Abstract
Enzymatic modification of ramie fibers was carried out and its influence on the performance of ramie-poly(butylene succinate) biocomposites was studied. Ramie fibers were treated with bacterial cellulase (Brevibacillus parabrevis and Streptomyces albaduncus) for the modification of surface properties. Effect of different reaction parameters such as carbon sources, nitrogen sources, pH, temperature and incubation period on the hydrolysis of ramie cellulose was studied. Modified ramie fibers were characterized by scanning electron microscopy and X-ray diffraction techniques. Thermal stability and moisture absorption properties of modified ramie fibers were also studied. Enzymatically modified ramie fibers were used as reinforcing material for the development of poly(butylene succinate) biocomposites. Enzymatic treatment results in the removal of gum and polysaccharide layers from the surface of ramie fibers and contributed towards the improved compatibility between fibers and polymer matrix.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural fibers offer various green advantages over the synthetic fibers such as biodegradability, renewability, non-abrasive nature, low-cost, easy availability. Use of natural fibers in composite materials has been increased to develop the composites with green image. The properties of composites depend upon interfacial adhesion between reinforcing material and matrix [1]. Natural fibers are hydrophilic in nature, which results into poor interfacial adhesion with hydrophobic matrix and leads to the formation of poor quality composite materials. Pretreatment with chemical or green methods is an essential requirement in order to decrease the hydrophilic nature of natural fibers. Enzymatic surface modification of natural fibers is a best approach as per the concept of green chemistry [2]. Bacterial cellulase is a synergistic enzyme, known for the cellulose hydrolysis [3]. Bacterial cellulase attacks the β-(1,4)-glycosidic bond of cellulose unit to convert the cellulose into glucose units.
Enzymatic treatment results in various changes in physical, chemical and mechanical properties of the natural fibers. Bacterial cellulase changes the pore structure of natural fibers and results in degumming, i.e. the removal of gum like materials from the surface of natural fibers [4, 5]. Enhanced crystallinity, thermal stability and surface smoothness were observed due to the bacterial cellulase treatment of natural fibers [6, 7].
Poly(butylene succinate) (PBS) is one of the emerging biopolymers and characterized by an aliphatic structure with a low melting temperature of 114 °C. The low melting temperature of PBS is favorable to process the ramie fibers, which are generally characterized by low thermal stability. PBS has yield strength similar to that of polypropylene, while its stiffness is between those of low-density and high-density polyethylenes. Moreover, PBS is characterized by good thermal stability and high toughness related to its low glass transition [8].
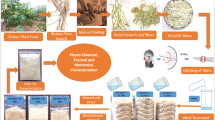
In present work, we have studied the effect of various reaction conditions on the enzymatic hydrolysis of ramie fiber cellulose. The effect of bacterial cellulase on the hydrophilicity and thermal stability of ramie fibers was also studied. Modified ramie fibers were used as reinforcing material in polybutylene succinate (PBS) matrix for the synthesis of biocomposites with better mechanical properties.
Experimental
Materials
Ramie fibers were supplied by Ramie Research Station, Sorbhog, Assam (India). Bacterial strains Brevibacillus parabrevis (MTCC No. 2708), Streptomyces albaduncus (MTCC No. 1764) were purchased from Institute of Microbial Technology, Chandigarh, India. Yeast extract, beef extract, malt extract, peptone, glucose, fructose, sucrose, mannitol, ammonium sulphate, sodium nitrate, nutrient broth, nutrient agar, NaCl and NaOH, all chemicals were purchased from HI-Media and were used as received. Poly(butylene succinate) (PBE003, NaturePlast, France, Mw = 84,100 g mol−1, PDI = 2.3) was used as received.
Methods
Enzymatic surface modification of ramie fibers
The standard growth mediums for respective bacteria were prepared for the bacterial growth. Started culture was first autoclaved at 121 °C for 1 h and then inoculated with bacterium strain upon cooling. The flasks were put in static conditions in an incubator. The turbidity in the growth media indicated the growth of strain after 1–2 days of bacterial incubation. Ramie fibers (0.5 g) were immersed in 250 ml conical flasks containing 90 ml of culture media of B. parabrevis (1 g/L beef extract, 2 g/L yeast extract, 5 g/L peptone and 5 g/L NaCl) and S. albaduncus (4 g/L yeast extract, 10 g/L malt extract and 4 g/L glucose). After autoclaving at 121 °C for about 1 h, flasks were inoculated with 10 ml of old broth of previous culture of bacteria. Flasks were then placed in incubator shaker (150 rpm) at 37 °C for 1 week. Treated ramie fibers were purified in 0.1 M NaOH at 80 °C for 20 min to remove all microorganisms, medium components and soluble polysaccharides. After filtration, the fibers were thoroughly washed with distilled water until the neutral pH [6]. The effect of different carbon sources, nitrogen sources, pH, incubation period and temperature on the hydrolysis of ramie fibers was observed w.r.t. the weight loss of fibers. The percentage weight loss of fibers as a result of hydrolysis was determined as follows:
where wi is the initial weight of fibers and wf is the weight of fibers after cellulase treatment.
Characterization techniques
Surface morphology of raw and treated ramie fibers was examined by Jeol JSM-6610LV Scanning Electron Microscope. Thermogravimetric analysis (TGA) was done at heating rate of 10 °C/min in an inert atmosphere using a Perkin-Elmer TGA 7 (gas flow 40 ml/min). A constant heating rate over a temperature range of 0–800 °C applied to the samples to get the resultant TGA curve. X-ray diffraction of raw and modified fibers was done on a Brucker D8 Advance X-ray diffractometer under ambient conditions. In cellulose, peak intensities close to 22° and 18° represent the crystalline and amorphous material, respectively. Percentage crystallinity (% Cr) was calculated as per following formula [7]:
where I22 and I18 are the crystalline and amorphous intensities at 2θ scale close to 22° and 18° respectively.
Moisture absorption study
Moisture absorption study of raw and treated ramie fibers was performed in a humidity chamber. Raw and modified fibers were dried at 80 °C for 4 h to attain the equal moisture level. Fiber samples were then exposed to 55 and 75% relative humidity (RH) at 23 °C in the humidity chamber. Fibers were quickly removed from the chamber after every hour and weighed till the constant weight was obtained. The percentage weight gain was calculated as follows [8]:
where w i and \(w_{f}\) are the initial and final weights of fibers, respectively.
Synthesis of ramie fiber reinforced biocomposites
Poly(butylene succinate) (PBS) based biocomposites were prepared by crushing the PBS to powder form. PBS and ramie fiber samples were then dried overnight at 60 °C for the removal of all the moisture content. Ramie fibers and PBS were weighted in required proportion and then transferred to the mold cavity of hot press. Three different heating cycles were chosen for complete soaking, mixing and adhesion of fibers with PBS resin. Initially, the mold was heated from room temperature to 70 °C and maintained there for 15 min. The temperature of the mold was then raised to 100 °C and kept there for 30 min and finally mold was heated at 125 °C for about 20 min. After three heating cycles, the mold was allowed to cool to room temperature. In order to avoid any structural disorder, a constant pressure (100 MPa) was maintained during all the thermal and cooling treatments. The manufactured composite sheet was then removed from the mold and cut into the required dimensions for further mechanical testing. The tensile and flexural properties of manufactured biocomposites strips (100 × 10 × 3 mm) were performed in Universal Testing Machine (UTM) (H5KT Tinius Olsen, 5 KN) with a crosshead speed of 5 mm/min [9, 10].
Results and discussion
Mechanism of enzymatic hydrolysis of ramie fibers
Natural fibers consist of cellulose, hemicelluloses, lignin, pectin and waxes. Ramie fibers composed of ~67 to 76% cellulose, 13 to 16% hemicelluloses, 0.6 to 0.7% lignin, 1.9% pectin and 0.3% waxes [11]. Cellulose forms the major portion of the ramie fiber composition. Cellulose is a polysaccharide which consists of β-d-glucopyranose units joined together by β-1,4-glycosidic bond. These cellulose units bonded together by strong hydrogen bonding between hydroxyl groups. Enzymatic hydrolysis of cellulosic units can modify the properties of natural fibers. Hydrolysis of natural fiber cellulose by bacterial and fungal cellulase is a green method to alter the properties of natural fibers.
Cellulase enzyme hydrolyzes the β-1,4-glycosidic linkage between polysaccharide molecules to convert cellulose into glucose units. Cellulase consists of three components endoglucanases, exoglucanases and β-glucosidase, which are required for the hydrolyzing process of cellulose. Endoglucanases perform the cleavage of internal β-1,4-glycosidic linkage to generate oligomer chains of different lengths having reducing and non-reducing ends. Exoglucanases attack the oligomer chains to produce the glucose or cellobiose. β-glucosidase converts the small chains and cellobiose to the glucose units (Fig. 1) [12, 13].
Effect of different reaction parameters on enzymatic hydrolysis of ramie fibers
Carbon sources
Cell metabolism and cellulase activation are majorly affected by the carbon sources available in the growth media. Different carbon sources such as glucose, fructose, sucrose and mannitol were used in the growth media of B. parabrevis and S. albaduncus. In case of ramie fibers treated with cellulase enzyme (B. parabrevis), the maximum weight loss due to enzymatic hydrolysis was 14.8% with fructose as a carbon source (RB-01) (Fig. 2a). Fructose plays a vital role in the metabolic pathways of bacteria. Fructose induced the phosphotransferase system of sugar uptake of bacteria. Fructose gets phosphorylated and performed the glycolysis to provide energy to bacterial cell system [14]. Fructose was reported as best carbon source during the production of cellulase enzyme from the bacteria [15, 16]. In case of ramie fibers treated with cellulase enzyme (S. albaduncus), the maximum weight loss due to enzymatic hydrolysis was 13.5% with sucrose as a carbon source (RS-01) (Fig. 2b). In case of bacteria, fructose and sucrose was proven to be a good inducer for the invertase enzyme, which is responsible for the hydrolysis of sugar molecules uptake by the bacteria. The uptake of carbon sources or sugar molecules increased after the activation of invertase enzyme. Fructose and sucrose were digested more rapidly by bacteria than other carbon sources. Cellulase enzyme hydrolyzes the cellulose content of fibers to glucose units by cleaving the glycosidic bonds. The hydrolysis results in a modified surface morphology and other properties of the ramie fibers [7, 17].
Nitrogen sources
Effect of nitrogen sources on the production of cellulase enzyme by bacteria was analyzed by varying the nitrogen sources in the growth media of bacteria. Two organic (peptone, yeast extract) and two inorganic (sodium nitrate and ammonium sulphate) nitrogen sources were studied in fermenting media of bacteria. The maximum weight loss (15.6 and 11.2%) was obtained when peptone was used as nitrogen source for the treatment of ramie fibers with B. parabrevis (RB-02) and S. albaduncus (RS-02), respectively (Fig. 3a, b). Peptone is an organic nitrogen source, which provides free amino acids and peptides necessary for the bacterial growth. Higher production of cellulase results in active cellulosic hydrolysis of ramie fibers. Addition of organic nitrogen such as peptone, beef extract, yeast extract resulted in enhanced growth of bacteria and production of cellulase [18].
Effect of pH
The pH of fermenting media is an important factor, which influenced the growth and activity of enzyme to a large extent by affecting the transportation of chemicals and enzymes across the cell membrane. The pH values were varied in the growth media of B. parabrevis and S. albaduncus to study the effect on enzymatic activity. The maximum weight loss 12.8 and 21.6% was obtained at pH 6 and pH 7 in case of ramie fiber treated with B. parabrevis (RB-03) and S. albaduncus (RS-03), respectively (Fig. 4). Maximum enzymatic activity of B. parabrevis and S. albaduncus was obtained at their optimum pH values of pH 6 and pH 7, respectively. At optimum pH, cellulase was most effective in performing cellulosic hydrolysis. The change in pH from the optimum value causes the deactivation of the enzyme and results in a less hydrolysis of cellulose.
Effect of temperature
An optimum temperature is required for the growth of every bacterium. In present study, cellulase activity was studied with respect to different temperatures. Fermentation mediums were placed at 25, 35, 45 and 55 °C in incubator shakers. The maximum weight loss [13.6 and 37.6% was observed at 35 °C after 5 days for B. parabrevis (RB-04) and S. albaduncus (RS-04), respectively (Fig. 5). It can be analyzed from the Fig. 5a, b that weight loss was very less at higher temperatures in comparison to the optimum temperature (35 °C). This showed that cellulase activity was decreased at higher temperature due to the collapsing of bacterial and enzymatic cells. The bacteria remain inactive at lower temperature (25 °C). Bacteria and enzyme are very much sensitive towards the temperature. Microorganism can grow only at optimum temperature and becomes inactive at other temperatures [19].
Effect of incubation period
Incubation period is an important parameter for the enzyme production by microbial strains. In the present study, incubation or fermentation was carried out for 6 days and weight loss of ramie fibers was measured at 24 h interval. Maximum weight loss in ramie fibers (13.4 and 14.6%) was observed on 4th day and 5th day in case of cellulase enzyme produced by B. parabrevis (RB-05) and S. albaduncus (RS-05), respectively (Fig. 6). A small weight loss was observed initially due to the less secretion of enzyme. After the complete consumption of all nutrients, more and more enzyme was available for the hydrolysis of cellulose. After optimum incubation period, activity of enzymes was decreased due to the production of proteases at prolonged time, which may be responsible for the inactivation of enzymes [20].
Characterization of modified ramie fibers
Morphological analysis
Surface of ramie fibers like other natural fibers remain covered with a protective layer consisting of lignin, hemicelluloses, pectin and waxes. Bacterial cellulase treatment is an environment friendly method for the removal of protective layer by degumming process. It can be analyze from the Figs. 7 and 8 that surface of enzymatically treated ramie fibers is cleaner and smooth due to the removal of wax layers in comparison to the surface of untreated ramie fibers. Surface of untreated ramie fibers is being covered with waxy impurities and appears to be irregular (Figs. 7a, 8a). The change in the surface morphology confirms the effective enzymatic treatment of ramie fibers.
Crystallinity
Effect of bacterial cellulase treatment on the crystalline nature of the ramie fibers was studied. It has been observed that %Cr was enhanced due to the enzymatic treatment of ramie fibers. The crystallinity of untreated ramie fibers was found to be 83.6%. Ramie fibers treated with B. parabrevis cellulase showed 87.1% (RB-01), 87.9% (RB-02), 87.5% (RB-03), 87.3% (RB-04) and 86.2% (RB-05) crystallinity (Fig. 9a). Whereas, 88.0, 86.6, 87.1, 86.6 and 86.6% crystallinity was obtained in case of RS-01, RS-02, RS-03, RS-04 and RS-05, respectively (Fig. 9b) (Table 1). The increased %Cr of ramie fiber was due to the enzymatic hydrolysis of amorphous cellulose and the removal of polysaccharide layers from the fiber surface. The amorphous cellulose region is more prominent for the enzymatic hydrolysis [6, 21, 22]. Enzymatic treated ramie fibers with increased crystallinity have a great potential as fiber reinforcement in PBS matrix composites materials.
Properties of enzymatically modified ramie fibers
Thermal properties
Thermogravimetric analysis of ramie fibers was performed to study the effect of bacterial cellulase treatment on thermal properties of the fibers. Thermal stability was not disturbed very much even after the treatment of ramie fibers with B. parabrevis (RB) and S. albaduncus (RS). Untreated ramie fibers showed first decomposition stage at 257 °C with 6% weight loss and final decomposition stage at 411 °C with 60% weight loss. In case of RB-01, RB-02, RB-03, RB-04 and RB-05, the initial decomposition stage was observed at 251 °C (3.3% weight loss), 255 °C (5.1% weight loss), 260 °C (4% weight loss), 263 °C (4.6% weight loss), 257 °C (5% weight loss) and final decomposition stage was observed at 402 °C (65.5% weight loss), 390 °C (59.3% weight loss), 411 °C (65.5% weight loss), 406 °C (65.2% weight loss), 408 °C (65.2% weight loss), respectively (Fig. 10a). Similarly, ramie fibers treated with S. albaduncus cellulase have shown the two stage decomposition during thermal analysis (Fig. 10b). In case of RS-01, RS-02, RS-03, RS-04 and RS-05, the first decomposition stage was observed at 258 °C (4.7% weight loss), 259 °C (4.3% weight loss), 268 °C (5.6% weight loss), 260 °C (7.3% weight loss), 266 °C (5.6% weight loss) and final decomposition stage was observed at 425 °C (73.2% weight loss), 397 °C (62.8% weight loss), 399 °C (62.2% weight loss), 402 °C (61.8% weight loss), 404 °C (63.2% weight loss), respectively. First stage of decomposition attributed towards the removal of water, i.e. evaporation and second stage of decomposition occurred due to the enzymatic hydrolysis of cellulose fiber [7, 23]. A negligible change in the thermal behavior of the treated ramie fibers was observed in comparison to the untreated ramie fibers. It can be concluded from this study that enzymatic treatment is a slow process, which does not affect the thermal behavior of the ramie fibers to a large extent.
Moisture absorption property
In moisture absorption studies, there is diffusion of moisture from the outer atmosphere to the surface of fibers followed by diffusion from the surface to the individual fiber surface. Finally, diffusion of moisture to the interior of fiber took place. Change in the surface functionality, morphology [24] and pore structure [22] attributed towards the change in the moisture diffusion process. Moisture absorption study of raw and bacterial cellulase treated ramie fibers was performed in order to analyze the change in hydrophilicity of ramie fibers. Moisture absorption graphs of the study have been shown in Figs. 11a, b and 12a, b. It has been observed that there is a decrease in the moisture absorption capacity of the ramie fibers due to the cellulase enzyme treatment. Cellulase enzyme results in the hydrolysis of amorphous cellulose by cleaving the β-1,4 glycosidic linkage between monomer units of cellulose. This enzymatic hydrolysis results in the removal of subsequent layers and fibrils from the surface. Due to the peeling effect, surface of the fibers becomes less hydrophilic in nature and decreases the moisture absorption capacity of fibers [6].
Ramie fiber reinforced biocomposites
Mechanical properties of synthesized biocomposites
Tensile load-strain curves and tensile strength (MPa) of composites are depicted in Figs. 13 and 14. Tensile and flexural testing of neat PBS, ramie fiber reinforced biocomposites were carried-out to study the effect of enzymatic treatment on the mechanical strength of biocomposites. It has been observed from the Figs. 13 and 14 that extra tensile force was required to break the composites reinforced with modified ramie fibers in comparison to the neat PBS (7.3 MPa) and raw ramie fiber reinforced composites. The fiber content and their orientation also play an important aspect in the mechanical properties of composites (Figs. 13c, 14c). Ramie fibers were randomly oriented in the synthesized composites. Tensile strength was increased with the increase in the fiber content from 0.5 to 1%. In case of raw ramie fibers, tensile strength was found to be 8.57 MPa (0.5% fibers) and 9.6 MPa (1% fibers). In case of ramie fibers treated with B. parabrevis (RB) and S. albaduncus (RS), the tensile force was 15.54 and 12.86 MPa, respectively with 0.5% fiber content. The tensile strength was increased to 21.5 and 19.14 MPa, respectively with the increase in the fiber content, i.e. 1%. Stress–strain curves and flexural strength (MPa) strain curves of neat PBS, raw and modified ramie fibers reinforced composites have been shown in Figs. 13d, e and 14d, e. It can be analyzed from the Figs. 13d, e and 14d, e that flexural strength of biocomposites reinforced with modified ramie fibers was found to be increased in comparison to the raw ramie fiber reinforced biocomposites and neat PBS (6.8 MPa). Flexural strength was also increased with the increase in concentration of fibers. In case of raw ramie fibers reinforced biocomposites, flexural strength was found to be 7.7 MPa (0.5% fibers) and 9.52 MPa (1% fibers). In case of ramie fibers treated with B. parabrevis (RB) and S. albaduncus (RS), the flexural strength was 10.22 and 10.76 MPa, respectively with 0.5% fiber content and 14.24 and 13.5 MPa, respectively with 1% fiber content.
Mechanical studies of biocomposites: a tensile load-strain curves with 0.5% fiber content, b tensile load-strain curves with 1% fiber content, c effect of fiber content on tensile strength, d flexural load-strain curve with 0.5% fiber content, e flexural load-strain curve with 1% fiber content, f effect of fiber content on flexural strength
Mechanical studies biocomposites: a tensile load-strain curves with 0.5% fiber content, b tensile load-strain curves with 1% fiber content, c effect of fiber content on tensile strength, d flexural load-strain curve with 0.5% fiber content, e flexural load-strain curve with 1% fiber content, f effect of fiber content on flexural strength
Improved tensile and flexural strength of composites reinforced with modified ramie fibers was due to the increase in the compatibility and even stress distribution between PBS resin and modified ramie fibers [1]. Cellulase enzyme resulted in the removal of gum and polysaccharide layers from the surface of fibers and contributed to the changes in surface of ramie fibers for the increased compatibility between fibers and matrix [6, 7, 25, 26]. Strong interfacial adhesion between two phases was responsible for better mechanical properties.
Fractured surface morphology of biocomposites
Fractured surface morphology of raw and modified ramie fiber reinforced biocomposites was studied (Fig. 15a–c). A better interfacial adhesion was observed between the modified fibers and polymer matrix in comparison to the raw fibers. The gaps were identified around the raw fiber reinforced composites, which shows the poor adhesion between two phases. Different polarity of two phases is also responsible for the poor interlocking, as PBS matrix is hydrophobic and ramie fiber is hydrophilic in nature [27]. On the other hand enzymatically treated ramie fibers have shown the effective adhesion with the polar matrix. Cellulase hydrolyze the β-1,4 glycosidic bonds present in the ramie fibers and removes the subsequent layers of the fibers, which provides a smooth appearance to the surface. As a result, enzymatically treated ramie fibers become more compatible towards hydrophobic PBS matrix. This resulted in a better interlocking between the fibers and matrix.
Conclusions
Enzymatically surface modification of natural fibers is a better alternate green treatment for the development of biocomposites with better mechanical properties. Bacterial cellulase treatment resulted in the degumming of ramie fibers and reduces the hydrophilicity. Improved mechanical properties of biocomposites reinforced with modified ramie fibers were observed due to the better compatibility and even stress distribution between polymer matrix and enzymatically modified ramie fibers.
References
Yu T, Ren J, Li S, Yuan H, Li Y (2010) Effect of fiber surface-treatments on the properties of poly(lactic acid)/ramie composites. Compos A 41:499–505. doi:10.1016/j.compositesa.2009.12.006
Kalia S, Thakur K, Celli A, Kiechel MA, Schauer CL (2013) Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: a review. J Environ Chem Eng 1:97–112. doi:10.1016/j.jece.2013.04.009
Chellapandi P, Jani HM (2008) Production of endoglucanase by the native strains of Streptomyces isolates in submerged fermentation. Braz J Microbiol 39:122–127. doi:10.1590/S1517-838220080001000026
Saikia R, Boruah P, Samanta R (2009) Microbial degumming of decorticated ramie and its fiber characteristics. Indian J Fiber Text Res 34:187–190
Park S, Venditti RA, Abrecht DG, Jameel H, Pawlak JJ, Lee JM (2006) Surface and pore structure modification of cellulose fibers through cellulase treatment. J Appl Polym Sci 103:3833–3839. doi:10.1002/app.25457
Kalia S, Vashishta S (2012) Surface modification of sisal fibers (Agave sisalana) using bacterial cellulose and methacrylate. J Polym Environ 20:142–152. doi:10.1007/s10924-011-0363-8
Kalia S, Sheoran R (2011) Modification of ramie fibers using microwave-assisted grafting and cellulase enzyme-assisted biopolishing: a comparative study of morphology, thermal stability, and crystallinity. Int J Polym Anal Charact 16:307–318. doi:10.1080/1023666X.2011.587946
Frollini E, Bartolucci N, Sisti L, Celli A (2013) Poly(butylene succinate) reinforced with different lignocellulosic fibers. Ind Crops Prod 45:160–169. doi:10.1016/j.indcrop.2012.12.013
Kaith BS, Kalia S (2007) Grafting of flax fiber (Linum usitatissimum) with vinyl monomers for enhancement of properties of flax-phenolic composites. Polym J 39:1319–1327. doi:10.1295/polymj.PJ2007073
Thakur K, Kalia S, Kaith BS, Pathania D, Kumar A (2015) Surface functionalization of coconut fibers by enzymatic biografting of syringaldehyde for the development of biocomposites. RSC Adv 5:76844–76851. doi:10.1039/C5RA14891J
Mohanty AK, Misra M, Hinrichsen G (2000) Biofibers, biodegradable polymers and biocomposites: an overview. Macromol Mater Eng 276:1–24. doi:10.1002/(SICI)1439-2054(20000301)276:1<1:AID-MAME1>3.0.CO;2-W
Esteghlalian AR, Mansfield SD, Saddler JN (2002) Cellulases: agent for fiber modification or bioconversion? The effect of substrate accessibility on cellulase enzymatic hydrolyzability. Prog Biotechnol 21:21–36. doi:10.1016/S0921-0423(02)80005-3
Verenich S, Aromugam K, Shim E, Pourdeyhimi B (2008) Treatment of raw cotton fibers with cellulases for nonwoven fabrics. Text Res J 78:540–548. doi:10.1177/0040517507083308
Shimizu K (2013) Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochem 1:1–47. doi:10.1155/2013/645983
Sethi S, Datta A, Gupta BL, Gupta S (2013) Optimization of cellulase production from bacteria isolated from soil. ISRN Biotechnol 1:1–7. doi:10.5402/2013/985685
Sreeja SJ, Malar JPW, Joseph SFR, Tiburcius S, Immanuel G, Palavesam A (2013) Optimization of cellulase production by Bacillus altitudinis APS MSU and Bacillus licheniformis APS2 MSU, gut isolates of fish Etroplus suratensis. Int J Adv Res Technol 2:401–406
Romanov VI, Martinez-Romero E (1994) Sucrose transport and hydrolysis in Rhizobium tropici. In: Graham PH, Sadowsky MJ, Vance CP (eds) Symbiotic nitrogen fixation, vol 57. Springer, Netherlands, pp 91–96
Dias PVS, Ramos KO, Padilha IQM, Araujo DAM, Santos SFM, Silva FIH (2014) Optimization of cellulase production by Bacillus sp. isolated from sugarcane cultivated soil. Chem Eng Trans 38:277–282. doi:10.3303/CET1438047
Singh J, Banal S (2013) Combinative impact of effectors on production of celluolytic enzyme from Brevibacillus parabrevis (MTCC 2208). Eur J Exp Biol 3:484–490
Nirmala P, Sindhu A (2011) Production of endoglucanase by optimizing the environmental conditions of Bacillus circulans on submerged fermentation. Int J Appl Eng Res 2:472–481
Kumar P, Barrett DM, Delwiche MJ, Stroeve P (2009) Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind Eng Chem Res 48:3713–3729. doi:10.1021/ie801542g
Park S, Venditti RA, Abrecht DG, Jameel H, Pawlak JJ, Lee JM (2007) Surface and pore structure modification of cellulose fibers through cellulase treatment. J Appl Polym Sci 103:3833–3839. doi:10.1002/app.25457
Shan CW, Idris MI, Ghazali MI (2012) Study of flexible polyurethane foams reinforced with coir fibres and tyre particles. Int J Appl Phys Math 2:123–130. doi:10.7763/IJAPM.2012.V2.67
Gulati D, Sain M (2006) Fungal-modification of natural fibers: a novel method of treating natural fibers for composite reinforcement. J Polym Environ 14:347–352. doi:10.1007/s10924-006-0030-7
Pommet M, Juntaro J, Heng JY, Mantalaris A, Lee AF, Wilson K et al (2008) Surface modification of natural fibers using bacteria: depositing bacterial cellulose onto plant fibers to create hierarchical fiber reinforced nanocomposites. Biomacromol 9:1643–1651. doi:10.1021/bm800169g
Pickering KL, Li Y, Farrell RL, Lal M (2007) Interfacial modification of hemp fiber reinforced composites using fungal and alkali treatment. J Biobased Mater Biol 1:109–117. doi:10.1166/jbmb.2007.012
Dong A, Yu Y, Yuan J, Wang Q, Fan X (2014) Hydrophobic modification of jute fiber used for composite reinforcement via laccase-mediated grafting. Appl Surf Sci 301:418–427. doi:10.1016/j.apsusc.2014.02.092
Acknowledgement
Authors are highly thankful to Ramie Research Station, Sorbhog, Assam (India) for providing us the ramie fibers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Thakur, K., Kalia, S. Enzymatic modification of ramie fibers and its influence on the performance of ramie-poly(butylene succinate) biocomposites. Int J Plast Technol 21, 209–226 (2017). https://doi.org/10.1007/s12588-017-9178-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-017-9178-3