Abstract
Steel-cemented WC was prepared by ball milling, cold compacting and microwave sintering with Fe powder as the matrix, WC as the hard phase and the addition of rare earth Y2O3. The results show that the interface of the WC particles and Fe matrix exhibits excellent wettability and liquidity when the microwave sintering temperature reaches 1,280 °C. The density and mechanical properties of the steel bonded WC carbides could be greatly improved, the hard phases become finer and more uniform dispersed owing to the addition of Y2O3. With the increase of the Y2O3 contents, the grain becomes uniform and fine first, and then gathers and grows up. The relative density, microhardness and bending strength all rise first, reaching the maximum values of 97.29 %, HV1024 and 1,267.60 MPa at 0.5 % Y2O3, respectively, and then decrease. Moreover, the relative density and mechanical properties of the steel-cemented WC with nano-Y2O3 are higher than that with micron-Y2O3, which indicates that the effect of nano-Y2O3 is better than that of the micron-Y2O3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Steel cemented, first appeared at the initial of 1960s in America, with steel as the binder phase and carbide as the hard phase, becomes a new type engineering material which is the most suitable to work at heavy loading and high friction and wear environment, such as new type tools and molds, wear parts and machinery parts, etc. [1]. Therefore, the properties of high strength, high wear resistance, and enough toughness are required for the steel cemented. Now, the production and application of domestic steel cemented are restricted due to its poor stability and bad durability [2]. However, more and more attentions are paid to the application of steel cemented in aviation and automobile industry due to the variety of the steel matrix and lower price [3].
Microwave sintering, a new powder metallurgy method, possessing superior performance such as rapid heating, selectivity heating, and non-thermal effect [4, 5], is considered as the revolution of the sintering technology and paid more attentions in the past ten years [6, 7]. By far, there are only a few reports about the cemented carbides prepared by microwave sintering at home and abroad, such as the WC–Co-cemented carbides studied by the Pennsylvania State University [8], Central South University [9], and Wuhan University of Technology [10]. Moreover, the effects of sintering process on the microstructure and mechanic properties of steel-cemented WC prepared by microwave sintering were studied in our earlier report [11]. However, to the best of our knowledge, there are few reports about the effects of Y2O3 on the microstructure and mechanic properties of steel-cemented WC prepared by microwave sintering at home and abroad.
The rare earth elements were involved into cemented carbides since 1960s, reaching a study peak during the initial of 1980s to the end of 1990s [12–14]. The results showed that the rare earth-cemented carbides possessed much more advantageous properties compared with the traditional cemented carbides. However, many problems about the quality stability were observed in the process of actual production of the rare earth-cemented carbides. The main reason was that the study on the effect mechanism of the rare earth elements on the cemented carbides did not obtained the breakthrough progress, which brought much blindness to the research of the rare earth-cemented carbides.
In this paper, steel-cemented WC was prepared by ball milling, cold compacting and microwave sintering, with Fe powder as the matrix, WC as the hard phase and the addition of rare earth Y2O3. The effect mechanism of Y2O3 involved in the steel-cemented WC was also studied.
2 Experimental
Table 1 shows the composition of steel-cemented WC with different Y2O3 contents, where the size of WC particles is 1–3 μm, the carbonyl iron powder accounts for 30 % of total iron powder content, has a granularity of 1–3 μm; the reduced iron powder accounts for 70 % of total iron powder content, has a granularity of 20–40 μm; the size of nano-Y2O3 particles is 30–40 nm, and the micro Y2O3 particle size is 1–5 μm.
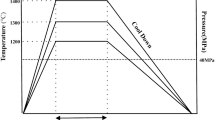
The powders were blended together to yield the different nominal compositions of steel-cemented WC as shown in Table 1. The blending process was carried out in a planetary ball mill using stainless steel as the container and stainless steel ball with the alcohol as the milling medium, the ball to powder weight ratio is about 6:1, and the rotation rate is 200 r·min−1 for 6 h. The ball milled and dried mixture powder was coldly compacted at a pressure of 300 MPa into cylindrical green compacts. The green compacts were sintered in a microwave sintering furnace under protection of high purity argon. The sintering temperature was 1,280 °C for 20 min with the heating rate of 15–35 °C·min−1, then cooling with furnace.
The sintered samples were carried out the heat treatment, spheroidize annealing first, and then quenching and tempering. The spheroidize annealing process was as follows: slowly heating to 850–880 °C for 3 h, furnace cooling to 730 °C for 5 h, further furnace cooling to 500 °C and then air cooling. The quenching and tempering process was 900 °C for 30 min, oil quenching, tempering at 200 °C for 2 h, air cooling.
The phase composition of the samples was determined by X-ray diffraction (XRD, D8ADVANCE), the element content of the sample was measured by energy dispersive spectroscopy (EDS, Oxford Model), the microstructure was observed by scanning electron microscope (SEM, QUANTA200), the density, microhardness, and bending strength were measured by Archimedes drainage method, HVS-1000 hardmeter, and electronic universal testing machine (WDW-50), respectively.
3 Results and discussion
3.1 Microstruture
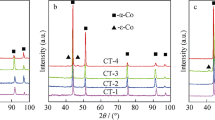
Figure 1 shows the XRD patterns of steel-cemented WC with different Y2O3 contents. It can be seen that all the samples except the 0 % Y2O3 sample are composed of α-Fe, Fe2W2C, and Y2O3 phase. The diffraction peaks of the Y2O3 increase with the increase of the Y2O3 contents, while the diffraction peaks of α-Fe and Fe2W2C rarely change. It can be concluded that the Y2O3 contents have little effect on the phase composition of steel-cemented WC.
The SEM images of steel-cemented WC surface with different Y2O3 contents are shown in Fig. 2. The hard phase particles can be clearly observed, which are Fe2W2C phases verified by XRD. The distribution of the hard phases exhibits nonuniform over the steel-cemented WC matrix without adding Y2O3 (Fig. 2a) and some defects, such as impurities and micropores, are also observed on the surface. When adding 0.5 % Y2O3 in the steel-cemented WC, the hard phases become finer and more uniform dispersed as well as the decrease of the defects. Further increasing the Y2O3 contents, although the hard phase particles are smaller and smaller, the aggregation of the hard phases obviously increases. At the same time, the pores over the steel-cemented WC gradually increase.
Figure 3 shows SEM image and EDS spectra of sintered steel-cemented WC. The microstructure of the steel-cemented WC is composed of matrix and dispersed hard phase, consistent with Fig. 2. Spectrum 1 (Fig. 3b) is the EDS analysis of the interface between the matrix and the hard phase, Spectrum 2 (Fig. 3c) is the EDS analysis of the hard phase, and Spectrum 3 (Fig. 3d) is the EDS analysis of the matrix. It can be seen that the matrix consists of Fe and C of 93.63 and 6.37 at%, respectively, the hard phase is composed of Fe, W, and C of 41.72 at%, 36.58 at%, and 21.70 at%, respectively, and the ratio between Fe, W, and C is nearly 2:2:1, which is consistent with the Fe2W2C phase. The result of spectrum 1 shows that the interface of the hard phase and the matrix is composed of Y, O, Al, Si, Ca, Mn, and Fe, where the elements of the Al, Si, Ca, and Mn are the impurity elements gathered in the interface, which perhaps come from the ball mill process or from the impurities of raw materials.
According to the results of Spectrum 1 (Fig. 3b), there are a large number of Y and impurity elements in the interface of Fe2W2C and Fe, which result from the addition of Y2O3. The rare earth additions possess low surface free energy and active chemical characteristic, which can eliminate micropores, refine crystalline grains and purify the interface, and then improve the properties of the sintered steel-cemented WC. The rare earth additions react with the O, Al, Si, Ca, and Mn element, forming the refractory compounds, which can decrease the gas evolution and formation of pores, purify the interface of Fe2W2C and Fe and improve its wetting property. At the same time, the refractory compounds deposit on the grain boundary of Fe2W2C, generate the pinning effect to the migration of Fe2W2C grains boundary, playing the role of refining grains, and then the hardness and bending strength of the samples are improved.
3.2 Density
Figure 4 shows the relation of Y2O3 contents with relative density of steel-cemented WC. It is clear that the relative density of the steel-cemented WC increases first, reaching a maximum value of 97.29 % at the addition of 0.5 % Y2O3, and then decreases with the increase of the contents of Y2O3. Moreover, the relative density of steel-cemented WC reaches a minimum value of 93.44 % without adding Y2O3 and the samples added nano-Y2O3 have higher relative density than those of the samples added micron-Y2O3.
3.3 Mechanical property
Figures 5 and 6 show the relation of Y2O3 contents with the microhardness and bending strength of the steel-cemented WC, respectively. It can be seen that the microhardness and bending strength of the steel-cemented WC have the same variation tendency with the increase of the Y2O3 contents, namely, the microhardness and bending strength reach maximum values of HV1024 and 1,267.60 MPa, respectively, with the Y2O3 content of 0.5 %, and then decrease with the further increase of the Y2O3 contents. When the Y2O3 content reaches up to 2.0 %, the microhardness and bending strength of the steel-bonded cemented carbide are even lower than those of without adding Y2O3 samples. At the same time, the samples added nano-Y2O3 have higher microhardness and bending strength than those of the samples added micron-Y2O3.
During the microwave sintering process, a large number of solid–liquid interfaces exist in the internal of the green compact, and the interface is not only geometry surface, but also is the area of several atoms thickness, in which there are a large number of microcosmic crystal defects, such as vacancy and dislocation, etc. [15, 16]. It is well known that the rare earth element is a surface active element and the atomic radius of Y (0.227 nm) is larger than those of Fe (0.172 nm) and W (0.202 nm), so the rare earth Y could be easily captured by the above-mentioned defects and segregated on the solid–liquid interface, named as “adsorption effect” [13, 17]. The experimental results show that the solidification and crystallization process of Fe matrix and the microstructure characteristics of the hard particle Fe2W2C are obviously influenced by the adsorption effect of rare earth. The number of adsorption on the different crystal face is different, generally, the higher the surface tension is, the more the number of adsorption is [18]. The number of adsorption of the surface activity element on the crystal face could be expressed as the following equation.
where Γ is the number of adsorption, mol·cm−2; c is the concentration of the surface activity element, mol·L−1; R is the constant of Planck; T is thermodynamic temperature, K; and γ is the surface tension of the crystal face, N.
Equation (1) indicates that the higher Γ value is, the higher c value is, so the bigger γ is, which causes the interfacial free energy to minimize. Therefore, the adsorption of the rare earth element on the crystal face not only decreases the free energy different values during the each crystal face, but also reduces the growth rate of the crystal face, equivalent to pinning or dragging effect for the migration of the grain boundary or phase boundary [16, 19], so Y2O3 can refine the grains.
It is clear from Figs. 4, 5, and 6 that addition of 0.5 % Y2O3 makes the relative density and mechanical properties of steel-cemented WC reach the optimum value. It can be explained as follows. First, during the process of ball milling, the cold weldings occur between the powder and powder, or powder and ball, or powder and wall due to the powders suffer intense collision and squeezing from grinding balls, which cause temperature rising locally. Rare earth elements could react with oxygen at room temperature due to its extremely active chemical properties, and form the stable rare earth oxides. This property could decrease the oxidation film on the powder surface during the sintering process, benefit the element interdiffusion between the atoms, accelerate the densification process, and improve the density of the alloy ultimately. Second, the rare earth elements could reduce the metastable eutectic reaction temperature, increase the crystallization degree of supercooling and the nucleation rate of the austenite, and refine the grains. On the other hand, oxygen sulfur compounds of the rare earth could refine grains of the matrix as the heterogeneous nucleation due to its low free energy, high melting point, lattice structure of NaCl, and the atomic separation very close to the austenite. At the same time, the rare earth element could seize the oxygen under the sintering temperature due to its active chemical property, absorb the particles surface during the sintering densification process, decrease the surface energy and the driving force of the grain growth. Therefore, the migration of grain boundary is impeded by the addition of rare earth element and the formation of its oxide, and causes the grains to refine and the number of the grain boundaries to increase, which provides more diffusion channel to the alloy elements and improve the density, mircohardness, and bending strength of the alloy. Finally, rare earth elements are surface active elements and could decrease the surface tension of the liquid phase, which is beneficial to the migration of the macrosubstance and accelerates the process of densification. Simultaneously, the rare earth elements could unite the impurities, purify the grain boundary and increase the interface bonding strength between the Fe and WC particles.
However, with the further increase of Y2O3 contents, the density and mechanical properties of the samples decrease. The reason may be that addition of the excessive Y2O3 would easily promote the rare earth compounds to aggregate on the grain boundary, deteriorate the continuity of the alloy matrix and impede the densification process, which results in the decline of the relative density of the alloy and the decrease of the mechanical properties.
It can be seen from Figs. 5 and 6 that the mechanical properties of steel-cemented WC added nano-Y2O3 are higher than that of added micron-Y2O3. The nano-Y2O3 possesses smaller size, and more particles can distribute on the interface interval of Fe and Fe2W2C or Fe2W2C and Fe2W2C, which is beneficial to improve the relative density of the samples. At the same time, the nano-Y2O3 can more uniformly distribute over the interface of Fe and Fe2W2C and Fe2W2C and Fe2W2C, playing roles in refining grains and eliminating the nonuniform growth-up grains.
4 Conclusion
During the preparation process of steel-cemented WC by microwave sintering, the interface of Fe2W2C and Fe matrix can be purified, the densification process of alloy can be improved and the bonding strength of the hard particles to matrix can be enhanced through adding the moderate Y2O3. With the increase of the Y2O3 contents, all the relative density, microhardness, and bending strength of steel-cemented WC increase first, reaching the maximum values of 97.29 %, HV1024, and 1,267.60 MPa, respectively, and then decrease. At the same time, addition of nano-Y2O3 exhibits better effect than that of micron-Y2O3.
The rare earth Y2O3 can decrease the surface tension and the growth rate of the grains, increase the nucleation rate and reduce the dimension of the grain; it also can suppress the phase boundary migrate of the hard phases, which makes it finer and more uniform dispersed over the matrix. At the same time, the rare earth Y2O3 possesses the adsorption effect on the impurities and defects, and plays a promotion role in the improvement of the density and microstructure uniformity of steel-cemented WC.
References
Kim HC, Shon IJ, Yoon JK, Do JM. Comparison of sintering behavior and mechanical properties between WC–8Co and WC–8Ni hard materials produced by high-frequency induction heating sintering. Met Mater Int. 2006;12(2):141.
Pan CZ, Zhang J, Zhang L, Zhao ZM. Investigation of solidification behavior of in situ TiC matrix composite ceramics. Trans Mater Treat. 2011;32(4):122.
Li ZL, Jiang YH, Yang H. Structure homogeneity of in situ TiC particles reinforced steel-based composite materials. Trans Mater Heat Treat. 2010;31(4):1.
Luo SD, Yan M, Schaffer GB. Sintering of titanium in vacuum by microwave radiation. Metall Mater Trans A. 2011;21:130.
Li YC, Xu F, Hu XF, Qu HY. In situ investigation of SiC powder’s microwave sintering by SR-CT technique. Sci China Tech Sci. 2011;54:1382.
Ai YL, He W, Liu CH, Cheng TC. Microwave sintering technology and microstructure of ZrO2(n)–Al2O3 composite ceramics. Heat Treat Met. 2010;35(2):24.
Guo FF, Li ZJ, Lin C, Xu Z, Peng F. Study on performance of Mg-B binary system in process of microwave sintering. Mater review. 2007;21(11A):88.
Agrawal D, Cheng J, Seegopaul P, Gao L. Grain growth control in microwave sintering of ultrafine WC–Co composite powder compacts. Powder Metall. 2000;43(1):15.
Bao R, Yi JH, Yang YJ, Peng YD. Research on microwave sintering of ultra-fine cemented carbide. Powder Metall Ind. 2010;20(2):23.
Liu WB, Zhou J. Study on technique and properties WC–10Co cemented carbide in microwave sintering. Wuhan: Wuhan University of Technology, 2007. 1.
Luo JM, Wei Z, Ai YL, Zhang JP. Effect of microwave sintering temperature on microstructure and properties of WC steel-bonded carbide. Trans Mater Heat Treat. 2011;32(7):31.
Xu CH, Hai AX, Huang CZ. Research and development of rare-earth ceented carbides. Int J Refrac Met Hard Mater. 2001;19(3):159.
Wang YM, He CX, Zhao BL. Research on including rare earth elements of hard alloy in China. China Tungsten Ind. 1999;14(5–6):186.
Zhang L, Huang BY, Chen S. Enrichment of rare earth on sintering skin of cemented carbide spherical powder. Mater Sci Eng Powder Metall. 2004;9(3):1.
Gu DD, Shen YF. Effects of La2O3 addition on structure and forming property of laser sintered (WC–Co) p/Cu metal matrix composites. Acta Metall Sin. 2007;43(9):968.
Zhai QJ, Guan SK, Shang QY. Alloy Thermodynamics Theory and Application. Beijing: Metallurgical Industry Press; 1999. 76.
Zhong HR. Steel Rare-Earth Chemical Heat Treatment. Beijing: Defense Industry Press; 1998. 102.
Yuan ZF, Ke JJ, Li J. Metal and Alloy Surface Tension. Beijing: Beijing Science and Technology Press; 2006. 38.
Wei QF, Sun J, Li CQ. Research on the application of RE additives in the cemented carbide. Rare Met Cem Carbides. 2002;30(2):33.
Acknowledgments
This work was financially supported by the Science and Technology Plan Projects of Jiangxi Province (No. 2011BBE50010) and the Project from the Jiangxi Province Key Laboratory of Copper Tungsten New Materials (No. 2011-TW-08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, JM., Xu, JL. & Zhong, ZC. Microstructure and properties of Y2O3-doped steel-cemented WC prepared by microwave sintering. Rare Met. 32, 496–501 (2013). https://doi.org/10.1007/s12598-013-0131-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-013-0131-0