Abstract
In the present research work we reported the highly dispersible TiO2/SnO2 nanoparticles produced via a simple aqueous solution growth technique with ~50 nm average grain size. The surface morphology of the material have been studied by using scanning electron (SEM) and atomic force microscopes (AFM). Using photoluminescence, interaction between ammonia gas and TiO2/SnO2 (60:40 composition) nanoparticles were investigated. The material shows average significant increment in the PL intensity with no shifting in peak, which conform its tremendous applicability in optical gas sensor to detect ammonia gas (20 ppm) using photoluminescence technique. The gas sensing was systematically performed through photoluminescence spectrometer for different concentration of ammonia at room temperature. The response and recovery time of these samples are found to be in the range of 12 and 50 s respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The TiO2 and SnO2 are N-type semiconducting metal oxide gas sensors. They are currently constitute one of the most investigated groups of gas sensors. TiO2 and SnO2 and some other oxide nanoparticles nanomaterial have attracted much attention due to their unique optical, dielectric and mechanical properties as well as numerous potential applications including photo catalysis, optical coating, and photoelectron chemical solar cells [1,2,3,4,5,6]. They have attracted much attention in the field of gas sensing under atmospheric conditions due to their low cost and flexibility in production; simplicity of their use; large number of detectable gases is possible. In addition to the radiative property changes of gas-sensing material, the detection of this reaction can be performed by measuring the change of optical characteristics, work function, capacitance, mass or reaction energy released by the gas/solid interaction [2,3,4,5,6]. The main attention in this paper will be focused on the optical characteristics of the TiO2/SnO2 semiconducting metal oxide for gas sensors. Photoluminescence spectroscopy as an important tool has been used to study TiO2/SnO2 sensing of gas.
There are various chemical methods for the synthesis of ultrafine oxide nanoparticles such as sol–gel, hydrothermal, aqueous solution growth technique flame combustion and precipitation. The aqueous solution growth technique is one of the best methods for fabricate material at low cost with mild temperatures, high purity materials with control in the nanostructure and surface properties. The synthesis involves the controlled heteronucleation of metal oxides in aqueous solutions [7,8,9]. Single-crystal nanostructures can give rise to novel usuful properties in different application fields, such as gas sensing. Indeed, the average surface-to-volume ratio, consequent on the reduced sized of nanostructures leads to an enhanced surface interaction [10,11,12,13,14,15]. In particular, sensors based on the variations of the photoluminescence (PL) emission in the presence/absence of gas molecules are attractive because they can provide high responses at room temperature and result stable with a rapid, reproducible and good sensitivity [16]. TiO2/SnO2 nanoparticles can also be produced by a variety of techniques ranging from simple chemical to mechanical to vacuum methods, including many variants of physical and chemical vapour deposition techniques for using as gas sensing elements [16,17,18].
A sensor is defined as a device able to convert a chemical or physical variation to be monitored into an easily processable signal. The sensing element (material) has to fulfill essentially two different tasks: first, it has to interact with the target gas through various mechanisms like surface adsorption, charge transfer, ionic exchange then this interaction has to be transformed into an easily processable signal, like a change in electrical conductivity or in optical properties. Particularly in metal oxides and carbon nanotubes, the mechanisms for recognizing the gases to be determined include absorption processes. There is a variety of sensing devices that rely on different chemical or physical phenomena: in fact the analyte presence can be detected through changes in electrical properties (conductivity, impedance, capacitance), optical properties (absorption, reflection, luminescence, refractive index) or other physical properties (mass, thermal conductivity, acoustic waves propagation), measuring the reaction heat analyzing specific electrochemical or biochemical recognition, and so on. The first and at the moment more established sensors are based on a conductometric interface: historically the birth of electrical sensor is dated 1962, when SnO2 has been assessed as good material for gas detection [19,20,21,22,23,24]. All the electrical sensors have limitation like contact problem and selectivity of gases but this problem can be solved through optical/Luminescent sensor.
In present work, TiO2/SnO2 nanopartical are prepared via simple aqueous solution growth technique which is assisted by ultrasonication and characterization of prepared TiO2 is done by SEM and AFM. The optical and sensing properties were studied through photoluminescence(PL) spectra at room temperature and the data were recorded using fluorescence spectrometer (F-7000 Hitachi) exciting with its Xenon lamp at 242 nm All the reagent used were of analytical grade and no further purification was done.
Experimental procedure
Reagent
Tetraisopropoxide (purity, 97%), Ti [OC3H7]4 and SnCl2·6H2O were used as a starting material and supplied by sigma-Aldrich company. Ethanol [C2H6O] and nitric acid [HNO3] from Merck. Distilled water was used for preparing all standard solutions. The chemicals were used without any further purification.
Synthesis process
In this research paper, TiO2/SnO2 nanopartical in the composition of 60:40 are prepared via simple aqueous solution growth technique which is assisted by ultrasonication. The aqueous solution synthesized TiO2/SnO2 was obtained from 0.1 M titanium(IV) Isopropoxide (TTIP) and 1.5 mg SnCl2·6H2O dissolved in 75 ml absolute ethanol and distilled water was added to the solution in terms of a molar ratio Ti:Sn:H2O = 0.6:0.4:4. Nitric acid was used to adjust the pH and for restrain the process of the solution. The solution was vigorously stirred for 30 min. After ageing 24 h, it transformed into precipitate. In order to obtain nanoparticle, the precipitate was dried under 90–100 °C for 3 h to evaporate water and organic material to the maximum extent. Then the dry precipitate was sintered at 450 °C for 4 h were subsequently carried out to obtain TiO2/SnO2 powder.
Gas sensing measurement
For optical gas sensing measurement, the sample was excited by a Xenon laser as light source [F-7000 Hitachi] at 242 nm excitation wavelength. PL spectra were acquired perpendicular to the sample surface using a single spectrograph. A filter was used to filter out secondary wavelength during the whole measurements. A sample holder is designed in lab to study the effect of gas on Photoluminescence of materials in situ. This sample holder is made from quartz tube with a cuvette vertically attached in its bottom. Sample was placed inside the quvette of quartz sample holder at room temperature; this sample holder was kept inside the spectrometer chamber for optical measurements. The data is recorded in the presence of simple air in chamber then the sample holder was filled with 20 ppm ammonia gas and data was again recorded after the exposure for comparison.
Characterization
Scanning Electron Microscopy (SEM, JEOL-JSM-6390) and Atomic Force Microscopy (AFM), NT-MDT Solver NEXT, was used to define the morphology chemical composition of as prepared samples. Optical properties and gas sensing were studied through Photoluminescence (PL) spectra at room temperature and the data were recorded using fluorescence spectrometer (F-7000 Hitachi) exciting with its xenon lamp at 242 nm.
Surface morphology
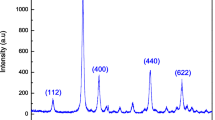
For investigating the morphology of the synthesized TiO2 nanopartical SEM and AFM were used. The SEM images are shown in Fig. 1 and AFM images are shown in Fig. 2 (a, b) on the scale of 200 nm.
Both SEM and AFM provides extraordinary topographic contrast and unobscured views of surface features. To analyze surface morphology, the 1 × 1 μm image was taken from the sample using an Atomic Force Microscope (AFM). Two-dimensional image and average grain size and average diameter of the sample is shown in Fig. 2b. From this image, it can be seen that dispersion of ZnO nanoparticles is relatively uniform. Average grain size and diameter of particle were determined with the help of Nova PX analysis software programming.
The average crystallite size of the ZnO sample was found to be 50 nm, the average aspect ratio is 26, average particle size is 60 nm. As seen in AFM images, the ZnO sample was formed from the particles like circles which are in good agreement with SEM results.. The particles are well distributed in the sample and the average roughness of sample was estimated as 1.62 nm using Nova PX software.
Photoluminescence and gas sensing study
Photoluminescence (PL) is a non-destructive technique used for the investigation of extrinsic and intrinsic properties of semiconductors. In this technique the semiconductor under investigation is excited optically and then the PL spectrum of the spontaneous emission from radiative recombination in the semiconductor band gap is obtained. During the PL process both radiative and non radiative recombination happen. The PL technique can be used to determine the bandgap, impurity levels, defects detection and recombination process in the semiconductor materials.
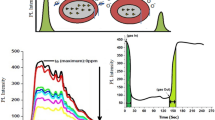
A Photoluminescence excitation spectrum is shown in Fig. 3. When the material is excited at 242 nm in the absence of ammonia gas, a strong emission peaks were observed around 422 nm due to violet emission which is attributed to the recombination of an electron and hole in the valance band. The second peak were observed at 465 nm (2.72 eV) due to blue emission. The blue emission might be related to the surface defect and confine particle size in TiO2/SnO2 thin film. The other two peak were observed at 512.4 and 545.4 nm (green emission). Due to oxygen vacancies and recombination of electron, oxygen vacancies occur in three different charge states, the neutral oxygen vacancy, the singly ionized oxygen vacancy and doubly ionized oxygen vacancy, only the singly ionized oxygen vacancy can act as the luminescent center, green emission decreased mainly due to decrease in the oxygen vacancies in the samples. When TiO2/SnO2 nanoparticle is again excited at 242 nm in the presence of 20 ppm ammonia gas, a strong emission peak is observed at same wavelength but this time the intensity of the peak increased due to ammonia gas which is reducing in nature [25,26,27]. The overlay plot of TiO2/SnO2 in presence of ammonia and in the absence of ammonia is given in Fig. 3.
Time response
Time response graph plotted between intensity and time for TiO2/SnO2 is shown in Fig. 4, and the curve is following a step change in composition from air to 20 ppm NH3 to again air at the room temperature. The response and recovery time are determined from this plot.
It is observed that when gas enter into the chamber it react with the oxygen present at the surface of TiO2/SnO2 due to which the radiative property of the TiO2/SnO2 start increasing and the intensity goes to its maximum and becomes constant in saturation state.
The response time (rise time) represents the time required by the sensitivity factor to undergo 90% variation with respect to its equilibrium value following a step increase in the test gas concentration and it was found to be 12 s in this case. Similarly, the recovery time (settling time) represents the time required by the sensitivity factor to return to 10% below its equilibrium value in air following the zeroing of the test gas ammonia and it was found to be around 50 s.
Sensing mechanism
TiO2/SnO2 is an n-type semiconductor where the majority charge carriers are electrons, and upon interaction with a reducing gas an increase in radiative property of material can occurs. Conversely, an oxidizing gas serves to deplete the sensing layer of charge carrying electrons, resulting in a decrease in radiative property. A p-type semiconductor is a material where the majority charge carriers are holes (Table 1) hence, the opposite effects are observed with the material and showing an decrease in the radiative property of material in the presence of an reducing gas. When O2 molecules are adsorbed on the surface of TiO2/SnO2, they would extract electrons from the conduction band Ec and trap electrons at the surface in the form of ions. This will lead a band bending and an electron depleted region. This electron depleted region is also known as space-charge layer, the thickness of which is the length of band bending region [28,29,30,31].
Reaction of these oxygen species with reducing gases or a competitive adsorption and replacement of the adsorbed oxygen by other molecules decreases and can reverse the band bending, resulting in an increased radiative property. In clean air the radiative property of TiO2/SnO2 is low because the conduction electrons are bound to surface oxygen, whereas in the presence of a reducing gas, electrons are no longer bound to the surface states and the radiative property increases. Therefore, the adsorption of gaseous species controls the surface radiative property of TiO2/SnO2 as well as that of grain boundaries.
Conclusion
The near spherical and well distributed TiO2/SnO2 nanoparticles have been synthesized using simple aqueous solution growth techniques from the solution TTIP and SnCl2·6H2O. The material show good Photoluminescence with a strong violet emission at around 420 nm. The material undergoes a significant increase in PL intensity upon exposure of ammonia and exhibits a fast response (12 s)and recovery time (50 s) at room temperature with only 20 ppm ammonia. These results shows good response of TiO2/SnO2 nanoparticles to be used as an analyte for Luminescence based ammonia gas sensor with fast response time. The mechanism of sensing gas by measuring change in luminescent properties is very innovative and effective. This mechanism is efficient for fast sensing of very small fraction of gases.
References
M. Parthibavarman, K. Vallalperuman, S. Sathishkumar, M. Durairaj, K. Thavamani, A novel microwave synthesis of nanocrystalline SnO2 and its structural optical and dielectric properties. J. Mater. Sci.: Mater. Electron 25, 730–735 (2014)
A. Umar, Edited Metal Oxide Nanostructures and their applications (American Scientific Publishers, CA, USA, 2010)
S. Suresh Kumar, B. Venkatachalapathy, L. Sujatha, T. Subba Rao, T.M. Sridhar, Influence of H2S concentration on Mn sensitized Tin dioxide nanoparticles based sensor-an impedance spectroscopic investigation. Sensor Lett. 14(9), 949–954 (2016)
K. Govardhan, A. Nirmala Grace, Metal/Metal Oxide Doped Semiconductor Based Metal Oxide Gas Sensors—A Review. Sensor Lett. 14(8), 741–750 (2016)
Z. Fozia, Haque and Padmini Pandey, light harvesting in dye-sensitised solar cells with advance in the design of dyes. Rev. Adv. Sci. Eng. 3, 320–330 (2014)
Y. Liu, Y. Jiao, Z. Zhang, F. Qu, A. Umar, X. Wu, Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation, smart gas sensor, and supercapacitor applications. ACS Appl. Mater. Interfaces 6(3), 2174–2184 (2014)
H.J. Kim, J.W. Yoon, K.I. Choi, H.W. Jang, A. Umar, J.H. Lee, Ultraselective and sensitive detection of xylene and toluene for monitoring indoor air pollution using Cr-doped NiO hierarchical nanostructures. Nanoscale 5(15), 7066–7073 (2013)
B.S. Khened, M.V.N. Ambika Prasad, M. Sasikala, Synthesis, characterization and liquid petroleum gas sensing of ZnFe2O4 nanoribbons doped polyaniline nanocomposites. Sensor Lett. 14(8), 817–823 (2016)
I. Madhi, B. Bouzid, B. Bessaïs, Effect of annealing temperature and Sn doping on structural, morphological and NO2 detection properties of nanostructured ZnO thin films. Sensor Lett. 14(4), 389–395 (2016)
M.K. Mishra, N. Singh, V. Pandey, F.Z. Haque, Synthesis of SnO2 nanoparticles and its application in sensing ammonia gas through photoluminescence. J. Adv. Phy. 5(1), 8–12 (2016)
R.K. Kumar, M. Husain, Structural properties of SnO2 nanostructures grown by rapid thermal chemical vapour deposition at lower temperature. Mater. Focus 3, 415–420 (2014)
S. Vadivel, G. Rajarajan, Effect of Mg doping on structural, optical and photocatalytic activity of SnO2 nanostructure thin films. J. Mater. Sci. 26, 3155–3162 (2015)
K.J. Choi, H.W. Jang, One-dimensional oxide nanostructures as gas sensing materials: review and issues. Sensors 10(4), 4083–4099 (2010)
A. Kolmakov, D.O. Klenov, Y. Lilach, S. Stemmer, M. Moskovits, Nano Lett. 5, 667 (2005)
M.M. Bagheri-Mohagheghi, M. Shokooh-Saremi, The influence of Al doping on the electrical, optical and structural properties of SnO2 transparent conducting films deposited by the spray pyrolysis technique. J. Phys. D: Appl. Phys. 37, 1248 (2004)
A. Roy, S. Dige, G.G. Umarji, M.D. Shinde, S.B. Rane, U.P. Mulik, D.P. Amalnerkar, R. Chauhan, Solvothermal syntheses of cadmium sulfide nanoparticles with varying concentration of ammonia and reaction time and their effect on optical properties. Mater. Focus 4, 142–149 (2015)
D. Xiang, H. Lin, L. Li, X. Chen, J. Ma, F. Qu, Flower-shaped SnO2 nanostructures for gas sensor application. Mater. Focus 3, 325–330 (2014)
A. Umar, M.M. Rahman, S.H. Kim, Y.B. Hahn, ZnO nanonails: synthesis and their application as glucose biosensor. J. Nanosci. Nanotechnol. 8(6), 3216–3221 (2008)
J. Liu, Y. Li, X. Huang, R. Ding, Y. Hu, J. Jiang, L. Liao, J. Mater. Chem. 19, 1859 (2009)
V. Pandey, N. Singh, M.M. Malik, and F.Z. Khan, SnO2, SnO2:Mg and SnO2:Ni Nanoparticles Based Luminescence Ammonia Sensors, Mater. Focus 6(3), 359–363 (2017)
V.V. Malyshev, A.V. Pislyakov, Investigation of gas-sensitivity of sensor structures to hydrogen in a wide range of temperature, concentration and humidity of gas medium. Sens. Actuat. B 134, 913–921 (2008)
T. Jayasree, M. Bobby, S. Muttan, Sensors for detecting ammonia from the exhaled breath of renal disorder patients. Sensor Lett. 14(10), 1003–1008 (2016)
M. Abaker, A. Umar, S. Baskoutas, G.N. Dar, S.A. Zaidi, S.A. Al-Sayari, A highly sensitive ammonia chemical sensor based on α-Fe2O3 nanoellipsoids. J. Phys. D Appl. Phys. 44(42), 425401 (2011)
S.S. Taimur Athar, M. Shafi, A. Ahmed Khan, Low-temperature growth and properties of NiO nano sheets decorated with small NiO nano rods via soft chemical process. Mater. Focus 3, 378–381 (2014)
P. Rajeshwaran, A. Sivarajan, Influence of Mn doping on structural, optical and acetone gas sensing properties of SnO2 nanoparticles by a novel microwave technique. J. Mater. Sci.: Mater. Electron 26, 539–546 (2015)
F.Z. Haque, N. Singh, P. Pandey, M.R. Parra, Study of zinc oxide nano/micro rods grown on ITO and glass substrates. Optik-Int. J. Light Electron Opt. 124(20), 4167–4171 (2013)
N. Singh, F. Syed, F.Z. Haque, Ionic liquid controlled growth of zinc oxide nano particles and their fluorescence study in the presence of NH3 gas. Mater. Sci. Res. India 11(1), 27–34 (2014)
R.H. Bari, S.B. Patil, A.R. Bari, Chemically sprayed SnO2-ZnO nanocomposites thin films for ethanol gas sensor. J. Nanoeng. Nanomanuf. 3, 1–5 (2013)
W. Izydorczyk, B. Adamowicz, Computer analysis of oxygen adsorption at SnO2 thin films. Optica Applicata. 37(4), (2007)
S. Jagtap, K.R. Priolkar, Investigation on flower like ZnO and effect of processing parameters on ‘green’ ZnO gas sensors. J. Nanoeng. Nanomanuf. 4, 221–229 (2014)
M. Acuautla, S. Bernardini, M. Bendahan, L. Gallais. Ammonia sensing properties of ZnO nanoparticles on flexible substrate in Proc.8th Int. Con.on Sensing Technology, Liverpool, UK, Sep. 2–4 2014
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, N., Pandey, V., Singh, N. et al. Application of TiO2/SnO2 nanoparticles in photoluminescence based fast ammonia gas sensing. J Opt 46, 199–203 (2017). https://doi.org/10.1007/s12596-017-0404-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12596-017-0404-3