Abstract
Female urinary incontinence mainly relates to damage of female urethra supporting structures, while its anatomy and function specially in which the connective tissue part are still unclear and controversial. We study it based on 4 thin-sectional, high-resolution, transverse sectional anatomical images [Chinese Visible Human (CVH) images] and 10 high-resolution MRI images from volunteers. The female urethral supporting structures and its adjacent structures were segmented and three-dimensional (3D) reconstructed with Amira software. The urethral supporting structures include muscular and connective tissue supporting structures. Muscular supporting structures are composed of levator ani muslce and striated urethral sphincter, the connective tissue supporting structures are composed of anterior vaginal wall, pubovesical muscle, pubovesical ligament, lateral vesical ligament, and tendinous arch of pelvic fascia (TAPF). The anterior vaginal wall includes tight and loose connections between urethral, bladder, and vagina. The lateral vesical ligament connects the proximal part of the urethra to the TAPF. The pubovesical muscle is crescent shaped and continues with the detrusor of the bladder superior and directly connects the TAPF laterally. The TAPF is an obvious fibrous structure that originates at the middle-posterior surface of the pubis, travels onto the parietal pelvic fascia, and inserts posteriorly onto the ischial spine. The anterior vaginal wall, the pubovesical muscle, the lateral vesical ligament, and the TAPF create the “hammock” structure and supplement DeLancey's theory. Its support to the proximal urethra and neck of bladder is crucial to maintain stability and urinary continence during increased abdominal pressure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pelvic floor dysfunction, including stress urinary incontinence (SUI) and pelvic organ prolapse (POP), is a common middle aged and old female disease that significantly affects female daily life. In the world, approximately 20% of post-menopausal females have this disease due to delivery, obesity, and increasing chronic abdominal pressure (Jelovsek and Barber 2006; Nygaard et al. 2014). A woman’s lifetime risk of surgery for POP is 12–19% and more than 300,000 prolapse surgeries performed annually in the US, which cost much money in each year (Barber 2016).
In the urinary continence system, closure and stability of the bladder outlet are very important and are chiefly provided by the urethra and its supporting structures. Therefore, we call the anatomical structures which are close to and support the urethra as the urethral supporting structures. Anatomical or functional impairment of the urethral supporting structures is regarded as the major cause of stress urinary incontinence and anterior pelvic organ prolapse (Delancey 2010).
Urethral supporting structures are complicated and too general structures including active supporting structure which is also called muscular supporting structure and passive supporting structure which is also called connective tissue structure (El-Sayed et al. 2007; Herschorn 2004). Muscular supporting structures include the levator ani muscle and the striated urethral sphincter, which are striated muscle and can be regulated actively by the human body. While the connective tissue supporting structures mainly include dense connective tissue and smooth muscle around the urethra, which create antagonistic force with increasing abdominal pressure and also play an important role in urethral supporting (Strohbehn et al. 1996). Impairment of urethral supporting structures promotes high mobility of the vesical neck, causing the vesical neck to move downward during rapid increases in intra-abdominal pressure and leading to stress urinary incontinence (SUI) or pelvic organ prolapse (POP) (Brandao et al. 2015; Patel et al. 2007).Clinicians and anatomists have long recognized that the support to the urethra is important in preventing the pelvic floor dysfunction (DeLancey 1989; Hodgkinson 1953).
In our previous research, we study the architecture of female urethral sphincter complex and levator ani muscle based on unreformed thin-sectional anatomical image in detail (Wu et al. 2015, 2018; Chen et al. 2017).
However, the detailed three-dimensional (3D) anatomic shape, subdivision, spatial relationship, and function of the urethral connective tissue supporting structures have many controversies and are still not well defined (Mauroy et al. 2000).
Many anatomists and gynecologists used different research approaches, including CT, MRI, and dissection, to study anatomy of female anterior pelvic floor (Kraima et al. 2013; Luo et al. 2014; Ramanah et al. 2012). However, MRI and CT have limited articulation and resolution, and it is difficult to identify the connective tissue of the pelvic floor. Dissection is prone to generate anatomy artifacts, including structure destruction and position changing, particularly in tiny facial and ligament structures.
Therefore, we plan to study the architecture of urethral connective supporting structures based on real-color, high-resolution, thin-sectional and unreformed sectional anatomical images (Chinese Visible Human images) (Wu et al. 2015; Zhang et al. 2003, 2004) and normal female pelvic MRI images, which can help to imaging diagnosis, surgical treatment, and clinical anatomy teaching.
Materials and methods
Data collection
Cross-section images from the iliac crest to the lower rim of the perineum were selected from the CVH female, including CVH2, CVH4, CVH5, and CVO. All cadavers were enrolled in the body donation program of the CVH project, which follows the scientific and ethical rules of the Third Military Medical University. The thickness of images ranged from 0.2 to 1.0 mm, and the maximal resolution was 4064 × 2704, and the pixel size can reach 0.12 mm × 0.12 mm × 0.2 mm. The detailed parameters of CVH images are presented in Table 1. No pathologies were found via CT and MRI scans before specimen milling.
Ten pelvic MRI images of healthy female volunteers were selected. Approximately 30 min before 3.0 T MRI scanning, the volunteers emptied their bladder (SIEMENS 3.0 Trio, four-channel surface coil). In MRI scanning, female volunteers were placed in a supine position. All women were asked to relax the pelvic floor muscle during the MRI examination. MRI scanning ranged from sacral promontory to the lower edge of perineum. A standardized protocol with the following parameters was used: turbo spin-echo (TSE) sequences, repetition time (TR) of 8610 ms, echo time (TE) of 9.8 ms, axial slice orientation with a field of view of 280 mm × 280 mm, pixel size of 0.55 mm × 0.55 mm, an image matrix of 512 × 512, and a slice thickness of 2 mm.
Segmentation and 3D reconstruction
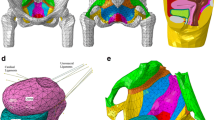
With Amira software (https://www.amiravis.com, version 5.3.3) (Fig. 1a), pelvic structures, including the pelvic bone, veins, bladder, compressor urethrae, urethrovaginal sphincter, urethral sphincter proper, medial and lateral part of pubovisceral muscle, superficial and deep part of the puborectal muscle, rectal wall, arcuate ligament, pubovesical ligament, lateral vesical ligament, pubovesical muscle, sacrotuberous ligament, sacrospinous ligament, piriformis, tendinous arch of pelvic fascia (TAPF), tendinous arch of levator ani (TALM), superior and inferior part of urethrovaginal septum, vesicovaginal septum, and cervicovaginal septum were segmented.
Criteria for segmentation included differences in tissue's natural color and fibrous septum. Specially, we segmented the levator ani muscle according to muscle fiber orientation and connective tissue septum between muscle fibers (Wu et al. 2015). Furthermore, we always inspected the corresponding sections in all other CVH specimens before proceeding to segmentation.
After segmentation, we used 3D reconstruct, smooth and simplify the urethral connective tissue structures with Amira software.
Creation of Interactive 3D-pdf
We exported the 3D models of female urethra and its supporting structures from the Amira software and imported them to CINEMA 4D (Maxon Computer GmbH, Friedrichsdorf, Germany). The ‘mesh’ function of CINEMA was used to create polygons that fitted the original AMIRA vertebral models. Furthermore, CINEMA 4D enabled the export of 34 modelled structures via ‘wrl export’ into an ADOBE portable device format (PDF) reader version 9 (https://www.adobe.com), which allows the generation of three-dimensional interactive PDF files (Supplementary Figure). The 3D-PDF allows the reader to three-dimensionally visualize all structures separately or as a whole to inspect their 3D shape and topographical relationship through Adobe Reader or Acrobat software.
Results
Four female pelvises (CVH 2, 4, 5 and CVO) were studied. Some available biometric details of the specimens are provided in Table 1.
We successfully performed an interactive 3D reconstruction of urethral supporting structures and studied them, including the anterior vaginal wall, the pubovesical muscle, the pubovesical ligament, the lateral vesical ligament and the TALM, and the adjacent active muscular structures including the striated urethral sphincter and the levator ani muscle. (Figs. 1b, 2a–f).
Shape and topographic relations of urethral muscular supporting structures. a–d Urethral muscular supporting structure. a Urethral muscular supporting structure in superior view; b urethral muscular supporting structure in lateral view; c urethral muscular supporting structure in inferior view; d urethral muscular supporting structure in lateral view with levator ani muscle transparent. B bladder, CU compressor urethra, DPR deep part of the puborectal muscle, EPV external layer of pubovisceral muscle, IPV internal layer of pubovisceral muscle, LAM levator ani muscle, R rectum, SPR superficial part of the puborectal muscle, U uterus, USP urethral sphincter proper, UVS urethrovaginal sphincter, V vagina
Active muscle supporting structure
Levator ani muscle
The levator ani muscle (LAM) was a funnel-shaped structure and was composed of the pubovisceral and the puborectal muscle. The pubovisceral muscle was bilayered: its internal layer and its outer, patchy layer reinforced the inner layer. The puborectal muscle included deep and superficial parts, which coincided with the “deep and superficial portion” of the external anal sphincter (EAS) (Fig. 2) (Wu et al. 2015).
The striated urethral sphincter
The striated urethral sphincter included the urethral sphincter proper, the urethral compressor, and the urethrovaginal sphincter. The sphincter proper encircled the urethra and contained a tendinous portion in the posterior midline. The U-shaped compressor muscle surrounded the urethra and the urethral sphincter anteriorly and laterally at the transition of the upper two-third into the lower one-third (Fig. 1). Posteriorly, the compressor muscle passed on the vaginal wall to insert into the anteroinferior border of the puborectal muscle with tendinous tissue. The distal part of the striated sphincter, the urethrovaginal sphincter, encircles both urethra and vagina.
Passive connective tissue supporting structure
Compared with active supporting structures, urethral passive supporting structures included the urethrovaginal septum, the pubovesical muscle, the pubovesical ligament, the lateral vesical ligament, and the TALM.
Anterior vaginal wall
The anterior vaginal wall is a strong urethral connective tissue support which has broad contact face. From top to bottom, this structure includes the cervicovaginal septum, vesicovaginal septum, superior part of urethrovaginal septum, and inferior part of urethrovaginal septum.
The cervicovaginal septum was 20.3 ± 6.1 mm in length. The vesicovaginal septum was 18.0 ± 5.6 mm in length. The superior part and the inferior part of the urethrovaginal septum were 18.3 ± 0.6 mm and 13.0 ± 5.0 mm in length, respectively. The cervicovaginal septum, vesicovaginal septum, and superior part of urethrovaginal septum are composed of loose connective tissue, and some vessels were located inside it, whereas the inferior part of urethrovaginal septum was composed of tight connective tissue (Figs. 3a–c, 4b, c). In the MRI images, the upper loose part showed higher signal due to the vessels, whereas the lower tight part showed low signal (Fig. 3d–f).
CVH sectional anatomical images and MRI of urethral connective tissue supporting structure. g The position of CVH and MRI transverse sections. The transverse sections show the transverse position of the lateral vesical ligament, superior part of urethrovaginal septum (a, d), pubovesical muscle, pubovesical ligament, lateral vesical ligament (b, e), inferior part of urethrovaginal septum (c, f) in CVH sectional image and MRI images. h, i The pubovesical ligament in VHP and CVH4 transverse anatomical images. AL arcuate ligament, Ante anterior, B bladder, CL clitoris, CU compressor urethra, LAM levator ani muscle, LVL lateral vesical ligament, Post posterior, PVL pubovesical ligament, PVM pubovesical muscle, R rectum, RS Retzius space, TAPF tendinous arch of pelvic fascia, U urethral, USP urethral sphincter proper, UVS urethrovaginal sphincter, Ante anterior, V vagina
Pubovesical muscle
The pubovesical muscle is a crescent-shaped smooth muscle with left to right direction. This structure was located anteoinferior to the bladder neck and anterior to the upper part of the main part of the urethral sphincter and continued with the detrusor of the bladder, which is like the apron of the detrusor. This muscle directly connects to the TAPF on the left and right side, and connects to the lateral vesical ligament posteriorly. Retropubic fat tissue was located between the pubovesical muscle and the anterior pubis and ended at urethral compressor muscle inferiorly. The urethral compressor muscle is located beneath the pubovesical muscle, but it does not connect with it (Figs. 3b, h, 4a, d, e). In MRI images, the structure was obvious and showed lower signal compared with that of the pelvic floor muscle (Fig. 5e).
Shape and topographic relations of urethral connective tissue supporting structures. a–f Urethral connective tissue supporting structure in posteosuperior view. a Urethral sphincter complex including compressor urethrae, urethrovaginal sphincter and urethral sphincter proper. Urethral connective tissue supporting structure includes pubovesical muscle (b), pubovesical ligament (c), lateral vesical ligament (d), tendinous arch of pelvic fascia (e) and urethrovaginal septum (f). Ante anterior, AVW anterior vaginal wall, CU compressor urethra, LVL lateral vesical ligament, PB pubic bone, Post posterior, PVL pubovesical ligament, PVM pubovesical muscle, TAPF tendinous arch of pelvic fascia, USP urethral sphincter proper, UVS urethrovaginal sphincter
Pubovesical ligament
The pubovesical ligament differed from the pubovesical muscle. This ligament was located between the pubis and the main part of the urethal sphincter and located in the the Retzius space, and directly connected to the pubovesical muscle. The shape of the pubovesical ligament exhibits wide variance. In the right part of CVH5 and VHP female, the structure was slim and originated from the anterior–lateral part of the main part of urethral sphincter, and ended in the posterior wall of pubis, and connected the upper part of the urethra to the pubis (Figs. 3b, h, 4a, 6c, d). In CVH2 and CVH4 female pelvises, the two side structures fused and were located in the middle of the Retzius space (Fig. 3i). While in the left part of CVH5 pelvis and volunteer MRI pelvis, this structure was fused with the anterior part of the TAPF (Fig. 3b, e). This ligament mainly keeps in anterior to posterior direction and the length was 12.3 ± 5.0 mm.
Shape and topographic relations of urethral connective tissue supporting structure. a Posteosuperior view of pelvis; b anterior view of urethrovaginal septum; c anterior view of urethrovaginal septum with bladder transparent; d and e anterior and right view of PVL, LVL, and PVM. AL arcuate ligament, Ante anterior, b bladder, CL clitoris, CS cervicovaginal septum, CU compressor urethra, Infe inferior, IUS inferior part of urethrovaginal septum, LAM levator ani muscle, LVL lateral vesical ligament, PB pubic bone, PVL pubovesical ligament, PVM pubovesical muscle, Post posterior, Supe superior, SUS superior part of urethrovaginal septum, TAPF tendinous arch of pelvic fascia, U urethral, USP urethral sphincter proper, UVS urethrovaginal sphincter, V vagina, VS vesicovaginal septum
Sectional anatomy and 3D topography of the TAPF. f The position of transverse image in a–c; d the posteosuperior view of the female pelvis; e the posteosuperior view of the structures which create hammock. AL arcuate ligament, AVW anterior vaginal wall, B bladder, CL clitoris, Co coccygeus, EPV external layer of pubovisceral muscle, IO internal obturator muscle, IPV internal layer of pubovisceral muscle, IS ischial spine, LAM levator ani muscle, LVL lateral vesical ligament, PVL pubovesical ligament, PVM pubovesical muscle, R rectum, SB sacral bone, SSL sacrospinous ligament, STL sacrotuberous ligament, TAPF tendinous arch of pelvic fascia, U urethral, V vagina
Lateral vesical ligament
The lateral vesical ligament originated from the lateral wall of the superior part of the urethral sphincter and the antero-lateral wall of the lower part of the vagina and ended in the TAPF, which affixed with the urethra to the TAPF and prevented the urethra from prolapsing downward. The lateral vesical ligament contained much dense connective tissue and vascular plexus which was mainly located in the medial part of the lateral vesical ligament, and connected the pubovesical ligament anteriorly. The antero-posterial length of the lateral vesicle ligament was 22.8 ± 5.7 mm in all CVH, and the superior-inferior length of the ligament to where attached the urethral sphincter proper was 13.0 ± 2.6 mm (Figs. 3a, b, 4a, d, 6c, d). In MRI images, the connective tissue part of the lateral vesical ligament was obvious and showed lower signal, and the vascular plexus part which was mainly located in the posterior part of the lateral vesical ligament and showed higher signal. The ligament originated from the antero-lateral part of the urethra and the posteo-lateral part of the bladder and ended in the levator ani muscle (Fig. 3d).
The tendinous arch of the pelvic fascia (TAPF)
The TAPF was a fibrous structure which began at the medial inferior part of posterior surface of the pubis and the beginning point was lateral to the pubic symphysis with the distance of 10.2 ± 3.0 mm. The TAPF was thicker part of endopelvic fascia and inserted posteriorly to the ischial spine. The TAPF was not direct line shape, but curve line shape. It was located beneath the tendinous arch of the levator ani muscle (TALM). The TALM conjoined with the TAPF at a point which kept the distance of 31.6 ± 18.6 mm from the ischial spine posteriorly.
The distance between this conjoint point and the ischial spine exhibited big variance. The TAPF was 81.1 ± 2.4 mm and was divided into two parts by this conjoint point. In its anterior part, the length was 53.8 ± 20.6 mm, and the width of its anterior portion was 4.0 ± 0.6 mm and that of its posterior portion was ~ 2.0 mm. In its posterior part, the length was 31.6 ± 18.6 mm and the width was 10.8 ± 3.1 mm, which was much thicker than that of the anterior part. The anterior part of the TAPF travelled onto the LAM and connected the lateral vesical ligament medially and its posterior part courses upward and travelled onto the internal obturator muscle (IOM) (Fig. 6a–e). The TAPF was not visible in MRI images because of its slimness.
The tendinous arch of the levator ani muscle (TALM)
The TALM was another tendinous arch in the pelvic wall, which was considerably thinner than the TAPF. The TALM was located in the superior side of the convergence of the IOM and the LAM, which was opposed to the abdominal cavity. In some individuals, the structure is almost invisible. The TALM originated from the pubis anteriorly and ended at the conjoining point with the TAPF posteriorly, and the distance between the starting point and the pubic symphysis was 24.8 ± 2.6 mm. The entire length of the TALM was 48.0 ± 17.4 mm. However, the location of the conjoining point exhibited significant variant between different individuals (Fig. 6a–e). In MRI images, the TALM was also not visible, similar to the TAPF.
Discussion
The support and stabilization of the vesical neck and urethra influences not only urinary continence but also the initiation of micturition. Therefore, this support is very important and consists of active muscular support from urethral sphincter complex and levator ani muscle (Wu et al. 2015, 2018), and the passive connective tissue support from urethrovaginal septum, pubovesical muscle, pubovesical ligament, lateral vesical ligament, and TAPF. Active muscular support can reinforce pelvic supporting function through pelvic floor muscle exercises. While the passive pelvic support including the connective tissue and smooth muscle is a passive support which plays a role with abdominal pressure increasing.
Muscular supporting structures
In muscular support, the sphincter proper’s contract will close the upper part of the urethra, and the compressor urethra is U shaped and its contraction produces a force on the urethra in a posteroinferior direction. Our study on levator ani muscle's four subparts including internal and external part of the pubovisceral muscle and superficial and deep part of the puborectal muscle is different from that on the traditional levator ani muscle's three subparts including the pubovisceral muscle, the puborectal muscle, and the iliococcygeal muscle. Contraction of the LAM will push the upper part of urethra forward. In our previous publication (Wu et al. 2015), we have described the levator ani muscle’s architecture in detail.
Contraction of the LAM compresses the rectum and moves the rectovaginal complex anteriorly and superiorly towards the urethra in a plane that lies parallel to, but superior of, that of the compressor urethra. Simultaneous contraction of the LAM and compressor urethra causes an anteriorly convex bend in the midurethra, which closes the midurethral lumen (Wallner et al. 2009; Wu et al. 2018).
Anterior vaginal wall
The anterior vaginal wall is a strong connective tissue support for urethra which has expansive connective surface between vagina and urethra and is also called the vesicovaginal connection or vaginal support (Occelli et al. 2001). Therefore, when the anterior vaginal wall prolapse, the urethra and bladder are usually pulled to prolapse by uterus and vagina.
Posteriorly, the upper part of the anterior vaginal wall mainly including vesicovaginal septum combines with the lateral vesical ligament and pubovesical muscle to create the hammock structure of the female urethra, which creates the upper supporting structure to the female urethra. In DeLancey's hammock, the continuity of the endopelvic fascia, vaginal wall, arcus tendineus fasciae pelvis, and levator ani forms the hammock-like structural layer. In our study, the lateral vesical ligament which is located lateral to the urethra and pubovesical muscle which is located anterior to urethra both belong to the endopelvic fascia around the urethra, which complies with and supplements DeLancey's hammock theory. Contraction of the levator ani muscles can elevate and contract the TAPF, and subsequently contract the vaginal wall directly and urethra through the lateral ligament which leads to close the urethral lumen.
We agree with Mauroy et al.’s opinion that the inferior part of the urethrovaginal septum is hardly separated from the anterior vaginal wall, because it mainly includes dense connective tissue (Mauroy et al. 2000). However, different with Mauroy et al.’s opinion that the proportion of this part to the whole urethrovaginal septum is two thirds, we found the proportion of this part is 0.16 (Mauroy et al. 2000).
Therefore, both the superior and inferior parts provide the strong urethral support. In the urethral middle part suspension, the gynecologists need to separate the vaginal wall and urethra between through urethrovaginal septum, the upper septum is relatively loose, so after water-cushion injection, they can separate it with gently blunt separation, but the middle and lower septum, it need to separate it with scissors' meticulously separation so as not to damage the urethra.
The lateral vesical ligament
The lateral vesical ligament, also named pubovesical ligament by Occelli et al. (2001), is a strong connection that attaches the superior part of the urethra to the TAPF, although it contains much vessels, like the cardinal ligament of uterus, which is a net can prevent the uterus and bladder from prolapsing downward (Mauroy et al. 2000). We think the word “the lateral vesical ligament” can show more accurate anatomical inserting and ending information than that of word “pubovesical ligament”. When the vagina and urethra were pulled down, the net was tight and created some force to prevent the urethra downward. This ligament belongs to the continuity of endopelvic fascia and is an important part of the “hammock” structure which connects the urethra with the ATFP. This ligament bridges the gap medially in the urogenital hiatus and supports the vesical neck and urethra (Ashton-Miller and DeLancey 2007; DeLancey 1994; Herschorn 2004). We disagree with Klutke et al.’s opinion that the urethra is suspended laterally by the urethropelvic ligament (Klutke and Siegel 1995) and we found there is no so-called urethropelvic ligament between vesical neck and levator ani muscle. We agree with De La Taille et al. that the uterus and bladder are held by the lateral vesical ligament through the TAPF. Inside this ligament, we cannot clearly identify two layers including the muscular and fascial layers, and found this ligament has only ligament layer, which is inconsistent with DeLancey et al. (1989).
Lateral vesical ligament
The lateral vesical ligament belongs to the endopelvic fascia, which connect s vesical neck, urethra, and the TAPF. In the anterior pelvic organ prolapse or stress urinary incontinence patients, gynecologists need to repair the lateral vesical ligament which supports the middle part of the urethra. Repairing the lateral vesical ligament to the TAPF that adjoins the pubic symphysis contributes to fixing the urethra and preventing it from prolapsing downward. Destruction of the lateral vesical ligament will lead to urethral prolapse. Therefore, to these prolapsed patients with ligament injury, gynecologists sew the vagina or uterus to the TAPF as a substitute for the lateral vesical ligament to strengthen the vaginal support and maintain the urethral stability.
Pubovesical muscle
The pubovesical muscle is composed of smooth muscle and is only loosely attached to its anterior surface with the pubovesical ligament, but it connects the TAPF through the lateral vesical ligament. Similar to other smooth muscles, the pubovesical muscles are delicate and easily torn. The pubovesical muscle is also a part of the hammock structure. The pubovesical muscle, the pubovesical ligament, and the lateral vesical ligament keep the position of the proximal urethra and create the primary positive support to the upper urethra, which can prevent the uterus and urethra prolapse. If the support to the upper urethra is injured, the mobility of the upper urethra will increase, and the orifice of the urethra does not easily close tightly with increasing abdominal pressure, which leads to stress incontinence. In Macura's opinion, they defined the paraurethral support into three parts including the periurethral ligament, the paraurethral ligament and the periurethral ligament (Macura et al. 2006). While in our opinion, these three ligaments nearly include pubovesical muscle and the lateral vesical ligament, because smooth muscle and connective tissue all has lower signal in MRI image.
Pubovesical ligament
The pubovesical ligament, which is also referred to as the pubourethral ligament or the posterior pubourethral (Zacharin 1963) or proximal pubourethral ligament (Abdel-Azim et al. 2009), is a ligament between the pubis and the proximal urethra. (DeLancey 1989) Compared with the vesicovaginal septum and lateral vesical ligament, it is a slim and a weak ligament that fixes the urethra and the bladder to the pubis. This ligament has big variance and in some pelvises, this ligament may converge with the TALM and is not visible. These findings are consistent with Mauroy et al. (2000) and inconsistent with DeLancey (1989). The pubovesical ligament and the pubovesical muscle are disparate structures, which is different from DeLancey et al.’s opinion in 1989 (DeLancey 1989). If this ligament destroys, the Retzius space will be expanded (Kim et al. 2003). The absence and hypoplasia of the pubovesical ligament will lead to weakening the fixation of the vesical neck or urethra, and then cause prolapse.
TAPF and TALM
The TAPF is a strong thickened fibrous structure that suspends the urethra and vagina and provides a strong support to the urethra and vagina. The destruction and relaxation of the TAPF lead to prolapse of the urethra and vagina. This structure can be palpated by the gynecologists through bimanual examination. However, the structure is not visible in MRI images, because of MRI's low resolution. The posterior part of the TAPF is much thicker than the anterior part, in contrast to the findings of Maarten et al. (Pit et al. 2003).
According to Richardson's technique, suturing of the vagina to the TAPF for paravaginal colposuspension allows a more physiological and resistant suspension of the bladder neck. Therefore, if this TAPF is weak, the uterus and bladder will prolapse, and the gynecologist may appeal to other operation methods. Because the TAPF is a clearly distinguishable string-like fibrous structure within the endopelvic fascia and it is a thicken part of endopelvic fascia, which are consistent with the findings of Maarten et al. (Pit et al. 2003).
The TAPF and TALM are completely different structures. Although confusing descriptions and illustrations are presented in the literature, clear anatomic differences are noted between the TAPF and the TALM (DeLancey and Starr 1990; Mostwin 1991). The TALM is much weaker than the TAPF, which nearly cannot provide support to urethral support, and did not belong to urethral supporting structures according to its position and relationship.
Based on clinical experience, the ability to prevent the descent of the proximal urethra during periods of increased abdominal pressure is crucial to maintaining urinary continence. Fixation to the pelvis and the pull-out strength of the anterior TAPF can explain reason why this structure has been used successfully as the anchor point for sutures in urethrosuspension procedures for urinary incontinence (Palma et al. 2001).
Conclusions
In our study, the urethra and its supporting structures were 3D reconstruct. We find the muscular support structures include the levator ani muscle and striated urethral sphincter, and the connective tissue supporting structures include the anterior vaginal wall, the pubovesical muscle, the pubovesical ligament, the lateral vesical ligament, and the TAPF. The pubovesical muscle, the anterior vaginal wall, the lateral vesical ligament, and the TAPF create the “hammock” structure, which complies with and supplements DeLancey’s theory. The support to the proximal urethra and vesical bladder may be crucial to maintaining stability and urinary continence during increased abdominal pressure.
Abbreviations
- CVH:
-
Chinese Visible Human
- VHP:
-
Visible Human Project
- 3D:
-
Three-dimensional
- TAPF:
-
Tendinous arch of pelvic fascia
- TALM:
-
Tendinous arch of levator ani muscle
- MRI:
-
Magnetic resonance imaging
- LAM:
-
Levator ani muscle
References
Ashton-Miller JA, DeLancey JO (2007) Functional anatomy of the female pelvic floor. Ann N Y Acad Sci 1101:266–296
Barber MD (2016) Pelvic organ prolapse. BMJ 354:i3853
Brandao S, Parente M, Mascarenhas T, da Silva AR, Ramos I, Jorge RN (2015) Biomechanical study on the bladder neck and urethral positions: simulation of impairment of the pelvic ligaments. J Biomech 48:217–223
Chen Z, Luo C, Shang X, Yang H, Yang L, Wulan H, Han Y (2017) Application of multislice computed tomography volume rendering and 3D printing technique of costal cartilage for auricular reconstruction. Zhonghua Zheng Xing Wai Ke Za Zhi 33:97–101
DeLancey JO (1989) Pubovesical ligament: a separate structure from the urethral supports (“pubo-urethral ligaments”). Neurourol Urodyn 8:53–61
DeLancey JO (1994) Structural support of the urethra as it relates to stress urinary incontinence: the hammock hypothesis. Am J Obstet Gynecol 170:1713–1720
DeLancey JO, Starr RA (1990) Histology of the connection between the vagina and levator ani muscles. Implications for urinary tract function. J Reprod Med 35:765–771
Delancey JO (2010) Why do women have stress urinary incontinence? Neurourol Urodyn 29(Suppl 1):S13–17
El-Sayed RF, Morsy MM, El-Mashed SM, Abdel-Azim MS (2007) Anatomy of the urethral supporting ligaments defined by dissection, histology, and magnetic resonance imaging (MRI) of female cadavers and of healthy nulliparous women. Am J Roentgenol 189:1145–1157
Herschorn S (2004) Female pelvic floor anatomy: the pelvic floor, supporting structures, and pelvic organs. Rev Urol 6(Suppl 5):S2–S10
Hodgkinson CP (1953) Relationships of the female urethra and bladder in urinary stress incontinence. Am J Obstet Gynecol 65:560–573
Jelovsek JE, Barber MD (2006) Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am J Obstet Gynecol 194:1455–1461
Kim JK, Yong JK, Choo MS, Cho KS (2003) The urethra and its supporting structures in women with stress urinary incontinence: MR imaging using an endovaginal coil. Am J Roentgenol 180:1037–1044
Klutke CG, Siegel CL (1995) Functional female pelvic anatomy. Urol Clin N Am 22:487–498
Kraima AC, Smit NN, Jansma D, Wallner C, Bleys RL, van de Velde CJ, Botha CP, DeRuiter MC (2013) Toward a highly-detailed 3D pelvic model: approaching an ultra-specific level for surgical simulation and anatomical education. Clin Anat 26:333–338
Luo J, Betschart C, Chen L, Ashtonmiller JA, Delancey JOL (2014) Using stress MRI to analyze the 3D changes in apical ligament geometry from rest to maximal Valsalva: a pilot study. Int Urogynecol J 25:197–203
Macura KJ, Genadry RR, Bluemke DA (2006) MR imaging of the female urethra and supporting ligaments in assessment of urinary incontinence: spectrum of abnormalities. Radiographics 26:1135–1149
Mauroy B, Goullet E, Stefaniak X, Bonnal JL, Amara N (2000) Tendinous arch of the pelvic fascia application to the technique of paravaginal colposuspension. Surg Radiol Anat 22:73–79
Mostwin JL (1991) Current concepts of female pelvic anatomy and physiology. Urol Clin N Am 18:175–195
Nygaard IE, Shaw JM, Bardsley T, Egger MJ (2014) Lifetime physical activity and pelvic organ prolapse in middle-aged women. Am J Obstet Gynecol 210:477.e1–12
Occelli B, Narducci F, Hautefeuille J, Francke JP, Querleu D, Crépin G, Cosson M (2001) Anatomic study of arcus tendineus fasciae pelvis. Eur J Obstet Gynecol Reprod Biol 97:213–219
Palma P, Riccetto C, Herrmann V, Dambros M, Mesquita R, Netto N Jr (2001) Tendinous vaginal support (TVS) using the porcine small intestine submucosa (SIS): a promising anatomical approach for urinary stress incontinence. J Urol 165:90
Patel PD, Amrute KV, Badlani GH (2007) Pelvic organ prolapse and stress urinary incontinence: a review of etiological factors. Indian J Urol 23:135–141
Pit MJ, De Ruiter MC, Lycklama ANAA, Marani E, Zwartendijk J (2003) Anatomy of the arcus tendineus fasciae pelvis in females. Clin Anat 16:131–137
Ramanah R, Berger MB, Chen L, Riethmuller D, Delancey JO (2012) See it in 3D!: researchers examined structural links between the cardinal and uterosacral ligaments. Am J Obstet Gynecol 207:437.e1–7
Strohbehn K, Quint LE, Prince MR, Wojno KJ, Delancey JO (1996) Magnetic resonance imaging anatomy of the female urethra: a direct histologic comparison. Obstet Gynecol 88:750–756
Wallner C, Dabhoiwala NF, DeRuiter MC, Lamers WH (2009) The anatomical components of urinary continence. Eur Urol 55:932–944
Wu Y, Dabhoiwala NF, Hagoort J, Shan JL, Tan LW, Fang BJ, Zhang SX, Lamers WH (2015) 3D Topography of the young adult anal sphincter complex reconstructed from undeformed serial anatomical sections. PLoS ONE 10:e0132226
Wu Y, Dabhoiwala NF, Hagoort J, Hikspoors JPJM, Tan LW, Mommen G, Hu X, Zhang SX, Lamers WH (2018) Architecture of structures in the urogenital triangle of young adult males; comparison with females. J Anat 233:447–459
Zacharin RF (1963) The suspensory mechanism of the female urethra. J Anat 97:423–427
Zhang SX, Heng PA, Liu ZJ, Tan LW, Qiu MG, Li QY, Liao RX, Li K, Cui GY, Guo YL et al (2003) Creation of the Chinese visible human data set. Anat Rec B New Anat 275:190–195
Zhang SX, Heng PA, Liu ZJ, Tan LW, Qiu MG, Li QY, Liao RX, Li K, Cui GY, Guo YL et al (2004) The Chinese Visible Human (CVH) datasets incorporate technical and imaging advances on earlier digital humans. J Anat 204:165–173
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 31771324 and 31671251; https://www.nsfc.gov.cn/), National Key Research and Development Program of China (No. 2016YFC0106403; https://program.most.gov.cn/) and Natural Science Foundation of Chongqing (No. cstc2018jcyjAX0537). The funders did not participate in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
YW and LL contributed to the concept and design, data analysis, and interpretation. JRL, NC, and HTX contributed to the segmentation of female urethral supporting structures. XH contributed to the creation of interactive 3D-pdf. JRL, YS, HTX, and YW contributed to the critical revision of the manuscript and figures.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, JR., Lei, L., Luo, N. et al. Architecture of female urethral supporting structures based on undeformed high-resolution sectional anatomical images. Anat Sci Int 96, 30–41 (2021). https://doi.org/10.1007/s12565-020-00554-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-020-00554-y