ln = La and Ce) nanorods by sonochemical method was investigated by SEM. Figure 4 shows SEM image of CePO4 synthesized by sonochemical method at pH 1 for 3–6 h. The morphologies of CePO4 depend on the reaction time. When reaction time is less than 3 h, the CePO4 nanoparticles with particles size less than 100 nm form. It should be noted that morphologies of CePO4 develop from nanoparticles to nanorods when the reaction time increases to 4 h. CePO4 nanorods have 100–200 nm in length and 50 nm in diameter. The CePO4 nanorods with the length of 1–2 μm were synthesized by sonochemical method at pH 1 for 6 h which is not significantly different for CePO4 nanorods with the reaction time of 5 h. Therefore, the reaction time on the formation of LnPO4 (Ln = La and Ce) nanorods by sonochemical method at pH 1 for 5 h is a suitable condition in synthesis of LnPO4 (Ln = La and Ce) nanorods.
Morphological information of the products was further characterized by transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) as shown in Fig. 5. Figure 5a shows a typical TEM image of the LaPO4 product. It clearly reveals that the product is composed of only nanorods with diameters of 20–60 nm and lengths of 1 μm, respectively. The HRTEM image in Fig. 5b shows that the nanorod is structurally uniform with an interplanar spacing of the (001) planes of LaPO4 and the LaPO4 nanorods grow along the [001] direction [23, 25], with the (−110) plane parallel to the growth direction. Similarly under the same synthetic conditions, CePO4 nanorods with diameters of 20–60 nm and lengths of up to 3 μm can be observed in Fig. 5c. The HRTEM image (Fig. 5d) shows the clearly resolved planes of (−110) which are parallel to the nanorod growth axis [23, 25].
A possible formation mechanism and schematic illustration as shown in Fig. 6 of 1D LnPO4 (Ln = La and Ce) phosphor nanomaterials are explained in Eqs. (3) and (4):
The H+ reacted with PO4 3− to form H n PO (3−n)−4 . After that the H n PO (3−n)−4 slowly released PO4 3− which reacted with Ln3+ (Ln = La and Ce) to form LnPO4 (Ln = La and Ce) nanorods. The H+ is the key ions in controlling the formation of 1D LnPO4 (Ln = La and Ce) nanorods. By adjusting the pH of the precursors to be 1, the concentration of H+ in the reaction system increases, and the chemical potential also increases. The preferential adsorption of H+ onto certain crystal facets is likely to raise the electrostatic potential on the crystal surfaces of 1D LnPO4 (Ln = La and Ce) nanomaterials. In order to reduce the surface energy, the atoms of the crystal surfaces rearrange themselves [26]. Finally, the 1D LnPO4 (Ln = La and Ce) nanorods are produced.
The UV–Vis spectra of LnPO4 (Ln = La and Ce) nanorods synthesized by the ultrasonic irradiation method are shown in Fig. 7. The absorption spectra of LaPO4 show a strong peak at 227 nm. La3+ does not have an electron transfer because it has zero electrons in the 4f shell, and thus there is only one possible optical state of PO4 3− clusters [27]. UV–Vis absorption spectra of CePO4 show two small shoulders at 225 and 278 nm due to the f–d electron transition of Ce3+ atoms in the lattice. The energy level for Ce3+ shows three electron transitions as 2F5/2 → 2F7/2, 2F5/2 → 2D3/2, and 2F5/2 → 2D5/2. The lowest energy transition (2F5/2 → 2F7/2) is a Laporte forbidden µ to µ transition corresponding to the fn–fn transition. The other two electron transitions, 2F5/2 → 2D3/2 and 2F5/2 → 2D5/2, are Laporte allowed nf to (n–1)f–d transitions. The two major absorption peaks of CePO4 nanorods at 225 and 278 nm are assigned to be 2F5/2 → 2D3/2 and 2F5/2 → 2D5/2 [28, 29]. Comparing with other reports, UV–Vis absorption spectra of the crystalline CePO4 nanofilaments show three peaks with maxima at 213, 256, and 273 nm due to f–d electron transitions in CePO4 [30]. However, the irregular CePO4 nanoparticles show a broad peak at 230–400 nm [31]. Therefore, the optical properties of lanthanide orthophosphate are controlled by morphology of the products.
4 Conclusion
In summary, single-crystal LnPO4 (Ln = La and Ce) nanorods were successfully prepared by a simple sonochemical method. It is found that the high aspect ratio of the products is able to be easily controlled by pH values of the solution precursors. This method is a candidate for green chemical synthesis of 1D nanomaterials.
References
Qian L, Du W, Gong Q, Qian X. Controlled synthesis of light rare earth phosphate nanowires via a simple solution route. Mater Chem Phys. 2009;114(1):479.
Yin YB, Shao X, Zhao LM, Li WZ. Synthesis and characterization of CePO4 nanowires via microemulsion method at room temperature. Chin Chem Lett. 2009;20(7):857.
Ma L, Chen WX, Zheng YF, Xu ZD. Hydrothermal growth and morphology evolution of CePO4 aggregates by a complexing method. Mater Res Bull. 2008;43(11):2840.
Damien B, Fabienne A, Thibault C, Dimitri S, Didier BA. Solid-state synthesis of monazite-type compounds LnPO4 (Ln = La to Gd). Solid State Sci. 2007;9(5):432.
Ye YX, Wei YH, Sheng WC, Chen M, Hua YQ. Luminescent properties of a new Nd3+-doped complex with two different carboxylic acids and pyridine derivative. Rare Met. 2013;32(5):490.
Ma MG, Zhu JF, Sun RC, Zhu YJ. Hydrothermal synthesis and characterization of CePO4/C core-shell nanorods. Mater Lett. 2009;63(28):2513.
Ma MG, Zhu JF, Cao SW, Chen F, Sun RC. Hydrothermal synthesis of relatively uniform CePO4@LaPO4 one-dimensional nanostructures with highly improved luminescence. J Alloy Compd. 2010;492(1–2):559.
Zhang Y, Guan H. Hydrothermal synthesis and characterization of hexagonal and monoclinic CePO4 single-crystal nanowires. J Cryst Growth. 2003;256(1–2):156.
Zhang Y, Guan H. The growth of lanthanum phosphate (rhabdophane) nanofibers via the hydrothermal method. Mater Res Bull. 2005;40(9):1536.
Nishihama S, Hirai T, Komasawa I. The preparation of rare earth phosphate fine particles in an emulsion liquid membrane system. J Mater Chem. 2002;12(4):1053.
Wang K, Zhang J, Wang J, Fang C, Yu W, Zhao X, Xu H. Growth defects and infrared spectra analysis of CePO4 single crystals. J Appl Crystallogr. 2005;38(4):675.
Zhang D, Du X, Shi L, Gao R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012;41(48):14455.
Yu C, Yu M, Li C, Liu X, Yang J, Yang P, Lin J. Facile sonochemical synthesis and photoluminescent properties of lanthanide orthophosphate nanoparticles. J Solid State Chem. 2009;82(2):339.
Thongtem T, Tipcompor N, Phuruangrat A, Thongtem S. Characterization of SrCO3 and BaCO3 nanoparticles synthesized by sonochemical method. Mater Lett. 2010;64(4):510.
Bhattacharyya S, Gedanken A. A template-free, sonochemical route to porous ZnO nano-disks. Microporous Mesoporous Mater. 2008;110(2–3):553.
Zhang D, Shi L, Fu H, Fang J. Ultrasonic-assisted preparation of carbon nanotube/cerium oxide composites. Carbon. 2006;44(13):2849.
Pan C, Zhang D, Shi L, Fang J. Template-free synthesis, controlled conversion, and CO oxidation properties of CeO2 nanorods, nanotubes, nanowires, and nanocubes. Eur J Inorg Chem. 2008;2008(15):2429.
Zhang D, Fu H, Shi L, Pan C, Li Q, Chu Y, Yu W. Synthesis of CeO2 nanorods via ultrasonication assisted by polyethylene glycol. Inorg Chem. 2007;46(7):2446.
Powder Diffraction File, JCPDS-ICDD, 12 Campus Boulevard, Newtown Square, USA, 2001.
Suryanarayana C, Norton MG. X-Ray Diffraction: a Practical Approach. New York: Plenum Press; 1998. 251.
Sumaletha N, Rajesh K, Mukundan P, Warrier KGK. Environmentally benign sol–gel derived nanocrystalline rod shaped calcium doped cerium phosphate yellow–green pigment. J Sol–Gel Sci Technol. 2009;52(2):242.
Yang M, You H, Zheng Y, Liu K, Jia G, Song Y, Huang Y, Zhang L, Zhang H. Hydrothermal synthesis and luminescent properties of novel ordered sphere CePO4 hierarchical architectures. Inorg Chem. 2009;48(24):11559.
Zhang YW, Yan ZG, You LP, Si R, Yan CH. General synthesis and characterization of monocrystalline lanthanide orthophosphate nanowires. Eur J Inorg Chem. 2003;2003(24):4099.
Wang X, Gao M. A facile route for preparing rhabdophane rare earth phosphate nanorods. J Mater Chem. 2006;16(14):1360.
Cao M, Hu C, Wu Q, Guo C, Qi Y, Wang E. Controlled synthesis of LaPO4 and CePO4 nanorods/nanowires. Nanotechnology. 2005;16(2):282.
Phuruangrat A, Ekthammathat N, Thongtem S, Thongtem T. Preparation of LaPO4 nanowires with high aspect ratio by a facile hydrothermal method and their photoluminescence. Res Chem Intermed. 2013;39(3):1363.
Mishra KC, Osterloh I, Anton H, Hannebauer B, Schmidt PC, Johnson KH. First principles investigation of host excitation of LaPO4, La2O3 and AlPO4. J Lumin. 1997;72–74:144.
Zhang F, Wong SS. Ambient large-scale template-mediated synthesis of high-aspect ratio single-crystalline, chemically doped rare-earth phosphate nanowires for bioimaging. ACS Nano. 2010;4(1):99.
Fang YP, Xu AW, Song RQ, Zhang HX, You LP, Yu JC, Liu HQ. Systematic synthesis and characterization of single-crystal lanthanide orthophosphate nanowires. J Am Chem Soc. 2003;125(51):16025.
Xing Y, Li M, Davis SA, Patil AJ, Mann S. Synthesis of cerium/cobalt phosphate nanostructures in catanionic reverse micelles. Soft Matter. 2006;2(7):603.
Zhang Y, Wang J, Zhang T. Novel Ca-doped CePO4 supported ruthenium catalyst with superior catalytic performance for aerobic oxidation of alcohols. Chem Commun. 2011;47(18):5307.
Acknowledgments
This study was financially supported by the Faculty of Science Research Fund, Faculty of Science, Prince of Songkla University, Thailand.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Phuruangrat, A., Thongtem, T. & Thongtem, S. Sonochemical synthesis and characterization of uniform lanthanide orthophosphate (LnPO4, Ln = La and Ce) nanorods. Rare Met. 34, 301–307 (2015). https://doi.org/10.1007/s12598-014-0297-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-014-0297-0