Abstract
Prosaposin (PS) is the precursor of four sphingolipid activator proteins, saposin A–D. PS is both a precursor protein and a neuroprotective factor, and is up-regulated in response to excitotoxicity induced by kainic acid (KA), a glutamate analogue. Excess glutamate release induces neuropathological disorders such as ischemia and seizure. Our group’s research revealed that PS immunoreactivity (IR) increased significantly in the hippocampal and cortical neurons on day 3 after KA injection, and high PS levels were maintained even after 3 weeks. The increase in PS, but not saposins, as detected by immunoblotting, suggests that the increase in PS-IR after KA injection was not caused by an increase in saposins acting as lysosomal enzymes after neuronal damage but, rather, by an increase in PS as a neurotrophic factor to improve neuronal survival. An 18-mer peptide (PS18) derived from the PS neurotrophic region significantly protected hippocampal neurons against KA-induced destruction. Furthermore, parvalbumin-positive GABAergic inhibitory interneurons and their axons exhibited intense PS expression. These results suggest that axonally transported PS protects damaged hippocampal pyramidal neurons from KA-induced neurotoxicity. Further in vitro studies that include the transfection of the PS gene will help with clarifying the mechanisms underlying the transport and secretion of PS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosaposin (PS) is the precursor protein of four small lysosomal glycoproteins, saposin A–D (Fig. 1; O’Brien et al. 1988; Sano et al. 1988). Each saposin activates specific lysosomal sphingolipid hydrolases (O'Brien and Kishimoto 1991; Kishimoto et al. 1992). Saposins and PS are widely expressed in various tissues, although the brain, skeletal muscle, and heart cells predominantly contain unprocessed PS rather than saposins (Sano et al. 1989, 1992; Kondoh et al. 1991, 1993; Hosoda et al. 2007; Terashita et al. 2007; Shimokawa et al. 2013). In addition, unprocessed PS is found in various secretory fluids, such as seminal plasma, bile, pancreatic juice, human breast milk, and cerebrospinal fluid (Hineno et al. 1991; Hiraiwa et al. 1992), and PS mRNA is strongly expressed in the choroid plexus (Saito et al. 2014).
In addition to its role as a saposin precursor, PS has been identified as a potent neurotrophic factor (O'Brien et al. 1994) that exists ubiquitously throughout nervous tissues (Kondoh et al. 1993; Morales et al. 1998). PS and peptides containing the neurotrophic activity domain of PS have been shown to exhibit neuroprotective and glioprotective functions in vitro (O'Brien et al. 1994; Campana et al. 1998; Tsuboi et al. 1998; Hiraiwa et al. 1997, 1999). Similarly, our group found neuroprotective and glioprotective functions using in vivo experiments; namely, PS and an 18-mer peptide facilitated transected sciatic nerve regeneration (Kotani et al. 1996a, b) as well as rescued ischemic hippocampal CA1 neurons (Sano et al. 1994; Kotani et al. 1996a) and MPTP-damaged dopaminergic neurons (Gao et al. 2013b). Moreover, we showed that levels of intrinsic PS and its mRNA increased in the facial nerve nucleus after nerve transection (Unuma et al. 2005; Chen et al. 2008) and decreased in the brain of mdx mice (Gao et al. 2013a).
Kainic acid (KA), a glutamate analogue, is a powerful neurotoxic agent (Olney and de Gubareff 1978) that stimulates excitatory neurotransmitter release (Ferkany et al. 1982). Systemic KA injection induces neuronal damage in many brain regions, especially in the hippocampus (Nadler and Cuthbertson 1980; Nadler et al. 1981; Schwob et al. 1980; Heggli et al. 1981; Lothman and Collins 1981). Neuronal damage induced by KA resembles that in some forms of ischemia or epilepsy; thus, KA is a good investigative drug for clarifying the mechanisms underlying neurodegeneration and neuroprotection (Coyle 1987; Lévesque and Avoli 2013; Wang et al. 2005).
Although PS receptors have been defined (Meyer et al. 2013), the transport of intrinsic PS in the nervous system remains unclear. In our previous studies, we showed that intrinsic PS was up-regulated in brain neurons and the choroid plexus after systemic KA injection (Nabeka et al. 2014), and that injection of PS18, an 18-mer peptide derived from the PS neurotrophic region, alleviated KA-induced neuronal damage (Nabeka et al. 2015). Axonally transported intrinsic PS may also protect damaged hippocampal neurons (Nabeka et al. 2017).
The materials and methods used are described in detail in our previous papers (Nabeka et al. 2014, 2015, 2017).
Determining the optimal KA dose
Rats were injected intraperitoneally with 0.3 mg/kg medetomidine, 4 mg/kg midazolam and 5 mg/kg butorphanol. KA dissolved in normal saline was injected subcutaneously (0, 2, 5, 8, 10, and 20 mg/kg) to determine the optimal dose for stimulating neurons without cell death. On day 7 after KA injection, each animal was anesthetized and perfused transcardially with 4% paraformaldehyde. Each tissue sample was embedded in paraffin, sectioned, and stained using the diaminobenzidine method. Viable neurons in the CA1 region were counted. Based on the results, we selected 5 mg/kg as the optimal KA dose for subsequent experiments (Fig. 2; Nabeka et al. 2014).
a–h Crude hippocampal extracts from tissues sampled from controls and animals injected with kainic acid (KA) on experimental days 1 and 3 were examined using anti-saposin D antiserum (a–c) and anti-PS immunoglobin (Ig) G (d–f). In samples stained with anti-PS (d–f), the single band at ~ 69 kDa (arrow), which is likely to correspond to PS, exhibited increased intensity after KA injection. Arrowheads in l–n indicate damaged neurons. o Percentages of pyramidal neurons with intact morphology along 1-mm lengths in the hippocampal CA1 region after injection of saline or KA. Scale bar = 20 µm. This figure is reproduced with permission from PLoS One (Nabeka et al. 2014)
Increases in PS after KA injection
Immunoblotting of the hippocampus using anti-PS immunoglobin (Ig) G showed that PS clearly increased after KA injection (Fig. 2a–c). PS immunoreactivity (IR) (Fig. 3a, b), as analyzed using NIH Image software, increased on day 1, peaked on days 3 and 7 after KA injection, and remained significantly elevated until day 21 (Fig. 3c). In the in situ hybridization experiment, PS mRNA expression in the hippocampus increased in all hippocampal areas on day 1 after KA injection (Fig. 3d, e), peaked on day 7, and remained significantly elevated until day 21 (Fig. 3f). Hybridization signals were localized mainly in the pyramidal neurons, but some strong signals were observed outside these layers. Based on the size and localization, the cells where the signals were observed appeared to be interneurons (Nabeka et al. 2014). Intense PS signals were also observed in the choroid plexus after KA injection (Fig. 3g, h). From these results, this KA-injection model was considered suitable for use in studies of changes in neurotrophic factors.
a, b Light micrographs of hippocampal CA1 neurons stained with anti-PS IgG and exhibiting PS immunoreactivity (IR) on day 3 after injection with 5 mg/kg KA. d, e In situ hybridization showing PS mRNA expression in the rat hippocampus on day 3 after injection with phosphate-buffered saline (PBS) (d) or KA (e). g, h PS mRNA expression levels were higher after KA injection than after PBS injection in all areas of the hippocampus as well as in the choroid plexus. Scale bars = 20 µm (a, b), 500 µm (d, e), or 50 µm (g, h). This figure is reproduced with permission from PLoS One (Nabeka et al. 2014)
Protective effects of PS or PS-18 in the KA-injection model
Injured or normal pyramidal neurons in the hippocampal CA1 region in rats injected with phosphate-buffered saline (PBS), 0.2 mg/kg PS18, or 2.0 mg/kg PS18 after KA injection were counted (Fig. 4a-d). Few injured neurons and more normal neurons were observed in PS18-injected rats than in PBS-injected rats (Fig. 4e, f). Our group has previously reported similar protective effects of purified PS in the ischemic hippocampus (Sano et al. 1994). Thus, these histological examinations revealed that PS or PS18 treatment rescued CA1 neurons from potential KA-induced degeneration.
a–d Photomicrographs of toluidine blue-stained hippocampal CA1 neurons in a normal control rat (a) and in rats that received injections of PBS (b), 0.2 mg/kg PS18 (c), or 2.0 mg/kg PS18 (d) after KA injection. c, d Injured CA1 neurons were rescued by PS18 treatment. e, f Effects of PS18 on injured (e) and viable (f) neurons in the hippocampal CA1 region in rats that received a subcutaneous injection of 12 mg/kg KA. PS18 treatment decreased the number of injured neurons (e) and increased the number of viable neurons (f) in a dose-dependent manner compared with PBS treatment. **P < 0.01. A P value < 0.05 was considered to indicate statistical significance. All data are expressed as the means ± standard error of the mean. Scale bar = 100 µm. This figure is reproduced with permission from PLoS One (Nabeka et al. 2015)
Immunofluorescence staining of PS and glutamate decarboxylase (GAD) after KA injection
In KA-injected animals, especially on day 3 after KA injection, intense PS-IR was observed in some cell types inside and outside the pyramidal layer (Fig. 5a–h). The cell bodies and nuclei were thinner than those of ordinal pyramidal neurons but larger than those of glial cells (Fig. 5a, b). To determine what these cell types were, colocalization of PS and GAD was examined by double immunostaining hippocampal tissues sampled on day 3 after KA injection. PS- and GAD-IR increased in intensity in the interneurons, and overall intensity levels were higher than those in CA1 pyramidal neurons (Fig. 5b). In addition to interneuronal cell bodies, numerous axon terminals exhibiting positivity for the double-labeling of PS- and parvalbumin (PV)-IR were observed around the pyramidal neurons (Fig. 6a, b; Nabeka et al. 2017).
a, b Immunofluorescence light micrographs showing PS-IR in hippocampal CA1 neurons stained with anti-PS IgG, anti-glutamate decarboxylase (GAD), or DAPI on day 3 after injection with PBS (a) or 5 mg/kg KA (b). The cell bodies and axon terminals of GABAergic interneurons in the CA1 region were stained green with anti-GAD antibodies. Arrows indicate interneurons with slender nuclei exhibiting very intense PS- and GAD-IR in the cytoplasm. Axon terminals exhibiting positivity for GAD-PS double-staining (arrowheads) can be found around almost all CA1 neurons after KA injection (b), but only around some neurons after PBS injection (a). The nuclei were stained blue with DAPI, and red and green areas indicate PS and GAD, respectively. Scale bars = 10 µm. This figure is reproduced with permission from IBRO Reports (Nabeka et al. 2017)
Double-labeled immunofluorescence light micrographs of tissues sampled from the rat hippocampal CA1 region on day 3 after KA injection stained with anti-PS and anti-parvalbumin (PV) antibodies (a, b). CA1 pyramidal layers are displayed at a higher magnification in b. PS is stained with anti-PS (red); cell bodies (arrows), axons, and axon terminals of inhibitory neurons are stained with anti-PV (green); and nuclei are stained with DAPI (blue) in these merged images. Many putative axon terminals exhibited strong PV-IR around the pyramidal neurons (b). Scale bars = 10 µm. This figure is reproduced with permission from IBRO Reports (Nabeka et al. 2017)
Double immunofluorescence staining of PS and Tau after KA injection
PS signal intensity in Tau-positive axons or terminal boutons around the pyramidal neurons in the hippocampal CA1 region increased significantly in KA-injected animals (Fig. 7b) compared with the controls (Fig. 7a). Approximately 90% and 60% of the granules in Tau-positive terminal boutons in the CA1 region exhibited PS-IR in KA-injected and normal animals, respectively. PS-IR fluorescence in Tau-positive axons, as analyzed using NIH Image software (Fig. 7d, f), increased significantly after KA injection (Fig. 7g). In particular, PS-IR granules were larger after KA injection than after saline injection (Fig. 7f).
a, b Double-labeled immunofluorescence light micrographs of the hippocampal CA1 region in normal control rats (a) and rats on day 3 after KA injection (b) stained with anti-PS and anti-Tau. Rectangles 1–6 in a and b are shown at a higher magnification below a and b. In 1–6R, nuclei are stained with DAPI (blue) and PS is stained with anti-PS (red). In 1–6G, axons and axon terminals are stained with anti-Tau (green). Merged images can be found in 1–6 M. Arrows indicate double-stained axon terminals containing PS. c–f Double-labeled immunofluorescence light micrographs of Tau-positive axons in the CA1 region of normal controls (c) and experimental animals on day 3 after KA injection (e). d and f are black-and-white images of the PS-IR observed in c and e, respectively, as analyzed using NIH Image (g). Granules exhibiting PS-IR (arrowheads) in the Tau-positive axons in KA-injected animals were larger than those in the controls. g Percentage of Tau-positive axons around the pyramidal layer of the CA1 region exhibiting PS-IR after injection with KA or saline. A significantly higher percentage of Tau-positive axons exhibited PS-IR after KA injection than after saline injection. **P < 0.01. Scale bars = 20 µm (a, b) or 10 µm (1R–6 M, c–f). This figure is reproduced with permission from PLoS One (Nabeka et al. 2014)
Triple immunofluorescence staining of PV, PS and Tau after KA injection
Examining PS-IR in PV-positive axon terminals using electron microscopy
Ultrathin tissue sections embedded in LR White resin were incubated in a solution containing rabbit anti-saposin D serum and mouse anti-PV IgG. Then, the sections were examined under a transmission electron microscope. Upon examination of electron micrographs of the pyramidal layer of the CA3 region, saposin D-IR gold particles were found to be localized in lysosome-like organelles in pyramidal neurons (arrows in Fig. 9c–e). However, saposin D-IR gold particles were observed more frequently in pale vesicles in PV-IR axons (arrows in Fig. 9b; Nabeka et al. 2017).
Electron micrographs of pyramidal neurons and axons in the pyramidal layer of the CA3 region double-labeled for PS-IR (15 nm) with anti-saposin D antibody and PV-IR (5 nm) with anti-PV antibody. Organelles exhibiting PS-IR in a are displayed at a higher magnification in b–e. b PS-IR gold particles were observed in pale vesicles (arrows) that do not resemble lysosomes in an axon exhibiting PV-IR. Staining with anti-saposin D antibody revealed the presence of PS-IR (15-nm gold particles) in lysosome-like organelles (arrows) in a PV-positive (arrowheads) axon. c–e PS-IR gold particles were observed in lysosome-like organelles (arrows) in three pyramidal neurons. Scale bars = 0.1 µm in d, e. This figure is reproduced with permission from IBRO Reports (Nabeka et al. 2017)
Conclusion and future perspectives
KA is a glutamate analogue, and KA injection causes neurotoxicity in animals. Moreover, PS, a neurotrophic factor, reportedly increases during neurotoxic events. We previously reported that PS was transported axonally in the cerebral cortex in a KA-injection rat model (Nabeka et al. 2014), which is, to date, the only report of axonal PS transport. In a follow-up study, PS18, the N-terminal peptide sequence of saposin C, was found to reduce neurotoxicity from KA injection in the hippocampus in the same KA-injection model (Nabeka et al. 2015).
Increases in PS were observed in the axons of PV-positive interneurons in rats after KA injection, and PS was concurrently secreted from synapses. Interneurons secrete PS around the pyramidal neurons of the hippocampus, protecting them from KA neurotoxicity (Nabeka et al. 2017). Figure 10 summarizes these findings.
Schematic representation of neuroprotection against excitotoxic stimulation. Pyramidal neurons can produce neuroprotective PS with a paracrine or autocrine function. PV-positive interneurons around the pyramidal layer can also produce PS, which is then transported to the axon terminals and secreted with GABA around the pyramidal neurons. Additionally, the choroid plexus produces PS and secretes it into the cerebrospinal fluid, where it responds to excitotoxic stimulation induced by KA. This figure is reproduced with permission from IBRO Reports (Nabeka et al. 2017)
Overall, neurotoxicity due to systemic KA injection in rats is particularly strong in the hippocampus, which exhibits increased PS levels. Increases in secretory PS were observed in PV-positive GABAergic inhibitory interneurons around pyramidal neurons and the choroid plexus, with higher levels of PS in the axons of interneurons. These findings suggest that axonal transport of PS results in neuroprotective activity in hippocampal pyramidal neurons.
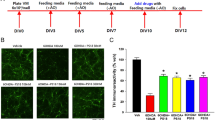
Future research will focus on the axonal transport and secretion of PS in cultured neurons. To this end, a DsRed-fused PS sequence has been constructed and transfected into cultured cells (Fig. 11). PS expression was confirmed using Western blotting with the anti-PS antibody. Further application of this method in tandem with time-lapse microscopy will help to clarify the mechanism underlying intracellular PS movement.
References
Campana WM, Eskeland N, Calcutt NA, MisasiMyers OʹBrien RRRJS (1998) Prosaptide prevents paclitaxel neurotoxicity. Neurotoxicology 19:237–244
Chen J, Saito S, Kobayashi N et al (2008) Expression patterns in alternative splicing forms of prosaposin mRNA in the rat facial nerve nucleus after facial nerve transection. Neurosci Res 60:82–94
Coyle JT (1987) Kainic acid: insights into excitatory mechanisms causing selective neuronal degeneration. Ciba Found Symp 126:186–203
Ferkany JW, Zaczek R, Coyle JT (1982) Kainic acid stimulates excitatory amino acid neurotransmitter release at presynaptic receptors. Nature 298:757–759
Gao HL, Li C, Nabeka H et al (2013a) Decrease in prosaposin in the Dystrophic mdx mouse brain. PLoS ONE 8:e80032
Gao HL, Li C, Nabeka H et al (2013b) Attenuation of MPTP/MPP(+) toxicity in vivo and in vitro by an 18-mer peptide derived from prosaposin. Neuroscience 236:373–393
Heggli D, Aamodt A, Malthe-Sørenssen D (1981) Kainic acid neurotoxicity; effect of systemic injection on neurotransmitter markers in different brain regions. Brain Res 230:253–262
Hineno T, Sano A, Kondoh K, Ueno S, Kakimoto Y, Yoshida K (1991) Secretion of sphingolipid hydrolase activator precursor, prosaposin. Biochem Biophys Res Commun 176:668–674
Hiraiwa M, Soeda S, Kishimoto OʹBrien YJS (1992) Binding and transport of gangliosides by prosaposin. Proc Natl Acad Sci USA 89:11254–11258
Hiraiwa M, Martin BM, Kishimoto Y, Conner GE, Tsuji S, OʹBrien JS (1997) Lysosomal proteolysis of prosaposin, the precursor of saposins (sphingolipid activator proteins): its mechanism and inhibition by ganglioside. Arch Biochem Biophys 341:17–24
Hiraiwa M, Campana WM, Mizisin AP, Mohiuddin L, OʹBrien JS (1999) Prosaposin: a myelinotrophic protein that promotes expression of myelin constituents and is secreted after nerve injury. Glia 26:353–360
Hosoda Y, Miyawaki K, Saito S et al (2007) Distribution of prosaposin in the rat nervous system. Cell Tissue Res 330:197–207
Kishimoto Y, HiraiwaOʹBrien MJS (1992) Saposins: structure, function, distribution, and molecular genetics. J Lipid Res 33:1255–1267
Kondoh K, Hineno T, SanoKakimoto AY (1991) Isolation and characterization of prosaposin from human milk. Biochem Biophys Res Commun 181:286–292
Kondoh K, Sano A, Kakimoto Y, MatsudaSakanakam SM (1993) Distribution of prosaposin-like immunoreactivity in rat brain. J Comp Neurol 334:590–602
Kotani Y, Matsuda S, Sakanaka M, Kondoh K, Ueno S, Sano A (1996a) Prosaposin facilitates sciatic nerve regeneration in vivo. J Neurochem 66:2019–2025
Kotani Y, Matsuda S, Wen TC et al (1996b) A hydrophilic peptide comprising 18 amino acid residues of the prosaposin sequence has neurotrophic activity in vitro and in vivo. J Neurochem 66:2197–2200
Lévesque M, Avoli M (2013) The kainic acid model of temporal lobe epilepsy. Neurosci Biobehav Rev 37:2887–2899
Lothman EW, Collins RC (1981) Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res 218:299–318
Meyer RC, Giddens MM, Schaefer SA, Hall RA (2013) GPR37 and GPR37L1 are receptors for the neuroprotective and glioprotective factors prosaptide and prosaposin. Proc Natl Acad Sci USA 110:9529–9534
Morales CR, Hay N, El-Alfy M, Zhao Q (1998) Distribution of mouse sulfated glycoprotein-1 (prosaposin) in the testis and other tissues. Journal of andrology 19:156–164
Nabeka H, Uematsu K, Takechi H et al (2014) Prosaposin overexpression following kainic acid-induced neurotoxicity. PLoS ONE 9:e110534
Nabeka H, Shimokawa T, Doihara T et al (2015) A prosaposin-derived peptide alleviates kainic acid-induced brain injury. PLoS ONE 10:e0126856
Nabeka H, Saito S, Li X et al (2017) Interneurons secrete prosaposin, a neurotrophic factor, to attenuate kainic acid-induced neurotoxicity. IBRO Reports 3:17–32
Nadler JV, Cuthbertson GJ (1980) Kainic acid neurotoxicity toward hippocampal formation: dependence on specific excitatory pathways. Brain Res 195:47–56
Nadler JV, Evenson DA, Cuthbertson GJ (1981) Comparative toxicity of kainic acid and other acidic amino acids toward rat hippocampal neurons. Neuroscience 6:2505–2517
OʹBrien JS, Kishimoto Y (1991) Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J 5:301–308
OʹBrien JS, CarsonSeo GSHC, HiraiwaKishimoto MY (1994) Identification of prosaposin as a neurotrophic factor. Proc Natl Acad Sci USA 91:9593–9596
OʹBrien JS, KretzDewji KAN, WengerEsch DAF, Fluharty AL (1988) Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science 241:1098–1101
Olney JW, De Gubareff T (1978) Glutamate neurotoxicity and Huntington’s chorea. Nature 271:557–559
Saito S, Saito K, Nabeka H, Shimokawa T, Kobayashi N, Matsuda S (2014) Differential expression of the alternatively spliced forms of prosaposin mRNAs in rat choroid plexus. Cell Tissue Res 356:231–242
Sano A, Radin NS, Johnson LL, Tarr GE (1988) The activator protein for glucosylceramide beta-glucosidase from guinea pig liver. Improved isolation method and complete amino acid sequence. J Biol Chem 263:19597–19601
Sano A, Hineno T, Mizuno T et al (1989) Sphingolipid hydrolase activator proteins and their precursors. Biochem Biophys Res Commun 165:1191–1197
Sano A, Mizuno T, Kondoh K et al (1992) Saposin-C from bovine spleen; complete amino acid sequence and relation between the structure and its biological activity. Biochem Biophys Acta 1120:75–80
Sano A, Matsuda S, Wen TC et al (1994) Protection by prosaposin against ischemia-induced learning disability and neuronal loss. Biochem Biophys Res Commun 204:994–1000
Schwob JE, Fuller T, Price JL, Olney JW (1980) Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience 5:991–1014
Shimokawa T, Nabeka H, Yamamiya K et al (2013) Distribution of prosaposin in rat lymphatic tissues. Cell Tissue Res 352:685–693
Terashita T, Saito S, Miyawaki K et al (2007) Localization of prosaposin in rat cochlea. Neurosci Res 57:372–378
Tsuboi K, Hiraiwa M, OʹBrien JS (1998) Prosaposin prevents programmed cell death of rat cerebellar granule neurons in culture. Brain Res Dev Brain Res 110:249–255
Unuma K, Chen J, Saito S et al (2005) Changes in expression of prosaposin in the rat facial nerve nucleus after facial nerve transection. Neurosci Res 52:220–227
Wang Q, Yu S, Simonyi A, Sun GY, Sun AY (2005) Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol 31:3–16
Acknowledgements
I would like to thank Seiji Matsuda and past and present members of Department of Anatomy and Embryology, Ehime Graduate School of Medicine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nabeka, H. Prosaposin, a neurotrophic factor, protects neurons against kainic acid-induced neurotoxicity. Anat Sci Int 96, 359–369 (2021). https://doi.org/10.1007/s12565-021-00605-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-021-00605-y