Abstract
In the current scenario of rapid conversion of tropical forests into human-dominated activities landscape, the present study was undertaken to understand the role of existing agroforestry systems in conservation of avian species. Three types of agroforestry systems namely tea gardens, homegardens and agrisilviculture were selected in the Brahmaputra North Bank Landscape of Assam, northeast India for the study. A total of 468 point counts revealed 164 bird species belonging to 51 families and 18 orders across all the three different types of agroforestry systems. Tea gardens showed significantly higher bird species richness followed by homegardens and agrisilviculture systems. As per IUCN, four species were categorized as Near Threatened and one species was categorized as Vulnerable. Out of 164 species recorded, 68.3% species were residents, 30.5% species were winter visitors and remaining were summer visitors (1.2%). Majority of the species (45.7%) were common to all the three types of selected agroforestry systems. The feeding guilds revealed that most of the species were insectivorous (50.0%) in habit. A total of 167 tree species belonging to 54 families and 24 orders was recorded across all the three different types of agroforestry systems. The highest tree species richness was recorded in the homegardens (160), followed by agrisilviculture systems (19) and least in tea gardens (11). No statistically significant correlation was found among bird species richness and patch size, tree species richness and number of bird species individuals. The selected agroforestry systems have provided shelter, nesting space, feeding and foraging grounds for birds during the winter season. This data can be useful for the policy makers, tea garden managers, homegarden owners for framing conservation policies of avian species in an era of natural wildlife habitat loss and degradation.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Tropical forests are rapidly decreasing in area and the available spaces are becoming fragmented. As a result, wild animals are becoming homeless. But they are trying to ensure their survival and propagation by being adapted to the available human-modified landscapes, viz., agroforestry systems. Since agroforestry system is emerging with shade trees amidsts various types of crop (Bhagwat et al. 2008) the adverse effect of deforestation is being subdued gradually. McNeely and Schroth (2006) have also reported that agroforestry systems reduce the house-hold use resource pressure in protected areas, extend the habitats for wildlife species, provide corridor facilities and connectivity between fragmented forest patches, thereby, highlighting the conservation value of the system. Several studies also confirmed that tree species planted in different combination and patterns in agroforestry systems (agrisilviculture, silvipasture, agrisilvipasture, homegardens, tea gardens, shelterbelts, windbreak, alley cropping, forested riparian buffers, etc.) have evidenced to provide opportunities to conserve wildlife species by contributing nesting sites, temporary refuge and migratory route, protective or escape cover against predators, access to breeding territory and food resources in all seasons and encourage beneficial species such as pollinators (Griffith 2000). Agroforestry systems are seen as tools in conjunction with appropriate conservation areas to buffer biodiversity loss, because agroforestry in some sites has 50–80% of the diversity that is comparable to natural forests and can help restrict the conversion of forests to grassland or other monospecific crops (Nobel and Dirzo 1997). Buck et al. (2004) in their review stated that agroforestry systems provide habitats for diverse populations of birds, with the greatest amount of evidence pointing towards the habitat value of shade-grown coffee and cocoa systems in Southeast Asia and Central America. Harvey et al. (2004, 2005) reported that silvopastoral systems also help to retain the regenerative capacity of the land. They stated that the presence of live fences and dispersed trees within pasture lands encourage the natural regeneration by serving as a source of seeds, attracting birds, bats and other animals that disperse seeds, providing appropriate microclimatic conditions for natural regeneration. Avian diversity in live fences was studied by Harvey et al. (2006) and reported low bird species diversity in live pollarded fences as compared to live fences with un-pruned canopies. Griffith (2000) studied the diversity and abundance of birds on the agrisilviculture systems to determine the role of agroforestry as a refuge for biodiversity conservation following fire in view of assemblages of 83 bird species. Gleffe et al. (2006) has recorded 253 nests in coffee plantations and stated that coffee plantation serves as refuge for reproductive activity and productivity of resident forest birds during the periods of widespread deforestation, lowering extinction rates. Harvey and Villalobos (2007) reported that diverse cacao and banana agroforestry systems contribute to conservation effort by serving as habitats to high numbers of bird and bat species including forest dependent and threatened species. Thus, based on the various studies mentioned above, agroforestry systems have proved to have high conservation value, due to their structural complexity, high floristic diversity and are similar to forest ecosystems (Schroth et al. 2004).
Homegardens are a part of age old traditional practices of local people living in Assam, northeast India. In Assam, almost all houses have a component of homegardens, tea gardens and agrisilviculture systems either singly or in various combinations. The homegardens are multi-strata agroforestry systems made up of four to five vertical canopy layers (Das and Das 2005; Saikia et al. 2012). These provide habitat and opportunity of foraging in homegardens for birds belonging to different food guilds (insectivore, omnivore, carnivore, granivore, frugivore and piscivore). Leakey (1998) reported that the multistrata Damar agroforests of Sumatra contain over 50% of all the regional pool of resident tropical birds, most of the mammals and about 70% of plant species. On the other hand, tea gardens comprising of water bodies, secondary growth forests and tea crops intermingled with shade trees form an ideal habitat for birds (Ahmed and Dey 2014). The proximity of tea gardens to the reserve forests increases the chances of birds to use them for forage or local movement. Bird species diversity in shaded plantations is always reported to be significantly higher than in other types of monoculture cultivation (Wunderle and Latta 1996; Petit et al. 1999; Tejeda-Cruz and Sutherland 2004). The agrisilviculture systems are composed of agricultural crops and trees providing shade, fuel, timber, fruits etc. These trees are planted either along the boundary or interspersed with agricultural crops. The habitat heterogeneity provided by trees, hedges etc. in the agricultural fields is considered beneficial for increasing diversity and density of birds in paddy field (Brenner 1991).
Therefore, the present study was designed to make an inventory of bird species occurring in selected agroforestry systems like homegardens, tea gardens and agrisilviculture systems to highlight their role and conservation value in terms of avian fauna conservation in era of habitat loss and degradation in Assam, India.
Materials and Methods
Study Site
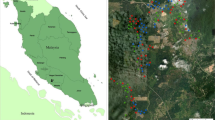
The present study was carried out in two districts viz. Lakhimpur and Sonitpur (Fig. 1a) as these districts had the desired agroforestry systems (tea gardens, homegardens and agrisilviculture systems) and were easily accessible by road. In Lakhimpur district, five villages viz. Naharani, Baligaon, Jajori, Bhogpur and Drupang (Fig. 1b) and in Sonitpur district, two villages viz. Chengeli Mari Gaon and Tarajan (Fig. 1c) which had homegardens and agriculture systems were chosen. Four tea gardens viz. Dufflaghar, Borhmajan, Boroi and Borgang (Fig. 1d) were also chosen from Sonitpur district. From each of these villages, nine homegarden plots and nine agrisilviculture systems plots were chosen. Thus, a total of 130 plots were selected comprising of 63 each for homegardens and agrisilvicuture systems plots and four tea garden plots to assess the avian diversity in these two districts. The entire study area comprised of 3955.9 ha wherein agrisilviculture, tea garden and homegarden systems constituted around 1044.8, 2824.5 and 86.7 ha respectively of the total area. Out of the whole study area (3955.9 ha), the sampling was done in 47.0 ha area wherein 15.8, 27.1 and 4.0 ha area were surveyed respectively as agrisilviculture systems, tea gardens and homegardens (Table 1).
Study Area. a Map showing district locations of study area. b Google map showing agrisilviculture systems, homegardens and adjoining reserve forest in Lakhimpur district. c Google map showing agrisilviculture systems, homegardens and adjoining Tiger Reserve in Sonitpur district. d Google map showing tea gardens and adjoining reserve forest in Sonitpur district
Lakhimpur district has a geographical area of 2967 sq. km. Geographically the district lies in between 26°48′–27°53′N and 93°42′–94°20′E. The northern side of the district is covered by tropical evergreen to semi-evergreen reserve forests (Ranga, Kakoi and Dulung) and the river Brahmaputra flows along the southern border. The temperature reaches a maximum of 35 °C during June-July and falls to a minimum of 8 °C in December-January. Average annual rainfall is around 2830 mm as against the average value of 2300 mm for the state as a whole (Hilodhari and Baruah 2011).
The district Sonitpur has a total area of 5103 sq. km and lies 100 m above the mean sea level. It is surrounded by Arunachal Pradesh in north, the Brahmaputra River and Morigaon, Nagaon, Jorhat and Golaghat districts in south, Darrang district in the west, Lakhimpur district in the east. Sonitpur district lies on the northern bank of the river Brahmaputra within 26°2′–26°6′N and 92°2′–93°5′E. Land use in the district is divided primarily among tropical semi-evergreen, moist deciduous, riverine forest, grassland, agricultural land and tea garden. The temperature ranges from 7 °C in January to as high as 38 °C in May. The annual rainfall in the district is 2393 mm (Dutta et al. 2010). Description of selected agroforestry systems is as follows:
-
1.
Homegarden agroforestry system: Homegardens are composed of trees like Bombax ceiba, Tamarindus indica etc. in emergent layer, Areca catechu, Artocarpus heterophyllus, Cassia siamea, Mangifera indica etc. in the canopy layer, Citrus sp., Cocos nucifera, Litchi chinensis in understory layer, Hibiscus rosa sinensis, Jasminum grandiflorum etc. in shrub layer and medicinal plants and herbs in herb layer (Das and Das 2005). These systems also have a pond, bamboo groves and thick forest groves in the backyard of the house.
-
2.
Tea garden agroforestry system: These include different habitats like water bodies, ecotone zones (the zone where the tea gardens end and the forest starts abruptly), secondary growth forests and tea gardens consisting of two-tier system (the shrub layer of Camellia sinensis (tea crops) and canopy layer of shade trees like Albizia procera, Acacia odoratissima, Melia azedarach etc. (Ahmed and Dey 2014).
-
3.
Agrisilviculture agroforestry systems: These are a mixture of agricultural crops and tree species like Mangifera indica, Dillenia indica, Bombax ceiba and bamboo groves etc. either on the boundary or intermixed with agricultural crops depending on the tree species.
All the three agroforestry systems had human habitation consisting of around 30–40 houses in and around their vicinities. The inhabitants in the study area belong to different ethnic groups like Assamese, Nepalis, Bengalis, Adivasis-tea garden tribes, Muslims, Mishing and Bodo.
Avian Diversity Assessment
Fixed-radius, point-count method (Bibby et al. 2000) of radius ranging from 10-20 m was employed to assess the avian diversity. In each village, two trails were laid covering nine homegarden plots and two trails were laid covering nine agrisilviculture system plots. Similarly, six trails were laid in each individual tea garden plots. More number of trails were laid in tea garden plots as the area of each tea garden plot was more than 500 ha (Table 1). Each trail was approximately 1 km in length. Each sampling trail had nine permanent point-count stations, making a total of 468 point counts (126, 216 and 126 point counts in agrisilviculture systems, tea gardens and homegardens respectively) (Table 1). Each two point count stations on the same trail had a minimum distance of 100 m between them and were monitored from 0600 to 0930 h. In homegardens, the sampling lines were laid on either side of the house (front and back), in agrisilviculture systems they were laid on either side of the accessible roads and in tea gardens they were laid on accessible roads. All sampling lines were at least 10 m from the edge of the habitat in homegardens and agrisilviculture systems. Each trail was sampled four times in the winter season from November 2013 to February 2015. Bird species observed visually were recorded for 10 min within a radius of 10 m from the point count station in homegardens and 20 m from the point count station in tea gardens and agrisilviculture systems. Birds flying above the canopy or across the landscape were not counted (Greenler and Ebersole 2015). A 7 × 50 binocular (Nikon) was used to observe birds. The opportunistic counts during other times of the day were also included. Identification and classification of birds into different feeding guilds were done using standard field guides (Grimmett et al. 2001; Ali 2002).
Vegetation Diversity Assessment
Vegetation was studied using quadrat method. Quadrats of 10 m × 10 m size for tree enumeration were laid randomly in different agroforestry systems of the study area (Muller-Dombois and Ellenberg 1974). Within each quadrat, an inventory of all the trees with girth at breast height (GBH) ≥10 cm (1.37 m above ground) with their common name was made. Plant species in the homegardens were identified by consulting different floras (Kanjilal et al. 1934–1940) and herbarium of the Botanical Survey of India, Eastern Circle, Shillong. A list of tree species richness of each agroforestry system under study was made.
Factors Affecting Avian Species Diversity
To analyze the effect of patch size on the bird species diversity and number of bird species individuals, the patch size of the selected agroforestry systems in the whole study area was derived using extraction tool of Google Earth. More details on the patch size of the selected study area have been highlighted in Table 1.
Data Analysis
The variables like tree species richness, the number of bird species individuals, bird species richness and patch size were subjected to normality tests. It was found that tree species richness, number of bird species individuals and patch size were normally distributed and therefore, were further assessed using parametric analysis (one-way ANOVA) (Zar 1999). This was followed by Turkey’s post hoc comparison. ANOVAs were carried out only when the assumptions like variable independence, normal distribution having equal variance were met by the variables. The bird species richness variable was not normally distributed and therefore, a non-parametric analysis was performed (Kruskal–Wallis). This was followed by Mann–Whitney U test which is used for post hoc comparison (Zar 1999). p < 0.05 was considered statistically significant for all the above-mentioned analyses. In order to explain the variation in bird species richness and number of bird species individuals across different agroforestry systems correlation analysis was applied. To evaluate the relationship between bird species richness, tree species richness, number of bird species individuals and patch size, non-parametric rank correlations (Spearman rank correlation matrix) was used. Similarly, to evaluate relationship between variables viz. number of bird species individuals and patch size, parametric rank correlation (Pearman’s rank correlation) was used. All analyses were run on SPSS (version 16.0 for Windows). For correlation analysis purpose, p < 0.01 was considered statistically significant. Means and standard errors of mean (±SE) are given in text and tables.
Results
Occurrence of Avian Species Diversity
A total of 164 bird species and 15,946 individuals belonging to 51 families and 18 orders were recorded across all the three different types of agroforestry systems (Table 2). Among them, highest, 68.3% species were resident followed by winter visitors, 30.5% and summer visitors, 1.2% (Fig. 2). The highest bird species richness was recorded in the homegardens (128) followed by tea gardens (123) and agrisilviculture systems (101) (Table 2). The three agroforestry systems revealed statistically significant difference in bird species richness among them (Kruskal–Wallis, X2(2) = 9.732, p = 0.008) (Table 3). The bird species richness mean rank scores were 16.0, 9.7 and 5.6 for tea gardens, homegardens and agrisilviculture systems respectively. The Mann–Whitney U test further clarified that the bird species richness in tea gardens was statistically significantly higher than the agrisilviculture system (U = 0.000, p = 0.008) and homegardens (U = 2.000, p = 0.023). Similarly, the bird species richness in homegardens was statistically significantly higher than the agrisilviculture systems (U = 11.000, p = 0.085). Thus, overall it can be concluded that tea gardens (80.8 ± 5.3) showed significantly higher bird species richness, followed by homegardens (63 ± 3.4) and least in agrisilviculture systems (50.7 ± 4.4) (Table 3).
The highest number of bird species individuals was recorded in the tea gardens (7258), followed by agrisilviculture systems (5030) and least in homegardens (3658) (Table 3). The number of bird species individuals recorded among the three agroforestry systems varied significantly (One-way ANOVA, F2,15 = 93.583, p = 0.000). Post-hoc pair-wise comparisons revealed that tea gardens had significantly higher number of bird species individuals (1815.0 ± 136.0, n = 4) as compared to that of agrisilviculture systems (719.0 ± 41.0, n = 7) and homegardens (523.0 ± 42.0, n = 7) (Table 3). However, difference noted between agrisilviculture systems and homegardens (p = 0.08) was statistically non-significant.
Out of the total patch size of 3955.9 ha of all the selected agroforestry systems in the study site, the highest patch size was found to be of tea gardens (2824.5 ha), followed by agrisilviculture systems (1044.8 ha) and least patch size was of homegardens (86.7 ha) (Table 1). The patch size derived between the three agroforestry systems varied significantly (One-way ANOVA, F2,15 = 71.853, p = 0.000). Post-hoc pair-wise comparisons revealed that tea gardens had significantly higher patch size (706.1 ± 86.9, n = 4) as compared to that of agrisilviculture systems (149.3 ± 31.9, n = 7) and homegardens (12.4 ± 1.8, n = 7) (Table 3).
Occurrence of Tree Species Diversity
A total of 167 tree species belonging to 54 families and 24 orders was recorded across all the three different types of agroforestry systems. The highest tree species richness was recorded in the homegardens (160), followed by agrisilviculture systems (19) and least in tea gardens (11). The tree species richness among the three agroforestry systems varied significantly (One-way ANOVA, F(2,15) = 238.30, p = 0.00). Post-hoc pair-wise comparisons revealed that homegardens had significantly higher tree species richness (81.4 ± 4.0, n = 7) as compared to that of agrisilviculture systems (12.0 ± 0.5, n = 7) and tea gardens (8.0 ± 0.0, n = 4) (Table 3). The difference noted in tree species richness between tea gardens and agrisilviculture systems was statistically non-significant (p = 0.619).
Occurrence of Threatened Avian Species
According to the IUCN status, four species were categorized as Near Threatened viz. Ashy-headed Green Pigeon (Treron phayrei) sighted in tea gardens adjacent to Behali reserve forest which is an Important Bird Area (IBA) and biodiversity area (Birdlife International 2016), Great Hornbill (Buceros bicornis) sighted in homegardens while Alexandrine Parakeet (Psittacula eupatria) was sighted in homegardens as well as agrisilviculture systems in fringe areas of Nameri Tiger Reserve and Red-breasted Parakeet (Psittacula alexandri) was sighted in all the three systems (Table 2), irrespective of their distance from the forest. One species viz. Lesser Adjutant (Leptoptilos javanicus) sighted in homegardens and agrisilviculture systems was categorized as Vulnerable as per IUCN status (Table 2). This species was also found at all the sites irrespective of their distance from the forest.
Factors Affecting Avian Species Diversity
To explain the bird species richness in different agroforestry systems, the correlation of bird species richness was analyzed against tree species richness and patch size. No statistically significant correlation was found among bird species richness and patch size (rs(16) = 0.141, p = 0.576), tree species richness (rs(16) = −0.293, p = 0.238) and number of bird species individuals (rs(16) = 0.0263, p = 0.291). However, patch size was significantly and positively associated with number of bird species individuals (r = 0.909, n = 18, p = 0.000), which explains the variation in number of bird species individuals between the different agroforestry systems. Therefore, the number of bird species individuals increased with the increasing patch size of agroforestry system.
Occurrence of Common and Exclusive Avian Species
Among the recorded species in different agroforestry systems of the study area, 45.7% species were common to all the three agroforestry systems, while 11.0% species were common in both tea gardens and homegardens only, 9.8% species were common in homegardens and agrisilviculture systems only and 3.7% species were common only in tea gardens and agrisilviculture systems. But, 15.2%, 12.2% and 3.0% species were restricted only to tea gardens, homegardens and agrisilviculture systems respectively (Fig. 3).
Occurrence of Avian Species Based on Feeding Habits
With regard to the avian feeding guilds in the three agroforestry systems studied, out of 164 species, the highest 50% were insectivorous, followed by 16.5% omnivorous, 11.6% frugivorous, 7.9% carnivorous, 6.7% piscivorous, 4.3% granivorous and 3.0% nectarivorous. As per feeding guilds and habit of species in tea gardens, out of 123 species recorded, the highest 55.3% were insectivorous, followed by 16.3% omnivorous, 12.2% frugivorous, 8.9% carnivorous, 3.3% granivorous, 2.4% nectarivorous and 1.6% piscivorous. In homegardens, out of 128 species, the highest 50.0% were insectivorous followed by 15.6% omnivorous, 11.7% frugivorous, 7.8% piscivorous, 5.5% carnivorous and granivorous and 3.9% nectarivorous. According to the feeding guilds observed in agrisilviculture systems, out of 101 species, 40.6% were insectivorous, followed by 23.8% omnivorous, 11.9% frugivorous, 8.9% piscivorous, 6.9% granivorous, 5.0% carnivorous and 3.0% nectarivorous (Fig. 4).
Discussion
Structurally complex agricultural habitats serve as substitute habitats for many wildlife species when the natural forests are depleted (Schelhas and Greenberg 1996) (Table 4). The present study records highest bird species richness in tea gardens. Similar avian diversity studies in tea gardens across the globe showed a record of 86 species in Sri Lanka (Kottawa-Arachchi and Gamage 2015), 88 species in Cachar valley, Assam, 59 species in southwestern China (Lin et al. 2012), which are lower than the current study. The reason for tea gardens supporting high bird species diversity may be because of diverse habitat of the same. The tea gardens constitute of shade trees like Albizia procera, Melia azedarach, Acacia odoratissima, Acacia procera and Acacia lenticularis forming the canopy layer and Camellia sinensis forming the shrub layer. There are also streams running criss-cross in the tea gardens which are lined by trees like Samanea saman, Terminalia arjuna, Terminalia bellirica, Ficus religiosa etc. The tea gardens, therefore, are two-layered vegetation structure with water bodies which attract the birds. Moreover, these tea gardens are adjacent to reserve forests viz. Behali and Singlijan reserve forests and therefore, may also serve as corridors for these species (Fig. 1d) (Estrada et al. 1997; Perfecto et al. 2003). This high species diversity recorded may also be on account of the largest patch size of tea gardens in the study area (Bellamy et al. 1996; Findlay and Houlahan 1997). The higher number of point counts stations established in tea gardens as compared to that of other agroforestry systems studied may also be one of the reasons for recording high species diversity in tea gardens.
Sidhu et al. (2010) reported higher abundance of birds in the tea gardens of Anamalai as compared to that of the surrounding forest. This supports the present study wherein the number of bird species individuals in tea gardens is high. Other reasons accounting for recording more number of individuals in tea gardens are patch size (largest as compared to other agroforestry systems studied) and biasedness in survey (higher number of point counts established in tea gardens as compared to that of other agroforesty systems studied).
Another reason for recording higher species diversity and number of individuals in tea gardens may be the winter (dry) season in which the survey was conducted. Out of a total of 50 species of winter visitors recorded in the study area, 42% were found to be present in tea gardens. Early and late winter season might be the time witnessing increase in density and diversity of birds as reported by (Greenberg et al. 1997a) in coffee plantations in Mexico. Winter visitors can comprise up to 70% of foliage-gleaning insectivores (Greenberg et al. 1997b). Out of the total 123 bird species found in the tea gardens in the current study, 55.3% belong to insectivorous feeding guild. This may be another possible reason for recording such high avian diversity in the tea gardens in the study area. The high record of insectivorous species in tea gardens may be due to the high availability of insects and tea pests which serve as a high value food resource for the birds in the area. This study reports higher insectivorous bird species than the study of Sinu (2011), who has reported 38 insectivorous bird species in the tea gardens of Sub-Himalayan plains of northeast India. High percentage of insectivores recorded might be due to the presence of a high number of infested plant species including tea plant (Sinu 2011). The scattered pattern of trees in tea garden may also serve as a good perching site for these insectivorous birds which is one of the important factor in capturing insects.
A complex forest structure provides different niches for wildlife than simple-structured vegetation (Lindberg et al. 1998). Similarly, homegardens are known to be multistrata and multispecies form of land use (Peyre et al. 2006) supporting higher number of bird species only next to tea gardens as is reflected in this study. Homegardens have greater diversity of shade and food providing trees for the birds. The fruit yielding species like Artocarpus heterophyllus, Ananas comosus, Litchi chinensis, Mangifera indica (Saikia et al. 2012), Carica papaya, Terminalia chebula, Musa sp., Syzygium sp., Zizyphus sp., Ficus sp., etc., nectar flowering species like Bombax ceiba, Erythrina indica, Moringa oleifera, Bahunia variegata, Callistemon citrinus, Delonix regia, etc., ponds supplying different varieties of fishes viz. Cirrhinus mrigala, Ctenopharyngodon idella, Hypophthalmichthys molitrix etc., are commonly found in homegardens in the study area. Thus, homegardens cater to the needs of a diverse species of birds having different foraging, nesting and perching site preferences. Compared to homegardens the other two systems have a more open form of tree cover and low species richness. The latter, therefore, not only provide less resources (Harvey et al. 2006) but also greater exposure to raptors (Estrada et al. 1997) and to extreme weather (Wilson et al. 2005). This may explain the higher bird and tree species diversity in homegardens as compared to that of agrisilviculture systems.
In the present study, agrisilviculture system presents the lowest bird species diversity in comparison to the other two systems. But this is higher than the study conducted by Mandan et al. (2014) who recorded 71 species in agrisilviculture systems of Ratnagiri district in Maharashtra. The agrisilviculture plots with various cropping patterns, viz. rice (Oryza sativa) crop in monsoon, Urad bean (Vigna mungo), Mustard seeds (Brassica juncea) etc. in winter and the associated pests serve as food reserves for gramnivorous, nectivorous and insectivorous species (Fujioka et al. 2010). This amy also be one of the reasons for recording high species diversity in our study area. Lower species diversity is reported in the field margins of agrisilviculture systems in Quebec, Canada (42 species) and Central Mexico (61 species) (Jobin et al. 2001; Zuria and Gates 2013). In both these studies the authors have recorded bird species which are present in the field margins composed of three different vegetation patterns only, whereas in our study, we have recorded the bird species present in the agricultural fields as well as field margins i.e. agrisilviculture system as a whole. This may be one of the reasons for the low number of bird species diversity in their studies.
Studies conducted on vegetation diversity in homegardens revealed tree species diversity (107) in Lakhimpur, Assam and Papumpare, Arunachal Pradesh (Zimik et al. 2012), 142 in Golaghat and Jorhat, Assam (Saikia et al. 2012), 71 in Barak valley, Assam (Devi and Das 2013) which is lower than the tree species richness recorded in the present study. This higher species diversity might occur due to the regional and homegarden owner’s preference difference. In the current study there were 11 tree species in the tea gardens, the lowest among the three agroforestry systems. Kessler et al. (2005) in a similar study reported lowest tree species diversity in cacao plantation (5) as compared to primary and secondary forests in Central Sulawesi, Indonesia. The low tree diversity in tea gardens in the current study also reflects the objective of the tea garden owner who plants trees only to provide shade for the tea plants which are main commercial product, whereas in homegardens the main objective of the owner is to plant trees which are not only economically beneficial but also cater to the daily household needs (Salam et al. 2000). Thus, increasing the tree diversity.
In the present study, we recorded Wreathed Hornbill (Rhyticeros undulatus) in addition to Great Hornbill in homegardens and Alexandrine Parakeet in homegardens and agrisilviculture systems adjacent to Nameri Tiger Reserve. All the three species are reported to be common residents found throughout the Nameri Tiger Reserve (Barua and Sharma 2005; Das and Deori 2010). During our surveys, we have occasionally seen both the species of Hornbill in a flock of 4–5 especially during the evenings. It seems that these birds use the homegardens as a stop over while returning back to the forest in the evening. Krishna et al. (2012) in their study reported that Wreathed Hornbills were sighted in disturbed and fragmented tropical forests of Arunachal Pradesh. Certain species like Brown Hawk Owl, Great Hornbill and Rufous Woodpecker are mainly forest specialist species. However, these species were recorded only in the homegardens and tea gardens adjacent to reserve forests viz. Behali and Singlijan reserve forests and Nameri Tiger reserve. Therefore, this study exhibits a good amount of evidence to the edge effect theory, especially in agroforestry systems which are in close proximity to the forests and also explains the high avian diversity. This may mean that the forest specialist birds may make use of this edge as a corridor or a space for finding food, shelter during the diurnal activity. Edge effect may have a positive effect on insect abundance and diversity. This statement is supported by the study conducted by Greenberg et al. (1997a) and Roberts et al. (2000) in coffee plantations where only edge specialist species were most common but the forest specialist species were absent in coffee plantations. Raman (2006) has also found typical rainforest bird species in coffee and cardamom plantations adjoining rainforest fragments. Morris et al. (2010) in their study in managed pine forest landscape in United States have witnessed forest edges to provide foraging opportunities for aerial-hunting bat species. Leptoptilos javanicus has been reported to be found in agricultural fields (Subaraj and Lok 2009) and nesting is seen in Bombax ceiba trees in Nameri Tiger Reserve (Barua and Sharma 2005) and Kaziranga National Park and its adjoining areas (Barua and Sharma 1999) and Mangifera indica (Choudhury 1991) which are commonly found trees in homegardens and agrisilviculture systems in the present study.
In the present study, the bird species richness is not affected by the tree species richness. But, in a similar study Osborne (1984), Harvey and Villalobos (2007) and Pulido-Santacruz and Renjifo (2011) found that species richness is positively correlated with vegetation structure and composition. So, this is in contradiction with the present findings. Scarcity of sensitive or tree diversity dependent species like frugivorous and nectarivorous feeding guilds (14.6%) and abundance of bird species independent of tree diversity like insectivorous, omnivorous, piscivorous, granivorous feeding guilds (85.4%) may be one of the reasons for no relation between tree species richness and bird species richness. This study reports the results confined to winter season alone and therefore, may not be able to draw a complete picture of correlation between different variables. In the present study, no relationship between bird species richness and patch size was found. This conforms with the studies conducted by Osborne (1984), Estrada et al. (1997) and Schmiegelow et al. (1997). Similar studies have also shown that species diversity is influenced by variables like distance from the forest (Estrada et al. 1997; Perfecto et al. 2003), density of roads (Findlay and Houlahan 1997), distance from roads (Eigenbrod et al. 2008), nature of surrounding landscape (Bellamy et al. 1996), human presence (Perfecto et al. 2003; Pautasso 2007) and management practices (Pulido-Santacruz and Renjifo 2011) which might have a correlation with bird species diversity in the present study. This study reports a correlation between number of bird species individuals and patch size. Studies conducted by Diamond (1970) and Jaenike (1978) on birds and insects also approve of this result.
It is also evident from this study that all the three agroforestry systems provide habitat suitable for the generalist bird species. All the three agroforestry systems are successful in providing space for birds which have specific habitat needs on account of their different zonation, vegetation structure and composition. We have recorded 45.7% habitat generalist species in our study. Compared to this study, Ahmed and Dey (2014) reported 34.1% generalist species in the tea gardens of Cachar district. The reason for recording low percentage of generalist species in their study may be because of the restriction of their study only in the tea garden whereas in our study two more agroforestry systems were considered apart from tea garden. This result also supports the synthesis of 53 published studies done by Scales and Marsden (2008) which stated that the agroforestry systems tend to support more of generalist species rather than specialists or rare species.
Among the 50 species of winter visitors recorded in this study, 80% were insectivorous. The study is conducted during the winter season, which supports the winter migrants. The winter migrants are known to survive by consuming an enormous amount of insects like large swarms of termites, beetles and arthropods like ants etc. (Sherry and Holmes 1995) and this may explain the high percentage of insectivorous birds recorded in this study. The study also shows a good representation of all categories of feeding guilds which may be possible because of the diverse habitats of the agroforestry systems, providing structural and compositional complex tree species, collective or scattered fruiting indigenous tree species, annual crops, water sources, etc. which provide food resource for birds belonging to various feeding guilds.
The most dominant guild in tea gardens in this study is insectivorous species followed by omnivorous species which conforms the study conducted in similar habitat by Githiru et al. (2009) in Kenya and Kottawa-Arachchi and Gamage (2015) in Sri Lanka. Similar studies conducted by Calvo and Blake (1998) in shaded coffee plantations in Guatemala and by Ahmed and Dey (2014) in the tea garden of the Cachar valley in Assam recorded higher abundance of omnivorous species than insectivorous species. The possible reasons for sighting high percentage of insectivorous birds is as already mentioned earlier. The presence of forests in the fringe areas and the labor colonies in tea gardens supplying different kind of food resources to which the omnivorous species are adaptable is one of the reasons for recording good presence of omnivorous species. The feeding guilds like frugivores, carnivores, granivorous, nectarivorous and piscivorous are very meagerly represented in the present study. A similar study conducted by Kottawa-Arachchi and Gamage (2015) in St. Coombs tea garden in Sri Lanka also shows the lesser presence of granivorous, nectarivorous and frugivores guilds. The low representation of these feeding guilds may be due to the presence of exotic species used as shade trees which mostly have unpalatable leaves and fruits as compared to the fruiting and palatable native species which are sparsely distributed. So such exotic trees temporarily support the frugivorous birds (Kottawa-Arachchi and Gamage 2015). This may explain the reason of a slightly higher presence of frugivorous species as compared to the other meagerly represented guilds. This study contradicts the study made by Wunderle and Latta (1996) as regards to the record of more nectarivorous species in shaded coffee due to the presence of flowering Inga vera—a pre-dominant species of over-story in the shaded coffee in the La Vega province of the Dominican Republic. The only available source of food for the granivorous birds are the labor quarters in the tea gardens and thus, explains the record of lowest percentage of granivorous birds as compared to homegardens and agrisiviculture systems which have abundant sources of grains. In the present study, piscivorous species are recorded to be the lowest in tea gardens as compared to the other two agroforestry systems studied. One of the reasons for this observation may be the streams running across the tea gardens which may be polluted with pesticides that are sprayed on tea bushes. These streams also may carry the factory effluents which may influence the fish availability negatively. Daniels (2003) has stated that heavy rainfall might wash-off most of the chemical pesticides used in the tea garden from the tea leaf or soil surface and reach the water bodies affecting the aquatic fauna within the habitat.
The homegardens with their complex and high structural and compositional diversity provides ample of niche and microhabitat for the invertebrates (Surasinghe and De Alwis 2010) and anthropods like ants, spiders and beetles which, according to Lemessa et al. (2015) are found in good numbers in tree-rich homegardens. It is also well known that traditional homegardens usually grow vegetables and other food crops organically, which means, no pesticides and insecticides are used and hence, forms a natural abode for the invertebrates. The high availability of protein rich food source for birds may be one of the reasons for such high presence of insectivorous birds in homegardens. The present finding contradicts with the study of (Wijesundara and Wijesundara 2014) as they reported more abundance of omnivores in homegardens adjacent to the Dekinda Forest Reserve, Balana in Srilanka. The reason for such difference may be due to the study sites. In Sri Lanka, the study was conducted adjacent to reserve forests alone, but in our case, the study sites are spread well across the two districts of Assam and only two villages are adjacent to forest. Another reason for such difference may be the season during which the study was carried out. The high number of insectivores and comparatively low number of frugivores and nectivores in homegardens also suggest that the introduction of exotic species for high timber yield by the homegarden owners may have affected the density of native plants which are good food sources for frugivores and other plant eating bird species like nectarivores (Wijesundara and Wijesundara 2014). In homegardens, the piscivorous species are recorded due to the presence of ponds containing fish reared by the homegarden owners for self-use.
The agricultural fields are most abundant with insects and pests belonging to groups of Auchenorrhyncha (cicadas, leafhoppers, treehoppers, plant hoppers), Coelptera (beetles, weevils), Heteroptera (bugs) etc. (Karim and Riazuddin 1999) which are preferential food items of insectivorous farmland birds (Moreby and Southway 1999), thus, validating the present result. Dissimilar results were obtained by Mandan et al. (2014) who reported 29.7% omnivorous and insectivorous species in agrisilviculture fields of Dapoli, Ratnagiri. The presence of low-density native tree species like Mangifera indica, Bombax ceiba, Syzygium cumini, Dillenia indica etc. in agrisivicultural systems support the frugivorous birds and this may explain recording of frugivorous feeding guilds in agrisilviculture system. The wet paddy fields are sources of fishes and grains which explains the recording of good percentage of piscivorous and granivorous birds in agrisilviculture systems.
From the present study, it can be concluded that the selected agroforestry systems have an important role in conservation of native and winter migratory bird species. In addition, it also provides secure and alternative habitat for avian species in era of habitat loss and degradation for sustaining their life.
References
Ahmed, A., and M. Dey. 2014. A checklist of the winter bird community in different habitat types of Rosekandy tea estate of Assam, India. Journal of Threatened Taxa 6(2): 5478–5484.
Ali, S. 2002. The book of Indian birds, 13th ed. Oxford: Oxford University Press.
Bakermans, M.H., A.D. Rodewald, A.C. Vitz, and C. Rengifo. 2012. Migratory bird use of shade coffee: The role of structural and floristic features. Agroforestry Systems 85: 85–94.
Barua, M., and P. Sharma. 1999. Birds of Kaziranga National Park, India. Forktail 15: 47–60.
Barua, M., and P. Sharma. 2005. The birds of Nameri National Park, Assam, India. Forktail 21: 15–26.
Bellamy, P.E., S.A. Hinsley, and I. Newton. 1996. Factors influencing bird species numbers in small woods in south-east England. Journal of Applied Ecology 33(2): 249–262.
Bhagwat, S.A., K.J. Willis, H.J. Birks, and R.J. Whittaker. 2008. Agroforestry: A refuge for tropical biodiversity? Trends in Ecology & Evolution 23(5): 261–267.
Bibby, C.J., D.A. Hill, N.D. Burgess, and S. Mustoe. 2000. Bird census techniques. London: Academic Press.
BirdLife International 2016. Important Bird and Biodiversity Area Factsheet: Behali Reserve Forest. Downloaded from http://www.birdlife.orgon 14/01/2016.
Brenner, L. 1991. Organic agriculture is for the birds. Journal of Pesticide Reform 11(4): 20–22.
Buck, L.E., T.A. Gavin, D.R. Lee, N.T. Uphoff, D.C. Behr, L.E. Drinkwater, W.D. Hively, and F.R. Werner. 2004. Ecoagriculture: A review and assessment of its scientific foundations. Ithaca, USA: Cornell University.
Calvo, L.A., and J. Blake. 1998. Bird diversity and abundance on two different shade coffee plantations in Guatemala. Bird Conservation International 8(3): 297–308.
Choudhury, A. 1991. Bird observations from Sibsagar district, Assam, India. Forktail 6: 35–42.
Daniels, R.J. 2003. Impact of tea cultivation on anurans in the Western Ghats. Current Science 85(10): 1415–1422.
Das, N., and S. Deori. 2010. The birds of Nameri National Park-Assam, India: An annotated checklist. Bird Populations 10: 37–55.
Das, T., and A.K. Das. 2005. Inventorying plant biodiversity in homegardens: A case study in Barak Valley, Assam, Northeast India. Current Science 89(1): 155–163.
Devi, N.L., and A.K. Das. 2013. Diversity and utilization of tree species in Meitei homegardens of Barak Valley, Assam. Journal of Environmental Biology 34: 211–217.
Diamond, J.M. 1970. Ecological consequences of island colonization by South Pacific birds. Volume 2. The effect of species diversity on total population density. Proceedings of the National Academy of Sciences, USA 67: 1715–1721.
Dutta, J., M. Nath, M. Chetia, and A.K. Misra. 2010. Monitoring of flouride concentration in ground water of small tea gardens in Sonitpur district, Assam, India: Correlation with physico-chemical parameters. International Journal of ChemTech Research 2(2): 1199–1208.
Eigenbrod, F., S.J. Hecnar, and L. Fahrig. 2008. Accessible habitat: An improved measure of the effects of habitat loss and roads on wildlife populations. Landscape Ecology 23: 159–168.
Estrada, A., R. Coates-Estrada, and D.A. Meritt. 1997. Anthropogenic landscape changes and avian diversity at Los Tuxtlas, Mexico. Biodiversity and Conservation 6: 19–43.
Findlay, C.S., and J. Houlahan. 1997. Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conservation Biology 11(4): 1000–1009.
Fujioka, M., S.D. Lee, M. Kurechi, and H. Yoshida. 2010. Bird use of rice fields in Korea and Japan. Waterbirds 33(sp1): 8–29.
Githiru, M., S. Karimi, and T. Imboma. 2009. Unilever Kenya Ltd. (Kericho): Avifaunal Assessment Report. Unilever Tea Kenya Ltd., Nairobi 15–27.
Gleffe, J.D., J.A. Collazo, M.J. Groom, and L. Miranda-Castro. 2006. Avian reproduction and the conservation value of shaded coffee plantations. Ornitologia Neotropical 17(2): 271–282.
Greenberg, R., P. Bichier, and J. Sterling. 1997a. Bird population in rustic and planted coffee plantations of eastern Chiapas, Mexico. Biotropica 29(4): 501–514.
Greenberg, R., P. Bichier, A.C. Angon, and R. Reitsma. 1997b. Bird populations in shade and sun coffee plantations in central Guatemala. Conservation Biology 11(2): 448–459.
Greenler, S.M., and J.J. Ebersole. 2015. Bird communities in tropical agroforestry ecosystems: An underappreciated conservation resource. Agroforestry Systems 89(4): 691–704.
Griffith, D.M. 2000. Agroforestry: A refuge for tropical biodiversity after fire. Conservation Biology 14: 325–326.
Grimmett, R., C. Inskipp, and T. Inskipp. 2001. Pocket guide to the birds of the Indian subcontinent. Oxford: Oxford University Press.
Harvey, C.A., A. Medina, D.M. Sanchez, S. Vilchez, B. Hernandez, J.C. Saenz, J.M. Maes, F. Casanoves, and F.L. Sinclair. 2006. Patterns of animal diversity in different forms of tree cover in agricultural landscapes. Ecological Applications 16(5): 1986–1999.
Harvey, C.A., and J.A.G. Villalobos. 2007. Agroforestry systems conserve species-rich but modified assemblages of tropical birds and bats. Biodiversity Conservation 16: 2257–2292.
Harvey, C.A., C. Villanueva, J. Villacis, M. Chacon, D. Munoz, M. Lopez, M. Ibrahim, R. Gomez, R. Taylor, J. Martinez, A. Navas, J. Saenz, D. Sanchez, A. Medina, S. Vilchez, B. Hernandez, A. Perez, F. Ruiz, F. Lopez, I. Lang, and F.L. Sinclair. 2005. Contribution of live fences to the ecological integrity of agricultural landscapes. Agriculture, Ecosystems & Environment 111(1–4): 200–230.
Harvey, C.A., N.I.J. Tucker, and A. Estrada. 2004. Live fences, isolated trees, and windbreaks: Tools for conserving biodiversity in fragmented tropical landscapes. In Agroforestry and biodiversity conservation in tropical landscapes, ed. G. Schroth, G.A.B. Da Fonseca, C.A. Harvey, C. Gascon, H.L. Vasconcelos, and A.-M.N. Izac, 261–289. Washington, DC: Island Press.
Hilodhari, M., and D.C. Baruah. 2011. Crop residue biomass for decentralized electrical power generation in rural areas (part 1): Investigation of spatial availability. Renewable and Sustainable Energy Reviews 15(4): 1885–1892.
Jaenike, J. 1978. Effect of island area on Drosophila population densities. Oecologia 36: 327–332.
Jobin, B., L. Choiniere, and L. Belanger. 2001. Bird use of three types of field margins in relation to intensive agriculture in Quebec, Canada. Agriculture, Ecosystems & Environment 84(2): 131–143.
Kanjilal, U.N., P.C. Kanjilal, A. Das, R.N. De, and N.L. Bor. 1934–1940. Flora of Assam. Vol. 1-5. Omsons Publications, New Delhi.
Karim, S., and S. Riazuddin. 1999. Rice insect pests of Pakistan and their control: A lesson from past for sustainable future integrated pest management. Pakistan Journal of Biological Sciences 2(2): 261–276.
Kessler, M., P.J.A. Kebler, S.R. Gradstein, K. Bach, M. Schmull, and R. Pitopang. 2005. Tree diversity in primary forest and different land use systems in Central Sulawesi, Indonesia. Biodiversity and Conservation 14: 547–560.
Kottawa-Arachchi, J.D., and R.N. Gamage. 2015. Avifaunal diversity and bird community responses to man-made habitats in St. Coombs Tea Estate, Sri Lanka. Journal of Threatened Taxa 7(2): 6878–6890.
Krishna, C.M., K. Sarma, and A. Kumar. 2012. Rapid assessment of Wreathed Hornbill Aceros undulatus (Aves:Bucerotidae) populations and conservation issues in fragmented lowland tropical forests of Arunachal Pradesh, India. Journal of Threatened Taxa 4(14): 3342–3348.
Leakey, R.R.B. 1998. Agroforestry for Biodiversity in Farming systems. In Biodiversity in Agroecosystems, ed. Collins, W.W., and C.O. Qualset, CRC Press. 129.
Lemessa, D., P.A. Hamback, and K. Hylander. 2015. The effect of local and landscape level land-use composition on predatory arthropods in a tropical agricultural landscape. Landscape Ecology 30(1): 167–180.
Lin, N., T.T. Nam, and J. Perera. 2012. Response of birds to different management types of tea cultivation in a forest-agriculture landscape, SW China. Proceedings of the Advanced Fieldcourse in Ecology and Conservation—XTBG 2012, Yunnan, China. 12–20.
Lindberg, J.E., V.R. Tolbert, A. Schiller, and J. Hanowski. 1998. Determining biomass crop managemeent strategies to enhance habitat value for wildlife. Poster presented at Bioenergy ‘98: Expanding Bioenergy partnerships, Madison, Wisconsin.
Mandan, J.K., V. Patil, S.S. Narkhede, R.P. Gunaga, and S. Bhave. 2014. Birds: A bio-control agent in farmland ecosystem. Indian Journal of Agroforestry 16(1): 15–20.
McNeely, J.A., and G. Schroth. 2006. Agroforestry and biodiversity conservation—traditional practices, present dynamics, and lessons for the future. Biodiversity and Conservation 15: 549–554.
Milder, J.C., F.A.J. DeClerck, A. Sanfiorenzo, D.M. Sanchez, D.E. Tobar, and B. Zuckerberg. 2010. Effects of farm and landscape management on bird and butterfly conservation in western Honduras. Ecosphere 1(1): 1–22.
Moreby, S.J., and S.E. Southway. 1999. Influence of autumn applied herbicides on summer and autumn food available to birds in winter wheat fields in southern England. Agriculture, Ecosystems & Environment 72(3): 285–297.
Morris, A.D., D.A. Miller, and M.C. Kalcounis-Rueppell. 2010. Use of forest edges by bats in a managed pine forest landscape. The Journal of Wildlife Management 74(1): 26–34.
Muller-Dombois, D., and H. Ellenberg. 1974. Aims and methods of vegetation ecology. New York: Wiley.
Nobel, I.R., and R. Dirzo. 1997. Forests as human-dominated ecosystems. Science 277: 522–525.
Osborne, P. 1984. Bird numbers and habitat characteristics in farmland hedgerows. Journal of Applied Ecology 21: 63–82.
Pautasso, M. 2007. Scale dependence of the correlation between human population presence and vertebrate and plant species richness. Ecology Letters 10: 16–24.
Perfecto, I., A. Mas, T. Dietsch, and J. Vandermeer. 2003. Conservation of biodiversity in coffee agroecosystems: A tri-taxa comparison in southern Mexico. Biodiversity and Conservation 12: 1239–1252.
Petit, L.J., D.R. Petit, D.G. Christian, and H.D.W. Powell. 1999. Bird communities of natural and modified habitats in Panama. Ecography 22: 292–304.
Peyre, A., A. Guidal, K.F. Wiersum, and F. Bongers. 2006. Dynamics of homegarden structure and function in Kerala, India. Agroforestry Systems 66: 101–115.
Pulido-Santacruz, P., and L.M. Renjifo. 2011. Live fence as tools for biodiversity conservation: A study case with birds and plants. Agroforestry Systems 81: 15–30.
Raman, T.R.S. 2006. Effects of habitat structure and adjacent habitats on birds in tropical rainforest fragments and shaded plantations in the Western Ghats, India. Biodiversity Conservation 15: 1577–1607.
Roberts, D.L., R.J. Cooper, and L.J. Petit. 2000. Flock characteristics of ant-following birds in premontane moist forest and coffee agroecosystems. Ecological Applications 10(5): 1414–1425.
Saikia, P., B.I. Choudhury, and M.L. Khan. 2012. Floristic composition and plant utilization pattern in homegardens of Upper Assam, India. Tropical Ecology 53(1): 105–118.
Salam, M.A., T. Noguchi, and M. Koike. 2000. Understanding why farmers plant trees in the homestead agroforestry in Bangladesh. Agroforestry Systems 50: 77–93.
Scales, B.R., and S.J. Marsden. 2008. Biodiversity in small-scale tropical agroforests: A review of species richness and abundance shifts and the factors influencing them. Environmental Conservation 35(2): 160–172.
Schelhas, J., and R. Greenberg. 1996. Forest patches in tropical landscapes. Washington, DC: Island Press.
Schmiegelow, F.K.A., C.S. Machtans, and S.J. Hannon. 1997. Are boreal birds resilient to forest fragmentation? An experimental study of short-term community responses. Ecology 78(6): 1914–1932.
Schroth, G., G.A.B. Da Fonseca, C.A. Harvey, C. Gascon, H.L. Vasconcelos, and A.-M.N. Izac. 2004. Agroforestry and biodiversity conservation in tropical landscapes. Washington, DC: Island Press.
Sherry, T.W., and R.T. Holmes. 1995. Summer versus winter limitation of populations: What are the issues and what is the evidence? In Ecology and management of neotropical migratory birds: A synthesis and review of critical issues, ed. D.M. Finch, and T.E. Martin, 85–120. Oxford: Oxford University Press.
Sidhu, S., T.R.S. Raman, and E. Goodale. 2010. Effects of plantations and homegardens on tropical forest bird communities and mixed-species bird flocks in the southern Western Ghats. Journal of the Bombay Natural History Society 107(2): 91–108.
Sinu, P.A. 2011. Avian pest control in tea plantations of sub-Himalayan plains of Northeast India: Mixed-species foraging flock matters. Biological Control 58(3): 362–366.
Soini, E. 2006. Bird diversity and land use on the slopes of Mt Kilimanjaro and the adjacent plains, Tanzania. African Zoology 41(2): 193–209.
Subaraj, R., and A.F.S.L. Lok. 2009. Status of the Lesser Adjutant stork (Leptoptilos javanicus) in Singapore. Nature in Singapore 2: 107–113.
Surasinghe, T.D., and C. De Alwis. 2010. Birds of Sabaragamuwa University campus, Buttala, Sri Lanka. Journal of Threatened Taxa 2(5): 876–888.
Tejeda-Cruz, C., and W.J. Sutherland. 2004. Bird responses to shade coffee production. Animal Conservation 7: 169–179.
Wijesundara, C., and M. Wijesundara. 2014. Bird diversity of Dekinda forest reserve, Balana, Sri Lanka: Implications for conservation. Ceylon Journal of Science 43(1): 137–146.
Wilson, J.D., M.J. Whittingham, and R.B. Bradbury. 2005. The management of crop structure: A general approach to reversing the impacts of agricultural intensification on birds. Ibis 147: 453–463.
Wunderle Jr., J.M., and S.C. Latta. 1996. Avian abundance in sun and shade coffee plantations and remnant pine forest in the Cordillera Central, Dominican Republic. Ornitologia Neotropical 7: 19–34.
Zar, J.H. 1999. Biostatistical analysis, 4th ed. Englewood Cliffs, New Jersey, USA: Prentice Hall Press.
Zimik, L., P. Saikia, and M.L. Khan. 2012. Comparative study on homegardens of Assam and Arunachal Pradesh in terms of species diversity and plant utilization pattern. Research Journal of Agricultural Sciences 3(3): 611–618.
Zuria, I., and J.E. Gates. 2013. Community composition, species richness and abundance of birds in field margins of Central Mexico: Local and landscape-scale effects. Agroforestry Systems 87: 377–393.
Acknowledgements
We are thankful to the Director and Head of Department of Forestry, North Eastern Regional Institute of Science and Technology for their administrative support and to the Council of Scientific and Industrial Research, New Delhi for providing financial support to carry out this work. We are grateful to the owners of the homegardens, agrisilviculture systems and tea gardens for providing permission to conduct the study. We owe tremendous thanks to all the field assistants who helped us in carrying out the field work smoothly. We are indebted to Mr. Manoj Singh, Mr. Parimal Chandra Ray and other colleagues for their useful comments to improve the manuscript. We also thank our anonymous reviewers for constructive comments which helped to improve the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yashmita-Ulman, Sharma, M. & Kumar, A. Agroforestry Systems as Habitat for Avian Species: Assessing Its Role in Conservation. Proc Zool Soc 71, 127–145 (2018). https://doi.org/10.1007/s12595-016-0198-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12595-016-0198-3