Abstract
Effect of magnetite (Fe3O4) nanoparticles on the performance of ethylene vinyl acetate (EVA) was prepared by a two-roll mixing mill. The morphology of the nanocomposites was carried out by transmission electron microscopy (TEM). The processing characteristics and the curing behavior of the nanocomposites were analyzed by Monsanto Rheometer. TEM images of the nanocomposite showed that nanoparticles were dispersed uniformly throughout the matrix up to 7 phr of filler and thereafter the dispersion decreased with further loading of fillers. The magnetite nanoparticle in EVA matrices was found to accelerate the curing process up to 26 % compared to unfilled sample. The transport characteristics of aromatic hydrocarbon through the nanocomposite were studied by simple sorption gravimetric analysis in the temperature range 28–70 °C. The solvent uptake was minimum for composites with 7 phr of filler and increased with increasing filler content. The transport phenomenon was found to follow an anomalous mode, the activation parameters were estimated and the molecular mass was calculated using Flory-Rehner theory. The sorption data were used to estimate the enthalpy, entropy and free energy of mixing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic- inorganic hybrids with well-defined morphology and structure at the nanometric scale represent a very interesting class of materials with unique properties. These materials represent a new class of polymeric materials, which combine the properties of the inorganic particle (mechanical strength, modulus,) flame and thermal resistance with processability and flexibility of organic polymer matrix [1–3]. These hybrid materials sometimes lead to unexpected new properties, which are not exhibited by individual compounds and thus open a new avenue for chemists, physicists and material scientists. In order to prepare such materials either organic molecule can be embedded into inorganic materials or vice versa.
Polymer nanocomposites consist of a blend of one or more polymer with various nanomaterials like nanoclays, carbon nanotubes, etc. A well-dispersed nanoparticle into the polymer is a prerequisite for achieving significant property enhancement. This is one of the challenging difficulties when preparing polymer nanocomposites [4, 5]. In this respect, a key factor turned out to be the compatibility between polymer matrix and reinforcement. Effort has to be put into rendering matrix polymer and nanofiller compatible. Among various polymeric materials for polymer/clay nanocomposites, ethylene vinyl acetate copolymer (EVA), a random copolymer consisting of ethylene and vinyl acetate (VA) as a repeating unit, has been recently adopted for its nanocomposite system due to its potential engineering application in the fields of packaging films and adhesives [6–9].
The sorption and transport behavior of various solvents through polymer–clay nanocomposite membranes has attracted the attention of many researchers [10–13]. Nanoparticles can be used to control the diffusion and permeation of molecules through the polymer matrix. It is necessary to understand their performance in various liquid atmospheres for the application of these polymer membranes. Molecular transport through polymer nanocomposite depends on number of factors such as the nature of polymer matrix, size of penetrates, temperature, content of nanoparticles, aspect ratio and degree of dispersion of nanoparticles. Materials based on nano-sized metal oxide provide a potential solution to meet the present and future technological demand in virtue and the novel properties. Among this magnetite (Fe3O4) and maghemite (Fe2O3) have been scientific and technological interest in last few decades. The traditional metal magnetic materials are replaced by magnetic elastomer because its own characteristics, such as high saturation magnetic induction, low coercivity, high permeability, low high-frequency loss and so on, can be widely used in the automobile, petroleum and chemical industries [14, 15]. EVA, being polar in nature, is a good choice for nanocomposites with the nano fillers and the system offers a lot of properties to explore. The present work therefore focused on the utilization of magnetite nanoparticles as filler in EVA with reference to their cure characteristics, morphology and the transport characteristics of aromatic hydrocarbons are studied.
Experimental
Materials and methods
EVA (Pilene 1802) was supplied by Polyolefin Industries Limited, Chennai, India. The solvents benzene, toluene and xylene were of reagent grade and were distilled before use to ensure purity. The crosslinking agent was dicumyl peroxide (DCP). Ferrous chloride tetrahydrate (FeCl2.4H2O) and iron trichloride (FeCl3) was obtained from Nice Chemicals, India. Magnetite (Fe3O4) nanoparticle with a size of 35 nm was prepared by the chemical process described previously [16]. The magnetic nanocomposites based on EVA have been prepared by adding various amount of Fe3O4 in a two roll mixing mill. EVA was mixed with ZnO (4 phr), magnetite naoparticles (0, 3, 5, 7 and 10 phr) and DCP (5 phr). For diffusion studies, circular samples of diameter 19.6 mm and 2 mm thickness were punched out from the vulcanized sheets. Weight and thickness of the samples were determined before sorption experiments. They were immersed in aromatic hydrocarbon (15–20 ml) in closed diffusion bottles, kept at constant temperature in an air oven. The samples were removed from the bottles at periodic intervals of 30 min, dried for 5–10 s between filter papers to remove the excess solvent on their surfaces and weighed immediately using an electronic balance that measured reproducibly within ± 0.0001 g.
Characterization
Cure characteristics were determined using a Moving Die Rheometer (MDR 2000) according to ASTM D 2084–93 at 160 °C. The composites were vulcanized to their respective cure time in a hydraulic press at 160 °C and a pressure of 6.7 MPa on the mould. Transmission electron micrographs of the samples were carried out using a Philips CM12 model with an acceleration voltage of 100 kV. The specimens were prepared using an ultra cut E cryomicrotone. Thin sections of about 100 nm were cut with a diamond knife at −100 °C.
Results and discussion
Cure characteristics
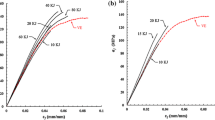
The torque values of filled and gum EVA compounds with different loading of magnetite are given in Fig. 1. It is clear from the figure that the maximum (Mh) and minimum torque (Ml) increases with the increase in concentration of nanoparticles and these values are found to be the maximum at a concentration of 7 phr of filler. The increment in torque values suggested that there are good interactions between the polymer and filler surfaces [17]. The Mh can be regarded as a measure of the composites modulus, which reveals the cross-link density of the fully vulcanized sample. Also, significantly increased torque value is an indirect hint for the enhanced interaction behavior and good interfacial adhesion between matrix and the filler. It is reported in the literature that for strongly bounded polymers with filler surfaces there will be an area of high density and thus, high modulus forms next to the surface [18]. Thus if all areas of the filler surfaces are capable of adsorption (high surface area), the segments of polymer chains will absorbed on the surfaces and form a layer close to the filler surfaces. This area would have combination properties of fillers (high modulus) and matrix. Minimum torque value is observed for EVA, which has the minimum reinforcement effect due to the free movement of polymer chain. Figure 1 shows that the addition of filler slightly increases the Ml value, which is due to the enhanced interaction between the filler-polymer that ultimately results in the restriction to molecular motion of the macromolecules [19]. It is obvious from Fig. 2 that the optimum cure time and scorch time decreased with increase in concentration of filler in EVA. Magnetite nanoparticle reinforced EVA is found to accelerate the vulcanization up to 26 % as compared to unfilled polymer matrix. The reduction in optimum cure time is due to the effect of increased thermal conductivity and filler’s surface area available for reaction leads to the cross linkage formation through coordination interaction between iron oxide nanoparticles and acetate groups of EVA. Similar results were reported in the literature that fillers play an important role in accelerating vulcanization process [20].
Transmission electron microscopy (TEM)
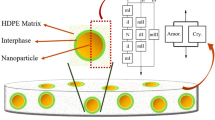
The transmission electron micrographs (TEM) of EVA/Fe3O4 nanocomposites with different dosages of nanoparticles are presented in Fig. 3. EVA compound with 5 phr Fe3O4 composite shows an irregular structure with globular particles is due to the poor dispersion and adhesion of Fe3O4 into the polymer matrix. It can be seen that in 7 phr of sample, the nanoparticles are well dispersed in the matrix with spherical in shape having very good uniformity and adhesiveness. The more ordered structure of polymer composite is due to the coordination interaction between 3d empty orbits of Fe atoms in Fe3O4 particles and the oxygen atoms on EVA has important impact on the formation of the global morphology [21]. When the concentration of filler increases, dispersion of nanoparticles decreases with an extensive stress wheeled region that extended deep into the sample and therefore the magnetite nanoparticles exists as large aggregates. The agglomeration and poor interfacial bonding of filler with polymer leads to poor reinforcing effect of the composite is due to the poor stress transfer from the matrix to filler resulting in the deterioration of nanocomposite properties. The above observation will be analyzed with the help of diffusion studies.
Effect of magnetite nanoparticles on sorption and diffusion
The diffusion curves of magnetite nanoparticles filled EVA, crosslinked by DCP is expressed as mol% uptake (Qt) of liquid vs. square root of time are plotted and given in Fig. 4. The experiment is conducted by using benzene as solvent at 28 o C. It can be seen from the figure that the loading of EVA with Fe3O4 reduces the solvent uptake is due to the reinforcement of polymer restricts the local freedoms of movement of macromolecular chain and thereby improves the solvent resistance. It can be observed that EVA showed the maximum solvent uptake and the composite with 7 phr Fe3O4 showed the least solvent uptake values. A similar trend is also found to be with other solvents. Nanoparticles form a chemical crosslinks with the polymer chains in certain zone and that leads to the immobilization of metal particles due to the coordination interaction between the Fe3O4 and acetate group of EVA. The coordination clusters formed would prevent the rearrangement of the macromolecular chains during solvent ingression and reduces the free volume in the composite, thereby causing resistance to the path of penetrants. Influence of different dosage of filler on the equilibrium uptake of solvents (benzene, toluene and xylene) is given in Fig. 5. There is a systematic trend on the sorption behavior of liquids of different molecular size. When the size of solvent molecule increases, the solvent uptake found to be decreases. It is well clear that among the solvent used, benzene uptake is maximum while xylene uptake is minimum and toluene uptake is intermediate. The decrease in solvent uptake with increase in size of solvents is due to the greater activation energy required for activating the diffusion process. Similar trend were observed for other studies [22]. It is also clear from the figure that the equilibrium uptake of the composite show a minimum with increase in the nanoparticle content. This would be attributed to the difference in the dispersion of filler particles in the matrix. TEM images shown in Fig. 3, clearly reveal that at higher filler loading, aggregation of filler particles occurs due to its poor dispersion in the matrix. Hence, a phase separation has formed between the polymer and magnetite nanoparticles, resulting in an increased uptake of solvents.
Temperature effects and arrhenius activation parameters
The dependence of sorption and diffusion behavior of 7 phr Fe3O4 filled EVA nanocomposite with various temperatures has been studied by carrying out the experiments at 30, 50 and 70 °C. The change in diffusivity values with temperature for different samples can be obtained from Fig. 6. The rate of diffusion and maximum solvent uptake increased with the increase in temperature. The disruption of long-range order in the crystalline region of EVA with enhanced segmental mobility of macromolecular chain at elevated temperature is responsible for the observed activation of transport process. The kinetic energy of the penetrants also increases with increasing temperature. All other systems showed the same trend. It is also found that the slope of the linear portion increases with temperature showing that the transport process is temperature activated.
The sorption, diffusion and permeation process are activated by temperature and therefore the energy of activation for diffusion and that for permeation process can be obtained by using Arrhenius equation [23]
where X is P (permeation coefficient, obtained as a product of diffusion coefficient and sorption coefficient) or D (diffusion coefficient) and X0 is P0 or D0 which is a constant, T is the temperature in absolute scale and R is the gas constant . Arrhenius plots of log P or log D vs. 1/T are constructed from the slope of the curves; the values of activation energy for diffusion, ED and the activation energy for permeation, EP were estimated by linear regression analysis. From the difference between EP and ED, the heat of sorption, ΔHS has estimated for benzene and the values are given in Table 1. The activation energy for diffusion and permeation for the polymer nanocomposite is higher than that of unfilled filled polymer membrane. The high aspect ratio of nanoparticles effectively increased the diffusion path, which ultimately result in high activation energy. It is obvious that extent of coordination interaction of filler in the composite increases, the extent of crosslink is higher and therefore the penetrant molecules require more activation energy to travel through the nanoparticles. The value of ΔHS gives additional information about the molecular transport through the polymer matrix. ΔHS is a composite parameter involving both Henry’s law and Langmuir type sorption mechanism. The positive value suggest that the Henry’s type mechanism predominates in the present system due to holes that are creating first within the polymer matrix and then the solvent molecules fill them; the formation of newer site involves and the process of sorption is endothermic [24]. The endothermicity of the reaction supports the fact that the rate of diffusion and maximum solvent uptake increase with increase of temperature.
Diffusion coefficient
Diffusion coefficient is a kinetic parameter related to the polymer segmental mobility, penetrant nature and extent of crosslinks present in a polymer matrix. The diffusion coefficient of a polymeric material immersed in a solvent can be calculated using the Equation
where θ is the slope of the initial portion of the plots of Qt versus √t, h is the initial thickness of polymer sample and Q∞ is the equilibrium mole % uptake. The calculated values of diffusion coefficients are given in Table 2. It is observed that composite exhibit reduced diffusion coefficient values. The rate of diffusion of a polymer primarily depends on the ease with which macromolecular chain segments exchange their position with penetrant molecule. Moreover, the mobility of the polymer depends on the amount of free volume in the matrix [25]. The value of diffusion coefficient shows that the impermeable nanoparticles produce a wreathed pathway for a penetrant to transverse the composite. Due to the uniform level dispersion of the organic and inorganic phases, the available free volume decreases and also the coordination interaction of Fe3O4 with EVA leads to cross-linked path that ultimately result in lower diffusivity values. The diffusion coefficient values first decreases upon the addition of nanoparticles up to 7 phr of Fe3O4 and thereafter the values increases with further loading of fillers. Composite with 7 phr of Fe3O4 exhibits low diffusivity value, indicates higher polymer filler interaction and it is also due to the restricted chain mobility of the composite. This fact is reflected in the study of morphology of the composite studied by TEM. The increase of diffusivity value at higher loading of nanoparticles can be explained in terms of aggregation of fillers that leads to an increase in free volume of the samples due to the poor interaction between the nanoparticles and EVA matrix.
Sorption mechanism
The mechanism of transport properties of polymeric membrane can be followed by using the empirical equation [26]
where Q t is the mol% solvent uptake at time t and Q ∞ is the equilibrium mol% uptake. The slope of the plot log Qt/Q∞ vs. log t gives the value of n, indicating the mechanism of transport and its y-intercept is the value of k, depends on the structural significance of polymer as well as its interaction with the solvent. The value of n suggests the mode of transport and the values of n and k for different systems are given in Table 3. It can be seen that the diffusion process in the present case deviates from the regular Fickian trend, observed with conventional rubbers, and can be classified as anomalous. These facts suggest that the nanoparticles effectively reduce the available free volume and hence the nanocomposite shows higher n values than the unfilled polymeric matrix. The nano level dispersion of metal particles in the polymer imparts a high degree of restriction to the macromolecular chain rearrangement. Thus the observed anomalous diffusion involves the retaliation between the ability of polymer segments to rearrange in the presence of solvents and the restriction imparted to the composite by the nanoparticles. The value of k implies the structural characteristics of polymer and gives an idea about the nature of interaction between the polymer and solvent. The k values decrease with increase in the molecular size of the penetrants from benzene to xylene, indicating decreased polymer- solvent interaction. The decrease in k values with increase in molecular size of the penetrants from benzene to xylene, indicating decreased polymer- solvent interaction.
Determination of crosslink density
The crosslink density of the vulcanized sample was determined from swelling data. The molecular weight between crosslinks (Mc) has estimated using the Flory–Rehner equation [27]
where ρp is the density of the polymer, V is the molar volume of the solvent, φ is the volume fraction of the polymer in the fully swollen state and χ is the polymer–solvent interaction parameter which is given by Eq. (5) [28]
in which, N is calculated from Eq. (6)
The M c values of EVA and Fe3O4 reinforced EVA nanocomposites are given in Table 4. The decrease in M c values of nanocomposite compared to EVA is due to the reinforcement of magnetite nanoparticles in the polymer and hence the stiffness of the material increases. The nanocomposite with 7 phr of sample shows the lower M c values indicating the strong interfacial interaction between the polymer and Fe3O4 nanoparticles and this result in lower swelling of the sample. This result is in good agreement with the rheometric data obtained from cure characteristics. It is well known that maximum torque is the measure of crosslink density [29]. In the present study, the maximum torque (from rheometric data) is increased with the increase in concentration of nanoparticles up to 7 phr and thereafter the maximum torque value found to be slightly decreases. Similarly in swelling studies, when the concentration of nanoparticles in EVA increased above 7 phr, the Mc values are found to be increases due to the poor strain of the network. It is also observed that the molecular mass (M c) changes with the solvent and this is due to the variation in polymer chain entanglements density or the filler packing between the macromolecular chains.
Conclusions
EVA/magnetite nanocomposites containing different filler loading have been prepared and the cure characteristics of nanocomposites were investigated. The rate of vulcanization and maximum torque value of the nanocomposites were higher than the gum compound. Morphology of the composites was determined by transmission electron microscopy (TEM). It was found that the incorporation of nanoparticles in EVA have profound influence on the morphology of the composites and composite with 7 phr of Fe3O4 exhibit uniform dispersion of nanoparticle. The uniform dispersion of nanoparticles decreased with an increase in the filler loading. The liquid transport characteristics of the nanocomposite were analyzed using benzene, toluene and xylene as probe molecules and the results were compared with unfilled sample. Nanocomposites showed a reduced swelling rate was due to the enhanced polar-polar interaction between filler and the polymer. The solvent uptake was minimum for 7 phr of filler show the high compatibility with EVA matrices in such a way that the polymer filler interface greatly hinders the diffusivity process. However, the solvent uptake tendency increased at higher filler loading. This can be ascribed in terms of poor interaction between the matrix and filler, leading to aggregation of nanoparticles. The transport coefficient and activation parameter showed a dependence on the size of penetrant molecules. The transport phenomenon was found to follow anomalous type mechanism in all the cases. The values of the polymer-solvent interaction parameter were used to calculate the molar mass between crosslinks using Flory–Rehner theory.
References
Chow WS, Neoh SS (2009) Mechanical, morphological and thermal properties of polycarbonate/SEBS-G-MA/montmorillonite nanocomposites. Polym Plast Technol Eng 49:62–68
Sukhorukov G, Ferry A, Mohwald H (2005) Iintelligent micro and nanocapsules. Prog Polym Sci 30:885–897
Ramesan MT (2013) Synthesis, characterization and conductivity studies of polypyrrole/copper sulfide nanocomposites. J Appl Polym Sci 128:1540–1546
Steurer P, Wissert R, Thomann R, Mulhaupt R (2009) Functionalized graphenes and thermoplastic nanocomposites based upon expanded graphite oxide. Macromol Rapid Commun 30:316–327
Sakinah ZAA, Ratnamb CT, Chuah AL, Yaw TCS (2009) Effect of mixing conditions on the tensile properties of ethylene vinyl acetate/waste tire dust (EVA/WTD) blend. Polym Plast Technol Eng 48:1139–1142
George JJ, Bhowmick AK (2008) Fabrication and properties of ethylene vinyl acetate-carbon nanofiber nanocompsoites. Nanoscale Res Lett 3:508–515
Bousmina M (2006) Study of intercalation and exfoliation processes in polymer nanocomposites. Macromolecules 39:4259–4263
Annet C, Sadiku ER, Vorster OC (2010) The Rheological and mechanical properties of ethylene-vinyl acetate (EVA) copolymer and organoclay nanocomposites. J Reinf Plast Compos 29:558–570
Yang F, Yngard R, Nelon GL (2005) Flammability of polymer clay and polymer silica nanocomposites. J Fire Sci 23:209–226
Krook M, Albertsson AC, Gedde UW, Hedenqvist MS (2002) Barrier and mechanical properties of montmorillonite/polyesteramide nanocomposites. Polym Eng Sci 42:1238–1246
Maiti M, Bhowmick AK (2007) Effect of polymer–clay interaction on solvent transport behavior of fluoroelastomer–clay nanocomposites and prediction of aspect ratio of nanoclay. J Appl Polym Sci 105:435–445
Chang JH, An YU (2002) Nanocomposites of polyurethane with various organoclays: thermomechanical properties, morphology, and gas permeability. J Polym Sci B Polym Phys 40:670–677
Feldman D (2013) Polymer nanocomposite barriers. J Macromol Sci Part A 50:441–448
Varga Z, Filipcsei G, Zrınyi M (2006) Magnetic field sensitive functional elastomers with tuneable elastic modulus. Polymer 47:227–233
Kong I, Ahmad SH, Abdullah MH, Hui D, Yusoff AN, Puryanti D (2010) Magnetic and microwave absorbing properties of magnetite–thermoplastic natural rubber nanocomposites. J Magnet Magn Mater 322:3401–3409
Ramesan MT (2013) Synthesis and characterization of magnetoelectric nanomaterial composed of Fe3O4 and polyindole. Adv Polym Technol 32:928–936
Vu VT, Mark JE, Pham LH, Engelhardt MJ (2001) Clay nanolayer reinforcement of cis-1,4- polyisoprene and epoxidized natural rubber. J Appl Poly Sci 82:1391–1403
Konar RB, Roy SK, Pariya TK (2010) Study on the effect of nano and active particles of alumina on natural rubber–alumina composites in the presence of epoxidized natural rubber as compatibilizer. J Macromol Sci Part A 47:416–422
Privalko VP, Ponomarenko SM, Privalko EG, Schon F, Gronski W (2003) Interfacial interactions-controlled thermoelasticity and Stress relaxation behavior of synthetic rubber/organoclay nanocomposites. J Macromol Sci Part B 42:1183–1196
Ramesan MT (2011) Effect of silica on uncompatibilised and compatibilised styrene butadiene rubber and nitrile rubber blends. Int J Polym Mater Polym Biomater 60:1130–1146
Ramesan MT (2013) Preparation and properties of Fe3O4/ polypyrrole/ poly(pyrrole-co-acrylamide) nanocomposites. Int J Polym Mater Polym Biomater 62:277–283
Prema KH, Kurian P, Anantharaman MR, Sunny V (2010) Cure kinetics and sorption characteristics of neoprene-based rubber ferrite composites. Int J Polym Mater Polym Mater 59:173–183
Aminabhavi TM, Khinnavar RS (1993) Diffusion and sorption of organic liquids through polymer membranes: 10. Polyurethane, nitrile butadiene rubber and epichlorohydrin versus aliphatic alcohols (C1–C5). Polymer 34:1006–1018
Kumar SA, Kumaran MG, Thomas S (2007) Sorption and diffusion of normal alkanes through poly(ethylene-co-vinyl acetate) membranes. J Polym Sci B 45:2470–2480
Harogoppad SB, Aminabhavi TM (1991) Interactions of substituted benzenes with elastomers. Polymer 32:870–876
Bhowmick AK, Stephens HL (1988) Handbook of elastomers. Marcel Dekker, New York
Sareena C, Ramesan MT, Purushothaman E (2012) Transport studies of peanut shell powder reinforced natural rubber composites in aromatic solvents. Polym Comp 33:1678–1692
Aminabhavi TM, Munnolli RS (1994) An assessment of chemical compatibility of bromobutyl rubber, chlorosulfonated polyethylene and epichlorohydrin membranes in the presence of some hazardous organic liquids. J Hazard Mater 38:223–242
Ramesan MT (2015) Processing characteristics and mechanical and electrical properties of chlorinated styrene butadiene rubber/ fly ash composites. J Thermoplast Comp Mater 28:1286–1300
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramesan, M.T. Effects of magnetite nanoparticles on morphology, processability, diffusion and transport behavior of ethylene vinyl acetate nanocomposites. Int J Plast Technol 19, 368–380 (2015). https://doi.org/10.1007/s12588-016-9130-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12588-016-9130-y