Abstract
Ni-base metal–intermetallic laminate composites were obtained from in situ reaction synthesis between Ni and Al foils by utilizing plasma activated sintering. The effects of Ni foil thickness on the microstructure and tensile properties of the composites were investigated. The results show that the phases forming during reaction synthesis are independent of the starting thickness of the Ni foils. However, thicker reacted layers are obtained in the samples fabricated from 100 μm Ni foils (Ni100) than those obtained in the samples from 50 μm Ni foils (Ni50) when treated at the same process. The tensile strength of Ni100 samples increases with the temperature increasing at the expense of ductility. Dissimilarly, Ni50 composites treated at higher temperatures exhibit enhanced strength and ductility. Both Ni50 and Ni100 laminate fracture in a similar mechanism. Cracking first occurs in the brittle intermetallic layers. These original cracks result in shear bands in Ni layers emitted from the crack tips, and thus producing local stress concentration, which initiates new cracks in adjacent intermetallic layers. The multiplication of cracks and shear bands leads to the failure of the laminates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The attainment of both strength and toughness is a vital requirement for most of the structural materials. Unfortunately, these properties are generally mutually exclusive. For these reasons, the development of strong and tough (damage-tolerant) materials is traditionally an exercise in compromise between hardness versus ductility [1]. To deal with this conflict, composites with different ductile reinforcement morphologies, including particles, fibers, and laminates, were developed [2, 3]. Specifically, for “laminate-like” composites, it was revealed that such architectures resulted in a greater level of toughening than fiber and particle reinforced composites [4]. One typical application is to fabricate metal–intermetallic laminate (MIL) composites which can be designed for structural use to optimize the unique properties and benefits of the constituent components. These composites usually combine high strength and stiffness of the intermetallic phase with high toughness of the metal [5].
Methods for the production of MIL composites include diffusion bonding [6] and magnetron sputtering [7]. However, these methods are time-consuming and/or are limited in the size and shape of the component that can be produced. To overcome these limitations, researchers at the US Bureau of mines exploited a processing technique that combustion reactions between dissimilar elemental metal foils were ignited to produce layered metal–intermetallic composites [8, 9]. The layered intermetallic microstructure promoted further work in fabricating Ti–Al [10, 11], Ni–Al [12, 13], and Nb–Al [14] MIL composites in a vacuum, in an argon atmosphere or even in the open air. However, a long treatment at higher temperature and pressure is necessary because the existence of high density of pores is inevitable for the combustion reactions. Recently, it has been shown that Ni–Al MIL composites can be produced through an improved process, pulsed-current hot pressing (PCHP) process, where the pulsed-current sintering (PCS), combustion synthesis and vacuum hot pressing were involved [15]. Through this process, MIL composites with Ni-aluminides can be fabricated in a notably shortened time (generally in 10 min). This modified process shows a great potential in rapid fabrication of the MIL composites and more details concerning the process need to be clarified.
In the present study, Ni–Al MIL composites were produced by utilizing plasma activated sintering (PAS) from the Ni and Al elemental foils. The PAS is an electric current assisted sintering equipment similar to the PCS [16]. In this study, the effects of the initial Ni foil thicknesses on the microstructure and tensile properties of these reaction synthesized laminate composites were investigated in detail.
2 Experimental
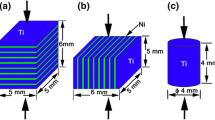
Commercially available foils of Ni (99.9 %) and Al (99.7 %) were cut into Φ40 mm disks and cleaned in the ultrasonic bath of acetone for 10 min. Any contamination on the surface of the foils was subsequently cleaned using degreased cotton swabs. After cleaning, 15 Ni foils and 14 Al foils were alternatively stacked into a cylindrical graphite die with an inner diameter of 40 mm and processed by PAS (Ed-PASIII, Elenix Ltd., Japan). The plies were initially heated to 973 K at an average heat rate of 5 K·s−1 under a pressure of 2.7 MPa and then were held for 90 s to insure that the reaction between the Ni and Al foils went to completion prior to the following heating. When the plies were heated to the given temperatures (1,073, 1,173, 1,273, or 1,473 K), the uniaxial loading was increased from 2.7 to 32.0 MPa and held for 600 s to insure high density of the laminates (Fig. 1). After sintering, the plies were furnace cooled to room temperature and the pressure was released at 873 K. To minimize the heat loss during the combustion reaction, the internal wall of the die was coated with a thin layer of boron nitride, and both ends of the stacked plies were thermally insulated by zirconia felt. Two kinds of laminates were produced by 50 μm thick Ni foils and 100 μm thick Al foils; 100 μm thick Ni foils and 100 μm thick Al foils, respectively. The former is hereafter called Ni50 and the latter is Ni100.
The characterization of the microstructure was performed by scanning electron microscope (SEM) with energy dispersive X-ray spectrometer (EDX). The thickness of the metal and intermetallic layer was identified based on the images of SEM. Flat dog-bone shape tensile specimens were electrodischarge machined from the as-processed MIL composites to determine the mechanical properties. The tensile tests were performed at room-temperature using an Instron screw machine with a strain extensometer at the strain rate of 5 × 10−3 s−1, and the tensile direction was parallel to the laminated layers. Before tensile, the lateral walls of the specimens were carefully polished initially with grade 800 abrasive papers and finally using 0.05 μm Al2O3 powders.
3 Results and discussion
3.1 Effect of Ni thickness on microstructure of laminates
Figure 2 shows a typical microstructure of the laminates prepared by PAS. It can be seen that the composites consist of alternating, well-bonded metal layers and reacted layers. EDX analysis elucidated that no unreacted Al was detected. The phases forming in the Ni50 and Ni100 samples are shown in Figs. 3 and 4, respectively. It can be seen that the phases forming in the reacted layers of the laminates processed by PAS show no dependence on the starting thickness of the Ni foils. After treated at 1,073 K, both Ni50 and Ni100 samples consist of identical phase, Ni2Al3, as the dominate phase (Figs. 3a, 4a). When the treated temperatures increase to 1,173 K, the primarily phase of both Ni50 and Ni100 samples is NiAl (51.63 at% Ni, Figs. 3b, 4b). Similarly, the dominate phase in both type of laminates treated at 1,273 K transforms into Ni-rich NiAl phase (67.13 at% Ni, Figs. 3c, 4c). When fabricated at 1,473 K, the reaction zones are composed of Ni-rich NiAl phase and Ni3Al phases. And part of Ni3Al is formed as a cellular structure when fabricated at this temperature (Figs. 3, 4). In light of the reaction temperature (1,473 K) far below the solidus line of NiAl (1,911 K) and Ni3Al (1,650 K) phases, Mizuuchi et al. [15] attributed this observation to the uniqueness of the PCHP process. A few voids concentrated along the centerline of the reacted layer can be observed (Figs. 3, 4). Previous works illustrated that this porosity may arise from the solidification shrinkage of transient liquid phase or from the evolution and entrapment of gaseous phases produced during the reaction [9]. Further, it can be seen that the densities and size of the pores decreases with the increase of the temperature. Then, to obtain pore-free composites, higher loads and temperatures are required for the samples after the combustion reaction.
Figure 5 shows the total thickness of the intermetallic layers and residual Ni layers of Ni50 and Ni100 samples treated at different temperatures. It can be seen that the reaction layer of the Ni50 samples is thinner than that of the Ni100 samples. Zhu et al. [17] found that the ignition temperature was independent of the thickness of the Ni foil varying between 12.5 and 150.0 μm. However, the rate of reaction increases with the decrease of the thickness of the Ni foil. Moreover, the maximum temperature of the reaction decreases rapidly when thicker Ni foils (>60 μm) is used. In view that during the PAS process the liquid Al was expelled inevitably from the edge of the samples due to the applied pressure, it is reasonable to elucidate that more liquid Al would be squeezed out from the Ni50 samples than that from the Ni100 samples. The former goes higher temperature, where the viscosity of the liquid Al is lower and easily expelled from the samples. As a result, the reaction layer of the Ni50 samples is thinner than that of Ni100 samples. Notably, the chemical composition and layer width of Ni3Al phase is nearly constant in Ni50 and Ni100 samples sintered at 1,173 and 1,273 K. This can be attributed to the narrow compositional region of these phases in Ni–Al phase diagram.
3.2 Effect of Ni thickness on tensile behavior of laminates
Tensile tests were carried out for Ni50 and Ni100 samples fabricated at different temperatures by PAS and the typical stress–strain curves of the examples were obtained at 293 K, as shown in Fig. 6. As can be seen in Fig. 6a, work hardening and plastic flow can be observed in the tensile curves. The tensile strength of all Ni100 samples increases with sintering temperature increasing in the range of 1,073–1,273 K with the expense of ductility (Table 1). Laminates with thick Ni layers tend to have the properties dominated by the metal. The increase of tensile strength and decrease of elongation of Ni100 samples are related to the decrease of the Ni volume fraction with temperatures increasing (Fig. 5b). In contrast, the composites having thinner Ni layers possess the properties dominated by the intermetallic. The elongation of Ni50 samples is far lower than that of Ni100 samples fabricated at the same temperature. In contrast, the ultimate strength of Ni50 samples is higher than that of Ni100 samples. When fabricated at 1,473 K, the tensile strength and the elongation of the Ni50 samples are enhanced compared with that of the composites fabricated at lower temperatures. This enhancement may relate to the solid solution hardening of the Ni matrix with the increase of Al content and/or to the improvement in ductility of Ni-aluminides due to the elimination of the pores in the intermetallic layers. These results indicate that the tensile behavior of the laminates is sensitive to the thickness of the Ni layers. It reveals that when the metal-intermetallic layered thicknesses are optimized, the mechanical properties of the layered composites can be tailored to be strong and tough at the same time.
3.3 Effect of Ni thickness on fracture mechanism of laminates
Fractography of the fractured surface and lateral surface (along the longitudinal direction and perpendicular to the layers) of the laminates after tensile tests was examined to reveal the failure mechanism of the laminates, and the results are shown in Figs. 7 and 8. When treated at lower temperatures (1,073 and 1,173 K), the fracture surfaces of Ni50 samples and Ni100 samples are extremely ragged. And the side view of the fracture surfaces resembles the sheets of opened books. The fracture of the metal–intermetallic laminates, where the ductile metal layers preferred to bridge the tunnel cracks originated in the intermetallic layers (Fig. 9), is a typical behavior of ductile phase-toughened matrix composites. The accompanying extensive deformation in Ni layer would result in shear bands generated from the crack tips and thus produced stress concentration points, which eventually induces crack initiation points which would otherwise cause local delamination in the adjacent intermetallic layers. Thus, the multiplication of the cracks and shear bands from the initial cracks is generated. Finally, the Ni layers lose their capability of further shear-strain, which would promote the failure of the composites. In addition, large scale delamination along the central line of the intermetallic layers where the residual pores locate occurs during the tensile process. Consequently, the metal layers separate from the adjacent intermetallic layers. The delamination occurs prior to significant plastic deformation in the metal layer [8] and subsequently relaxes the constraining forces around the metal layer, allowing extensive plastic deformation of the ductile metal layer (Figs. 7a, 8a). With the increase of the treated temperature (1,273 and 1,473 K), the delamination are rarely observed except very close to the fracture surfaces and the density of the cracks forming in the intermetallic layers is lower. For the samples treated at 1,473 K, it can be seen that transverse cracks forming in the center of the intermetallic layers are inhibited by the Ni3Al individual layers (Figs. 7g, 8g). It is suggested that the Ni3Al layers forming through in situ reaction synthesis may have a similar, low energy, coherent interface with the Ni layers [18]. Thus, the dislocations in the Ni layers can slide through the interface between the Ni3Al and Ni layers, and do not pile up at the interfaces. This can be verified by the side view of the occasional delamination at the Ni/Ni3Al interface (Fig. 7h) and the fracture surface of the Ni3Al (Fig. 8h). Thus, the stress concentration in the Ni3Al layers can be released to some extent, and will not result in the failure of the Ni3Al layers. As a result, the enhancement of both tensile strength and elongation of the laminates can be obtained.
The fracture surfaces of the intermetallic layers with Ni2Al3 as the primarily phase in Ni50 samples and Ni100 samples treated at 1,073 K show a transgranular cleavage character. When the dominate phase transforms into NiAl, it can be seen that the fracture surfaces of the intermetallic layers consist of a mixing mode of intergranular and transgranular cleavage facets. The fracture surfaces of the Ni layers in the samples treated at lower temperatures show serpentine glide characters. For the samples treated at higher temperatures (1,273 and 1,473 K), the Ni layers show a dimple fracture characteristic.
4 Conclusion
In this study, the effect of the initial thickness of Ni foils on the microstructure and mechanical properties of in situ reaction synthesis of metal–intermetallic laminate composites fabricated by PAS was investigated. It is shown that the phases forming in the laminates during the process are independent of the initial thickness of the Ni foils. Thicker reacted layers can be obtained in Ni100 samples than those in Ni50 samples during the same process. The tensile strength of the laminates with thick Ni layers (100 μm) increases with reaction temperature increasing at the expense of ductility. However, composites having thinner initial Ni layers (50 μm) have properties dominated by the intermetallic. It indicates that optimization of thickness ratios of Ni and Al foils may be important in improving mechanical properties of composites fabricated from Ni and Al foils.
Despite the different thickness ratios of Ni and intermetallic layer in the samples, these laminates fracture in a similar mechanism: cracking first occurs in the brittle intermetallic layers. The extensive deformation possible in the Ni layers results in shear bands being generated from the crack tips and thus producing stress concentration, enabling crack initiation points in adjacent intermetallic layers. The multiplication of cracks and shear bands leads to the failure of the laminates.
References
Ritchie RO. The conflicts between strength and toughness. Nature. 2011;10(11):817.
Kajuch J, Short J, Lewandowski JJ. Deformation and fracture behavior of Nb in Nb5Si3/Nb laminates and its effect on laminate toughness. Acta Metall Mater. 1995;43(5):1955.
Cai W, Feng X, Sui J. Preparation of multi-walled carbon nanotube-reinforced TiNi matrix composites from elemental powders by spark plasma sintering. Rare Met. 2012;31(1):48.
Li M, Soboyejo WO. An investigation of the effects of ductile-layer thickness on the fracture behavior of nickel aluminide microlaminates. Metall Mater Trans A. 2000;31(5):1385.
Vecchio K. Synthetic multifunctional metallic–intermetallic laminate composites. JOM. 2005;57(3):25.
Mumtaz K, Echigoya J, Nakata C, Husain SW. Effect of cold rolling and subsequent annealing on hot pressed Ni/Al laminates. J Mater Sci. 2001;36(16):3981.
Ma L. He Xd, Li Y. Optimized design and preparation of Ti/TiAl laminated composite. Trans Nonferrous Met Soc China. 2005;15(3):48.
Rawers J, Hansen J, Alman D, Hawk J. Formation of sheet-metal intermetallic composites by self-propagating high-temperature reactions. J Mater Sci Letts. 1994;13(18):1357.
Alman D, Dogan C, Hawk J, Rawers J. Processing, structure and properties of metal–intermetallic layered composites. Mater Sci Eng A. 1995;192–193(2):624.
Peng LM, Wang JH, Li H, Zhao JH, He LH. Synthesis and microstructural characterization of Ti–Al3Ti metal–intermetallic laminate (MIL) composites. Script Mater. 2005;52(3):243.
Patselov A, Greenberg B, Gladkovskii S, Lavrikov R, Borodin E. Layered metal–intermetallic composites in Ti-Al system: strength under static and dynamic load. AASRI Procedia. 2012;3:107.
Wang H, Han J, Du S, Northwood D. Reaction synthesis of nickel/aluminide multilayer composites using Ni and Al foils: microstructures, tensile properties, and deformation behavior. Metall Mater Trans A. 2007;38(2):409.
Konieczny M. Microstructural characterisation and mechanical response of laminated Ni-intermetallic composites synthesised using Ni sheets and Al foils. Mater Charact. 2012;70:117.
Chung D, Enoki M, Kishi T. Microstructural analysis and mechanical properties of in situ Nb/Nb-aluminide layered materials. Sci Technol Adv Mater. 2002;3(2):129.
Mizuuchi K, Inoue K, Sugioka M, Itami M, Lee J, Kawahara M. Properties of Ni-aluminides-reinforced Ni-matrix laminates synthesized by pulsed-current hot pressing (PCHP). Mater Sci Eng A. 2006;428(1–2):169.
Munir ZA, Quach DV, Ohyanagi M. Electric current activation of sintering: a review of the pulsed electric current sintering process. J Am Ceram Soc. 2011;94(1):1.
Zhu P, Li JCM, Liu CT. Adiabatic temperature of combustion synthesis of Al-Ni systems. Mater Sci Eng A. 2003;357(1–2):248.
Wang HB, Han JC, Du SY, Northwood D. Effects of Ni foil thickness on the microstructure and tensile properties of reaction synthesized multilayer composites. Mater Sci Eng A. 2007;445–446:517.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 51002115), the Special Fund for Basic Scientific Research of Central Colleges, Chang’an University (No. 2011JC139), and the Foundation of State Key Laboratory for Mechanical Behavior of Materials (No. 20121203).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, YJ., Shi, ZQ., Xu, YK. et al. Correlation between microstructure and tensile behavior of metal–intermetallic laminate compound with different initial Ni foil thickness. Rare Met. 33, 196–202 (2014). https://doi.org/10.1007/s12598-013-0203-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-013-0203-1