Abstract
During most of feather growth (anagen), the dermal papilla stimulates the collar epithelium to give rise to feather keratins accumulating cells that form most of the corneous material of barbs and the rachis. Aside from the induction of differentiated cells of the feather, the distal part of the papilla forms a loose connective tissue that nourishes the growing feather, termed the pulp. In the last stages of feather growth, the pulp undergoes a process of re-absorption and leaves empty cavities indicated as pulp cups surrounded by keratinized cells inside the calamus. The process of cornification of pulp cups in different species of birds has been described here by using electron microscopy and immunocytochemistry for keratins. Pulp cells accumulate bundles of soft (alpha)-keratin, but do not synthesise feather keratins as in the surrounding calamus cells. Cells of the pulp epithelium accumulate large amounts of lipids and form a softer keratinized epithelium surrounding the pulp. This type of keratinization resembles the formation of soft epidermis in apteric and interfollicular regions. The role of the cornified pulp epithelium is to limit water loss and to form a microbe barrier before the mature feather is moulted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During embryogenesis, the skin of birds forms a temporary embryonic epidermis, which is made of a basal layer, a subperiderm, an inner periderm and an outer periderm (Sawyer and Knapp 2003). These layers remain in the apteric (non-feathered areas) and in the interfollicular epidermis (between feathers), and in the epidermis of scales, claw and beak until shedding at hatching. In the embryonic epidermis of feathers, these embryonic layers are transformed into the sheath and the downfeather. While the sheath is shed at hatching, the downfeather is shed after been replaced by a juvenile feather (moulting). Feathers represent extremely complex epidermal derivatives that are generated through a complex process of morphogenesis (Matulionis 1970; Lucas and Stettenheim 1972; Chuong and Edelman 1985a, b; Alibardi 2002, 2005, 2006, 2007a, b, c; Alibardi and Sawyer 2006; Fig. 1).
Schematic drawing of the final phases of anagen of a feather. A Shows that most barb ridges (A1) and barbs have been formed and that the apex is largely free of the sheath with a definitive vane. The two squares detail the structure of the collar epithelium with the thinner germinal collar (B) that is the origin of the last barb ridges (C), in comparison to the upper part of the collar that will be transformed into a calamus (D) at the end of the anagen. Cells coloured in green represent those synthesisng beta-keratin [sauropsid Keratin Associated Proteins (sKAPs)], while cells coloured in pink do not produce sKAP and only alpha keratin remains. C The ramogenic collar (dashes on the branching area where the rachidialkrige is located) from which a circular epithelium (C1) is progressively folded (C2 and C3) into typical barb ridges (C4) in upper levels. After barb ridges are no longer formed, the epithelium becomes circular (E, E1), and a series of pulp cups is formed inside the calamus (1, 2, 3 in E2). A Axial plate, αC alpha-keratin cell, αk alpha-keratin, BP barbule plate, BR barb ridges, βc beta-keratin cell (accumulating sKAPs or feather keratins), βk beta-keratin (the specialised sKAPs of feathers), CO collar, DS degenerating sheath, FCL forming calamus, R ramus area, RA rachidial ridge, GE germinal epithelium, M marginal plate epithelium, PC pulp cup, PE pulp epithelium, S sheath, ss subperiderm cell stratum
This complex structure is regenerated at least one time per year through the proliferation of stem cells present in the dormant follicle of feathers (Yu et al. 2004; Yue et al. 2005). This stage of feather growth, like in hairs, is indicated as anagen (Spearmann and Hardy 1985). Feather regeneration occurs through the formation of new barb ridges in the basal part of the feather follicle, indicated as the ramogenic region of the collar, which is localised above the dermal papilla (Fig. 1A–C4). From the more external part of the collar, the sheath of the feather is produced, and at the end of anagen phase, also the calamus (Fig. 1A–D).
Cells of the dermal papilla through close dermal-epidermal cell interactions stimulate the collar epithelium to produce barb ridges in the ramogenic zone (Lucas and Stettenheim 1972; Chuong and Widelitz 1999). The formation of the rachis occurs by a process indicated as “helicoidal growth” (Prum 1999; Prum and Williamson 2001), where barb ridges produced in the ramogenic collar grow along the collar describing a helicoidal tract until they meet forming the rachis.
The dermal papilla also produces a connective tissue mixed with blood vessels and nerves that colonise the upper part of the follicle and of the feather germ, indicated as pulp. The pulp is vascularised and allows the nourishment of the ramogenic collar that produces barb ridges. At late periods of anagen, the pulp begins a periodical contraction as more cells are reabsorbed in its apical part than cells are produced at its base (Lillie 1943; Lucas and Stettenheim 1972). The final stage of reabsorbing of the pulp takes place in late periods of anagen, before the growth ends with the cessation of rachis formation (superior umbilicus) and the formation of the calamus. This is probably connected with the cessation of the activity of the dermal papilla that precedes the regression phase of the feather follicle (telogen, Spearmann and Hardy 1985). The resting follicle is made of few dermal cells surrounded by a narrow and flat collar epithelium surrounded by the lowermost corneous layer of the calamus (the inferior umbilicus).
The old feather remains functional for a variable period, but is eventually shed by the production of new, replacing feathers produced underneath from the reactivation of the dermal papilla (Chuong and Widelitz 1999). The interruption of the skin barrier after feather moulting could cause a distressful loss of water considering the loss of many feathers in a short period of time, and could also be a point of microbe penetration into the body in case the skin barrier is disrupted. The replacement of an old with a new feather takes place after the formation of a softer keratinized tissue that limits water loss and microbe penetration through the follicle, indicated as pulp cups (Lillie 1943; Lucas and Stettenheim 1972; Maderson and Alibardi 2000). The latter tissue may also represent a shedding layer since keratinocytes stop producing “feather keratins” like in the surrounding cells of the calamus, and seem to accumulate bundles of (alpha-)keratin filaments and lipids (Alibardi 2002).
The ultrastructural details of the formation of corneus cells of the pulp epithelium, confirming that only soft keratin is synthesised, have however yet to be demonstrated. The present microscopic and immunocytochemical study would provide the final evidence to confirm the periodic formation of a specialised waterproof layer at the base of feathers (Maderson and Alibardi 2000). The present study describes in detail the cytological phases of the process of cornification of the pulp epithelium by means of electron microscopy and immunocytochemistry for keratins.
Materials and methods
Embryos of zebrafinch of 15–16 days post-deposition, previously analysed for skin differentiation (Alibardi 2002), were further studied for pulp cup formation.
Adults and juvenile chickens (Gallus gallus) were killed in Spring, and the skin of wings, pectoral and dorsal regions, containing regenerating feathers was immediately fixed. Samples from adults of the zebrafinch (Taniatopigigia castanotis) and quail (Coturnix coturnix) were killed in Spring, and feathers from the pectoral and wing region were used for fixation. Adults ostriches (Struthio camelus), killed in a slaughtering house in mid Spring, were also utilised, but only the hair-like feathers (bristles) from the head and neck region were collected.
Feathers of different lengths from the species here utilised were above 5 mm in length despite the different sizes of birds, and still were implanted in the skin with their follicle. Fixation was done at 4°C for 8–12 h in 2.5% glutaraldehyde in 0.12 M phosphate buffer at pH 7.2–7.4. Tissues were rinsed in buffer for 20 min and post-fixed in 2% OsO4 in buffer for about 90 min. Samples were dehydrated in graded ethanol (70°, 90°, 95°, 100°), infiltrated in propilene-oxide for 1 h, and infiltrated for 4–5 h in a 1:1 mix of propilene oxide and Durcupan resin. Finally, tissues were left embedding in pure Durcupan resin for 24 h and cured in Durcupam resin at 60°C for 2 days.
The basal part of the implanted feather with the follicle was sectioned at 1–4-μm thickness (semithin sections) in longitudinal or in cross section with an ultramicrotome. The collected sections were stained with 0.5% toluidine blue for the light microscopic examination. Thin sections (60–90 nm thick) of interesting areas selected from follicles at different levels (from the basal part to the neck region before the emergence from the follicle) were also collected for the ultrastructural study. The collected thin sections were attached to copper or nickel grids, stained with uranyl acetate-lead citrate according to routine methods, washed and dried, and then observed under a Philips CM-100 or Hitachi-600 transmission electron microscope operating at 60–80 kv.
Other tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer at pH 7.2 for 3–4 h, rinsed in buffer, dehydrated until 80% ethanol, infiltrated in a 1:1 mix of ethanol and Bioacryl resin for 1–2 h and immersed in pure Bioacryl resin (until tissues sink). Tissues were embedded in gelatin capsules using Bioacryl resin (Scala et al. 1992). The resin was polymerised at 0–4°C under UV light for 2–3 days. Tissues were sectioned in longitudinal or cross planes using an ultramicrotome, and 1–3-μm-thick sections were initially collected for the light microscopic study after toluidine blue stain. Some representative feathers were cross-sectioned in order to obtain a serial sequence, from the exposed, fully cornified vane, to the bottom of the follicle. This study allowed visualisation of the progressive changes of the follicular histology at all levels and determination of which feathers were growing (anagen stage) or resting (telogen stage, Spearmann and Hardy 1985). From selected areas, some 40–90-nm-thick sections containing the basal part of the feather were collected on nickel grids for the immunocytochemical study.
The sections were incubated overnight in the primary antibodies at 4°C and diluted 1:200 in Tris buffered saline (TBS) with 1% cold water fish gelatin or 2% BSA. One primary antibody was rabbit polyclonal chick beta-keratin antibody (Beta-1), and also a goat polyclonal chick alpha-keratin antibody (A-1) was used. Both antibodies were a gift from Drs. R.H. Sawyer and L.W. Knapp (University of South Carolina, Columbia, SC; see Sawyer et al. 2000). In controls the primary antibody was omitted. Grids were rinsed in buffer and incubated with the secondary antibody (10-nm gold-conjugated IgG anti-rabbit or anti-goat, diluted 1:50) for 1 h at room temperature. Grids were rinsed in buffer and distilled water, and the sections were stained for 6 min in 2% uranyl acetate, dried, and observed with a Hitachi-600 electron microscope.
Results
General morphology and size of analysed feathers
Embedded feathers show the structure and shape of their vane through the transparent resin in which they are embedded. In the studied material from different species, follicles of different diameters are observed. Small feathers of the zebrafinch are 3–10 mm in length and 2–5 mm large. Feathers from quail are 5–15 mm in length and 3–8 mm wide. The shape of ostrich feathers corresponds to bristles of 5–15 mm in length, the type of feather present in the neck of this species. Adult chick feathers are 20 mm in length and 8–15 mm wide, but some larger chick feathers are above 30 mm in length. Feathers with different size show follicle diameters of variable dimension. In case of follicles with barb ridges in the anagen stage of growth, the diameter varies from 100–300 μm for the small feathers of the zebrafinch, to 100–400 μm for small to intermediate size feathers of the quail. In case of juvenile feathers in the chick, the diameter of the follicle varies between 100–500 μm, but could reach 1,000 μm for adult chick feathers. A diameter of 100–500 μm is present in feathers from the ostrich.
Pulp epithelium of zebrafinch downfeather
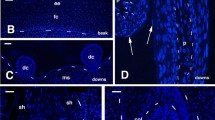
At 16–17 days post-deposition, embryonic downfeathers in zebrafinch are almost completely keratinized, and the pulp is concentrated at their base (Fig. 2a). The close microscopic analysis reveals that the pulp consists of a mass of cells among which numerous pycnotic nuclei are present (Fig. 2b). One to two corneous layers laying over flat epithelial cells surround the regressed pulp. The ultrastrucural examination of the regressed pulp shows that degenerating cells are rich in lipid droplets and pale vesicles. The pulp epithelium is made of one to two layers of flat but viable cells that are surrounded by strongly cornified cells of the basalmost part of the calamus (Fig. 2c). Therefore, the softer pulp cup is in continuity with the more intensely cornified calamus. The latter contains long and dense bundles of corneous material and paler cytoplasm remnants containing ribosomes. In the distal part of the calamus, located above the epidermis, the corneous material becomes compact, and no cytoplasm remains. Cells of the pulp epithelium contain tonofilament bundles that are immunoreactive for the A1-antibody, among numerous lipid vesicles (Fig. 2d, e). Sparse ribosomes and cisternae of the endoplamic reticulum are present in these cells. The A1 antibody labels the cornified cells in the more apical part of the pulp cup (Fig. 2f).
Pulp cups during downfeather maturation at 15–16 days post-deposition. a Longitudinal section of forming calamus (arrowheads) at the base of the follicle. The arrow indicates the top part of the first regressing pulp (bar 20 μm). b Detail of the thin corneous layer (arrows) of the forming pulp cup. Arrowheads indicate the solid calamus (bar 10 μm). c Ultrastructural detail of the regressed pulp surrounded by the pulp epithelium (arrows), also in the bottom part (double arrowheads) (bar 5 μm). d A1-immunogold labelling of keratin tonofilaments in cells of the pulp epithelium (bar 100 nm). e Detail of keratin tonofilaments (arrows) and lipid vesicles in cell of the pulp epithelium (bar 0.5 μm). f Detail of A1-immunolabelled cells of the stratum corneoum of the pulp epithelium (bar 100 nm). A1 Immuno-reaction using the A1 antibody, c corneous layer, ca calamus, dp dermal papilla, k keratin bundles, l lipid droplet, n nucleus, rp regressing pulp
Pulp epithelium of regenerating feathers
In favourable cases of regenerating feathers of zebrafinch, chick and quail, probably at the end of the anagen phase, a series of pulp cups separated by empty cavities are observed, with the last pulp cup still in the process of maturation (Fig. 3a–c). Beneath the pulp cup, the central pulp is in the phase of reabsorbing and terminates with a dermal papilla at the bottom of the follicle (Fig. 3b, c). The closer observation of forming pulp cups in regenerating feathers of zebrafinch and quail is initially described in longitudinal section. This analysis reveals that keratinocytes of the pulp epithelium are flat cells organised in three to four layers, while four to six layers of (alpha-)keratinocytes form the corneous layer (Fig. 3d–f).
Light microscopic view of longitudinal sections of pulp cups in the zebrafinch (a, f), chicken (b, c) and quail (d, e). a Two mature pulp cups (arrows) and a proximal forming pulp cup (arrowhead) are seen in this wing feather (bar 50 μm). b Base of a wing feather featuring the regressing pulp (arrow) that is continuous with the dermal papilla. The proximal pulp epithelium (arrowhead) is artefactually detached from the corneous layer (double arrowhead) (bar 30 m). c Details on the dermal apilla and regressing pulp made of fusiform fibroblasts. Arrows indicate the calamus wall (bar 20 μm). d Particular view of the forming, proximal pulp cup of a pectoral feather. Arrowhead: On the viable epidermis. Arrow: On the corneous layer (bar 20 μm). e Detail of the stratified corneous (arrow) and the viable epidermis (arrowhead) (bar 15 μm). f Detail of stratified pulp epithelium showing differentiating flat cells of the corneous layer (arrow) (bar 10 μm). ba Barb, ca calamus, dp dermal papilla, e epidermis, p pulp, pe pulp epithelium, rp regressing pulp
The study on cross sections of zebrafinch, quail, ostrich and chicken follicles shows the variation in stratification of the pulp epithelium moving from apical regions into more basal levels of the same follicle (progressive serial sections). A linear epithelium forming a ring of cells around the pulp dermis is seen in follicles of regenerating feathers containing mature barb ridges. In the case shown in Fig. 4a–d for a forming dorsal feather of the zebrafinch, representative sections are shown to illustrate the presence of a two-stratified circular epithelium at a distal level (Fig. 4a). This epithelium becomes folded into barb ridges in a lower level (Fig. 4b). This stratified epithelium is absent during the beginning of barb ridge or rachidial ridge formation when epithelial cells form the marginal plates. Therefore, the pulp epithelium rapidly disappears and become single layered as one moves from the top to the bottom of a regenerating, anagen feather. In these regions barb ridges become evident and are surrounded by a typical layer of cylindrical epithelium that forms the marginal plates (Fig. 4c). In further lower parts of the follicle, also barb ridges disappear, leaving a multi-stratified epithelium of the collar, in contact with the dermal papilla (Fig. 4d). Similar aspects are observed in follicles of quail, ostrich and chicken.
Light microscopic view of serial cross sections of regenerating feathers in zebrafinch (a–d) featuring the morphological variations of the epithelium contacting the dermal core. a In this distal level the epithelium is flat (double arrowhead on the basement membrane), and marginal plates (arrowheads) still separate barb ridges and barbule plates (arrows) (bar 20 μm). b At a lower level, the epithelium is folding (double arrowheads) around barb ridges and contacts marginal plates (arrows). Arrowheads indicate pigmented barbule plates (bar 25 μm). c At a lower level inside the follicle only the flat epithelium of marginal plates between barb ridges (arrows) remains, and no pulp epithelium is present (bar 15 μm). d Finally, at the base of the follicle barb ridges are absent, and the collar epithelium returns to being flat and circular. Arrows indicate differentiating cells of the intermediate layer (bar 10 μm). e Detail on the flat cells of the pulp epithelium (arrow) of ostrich bristle feather (bar 20 μm). a Axial plate of barb ridges, b barbule plates, co collar epithelium, de dermal papilla, f follicle space, m marginal plate, p pulp, pe pulp epithelium, ra ramus, s sheath, v barb vane ridge cells (future shedding layer)
The stratification of the pulp epithelium varies slightly in the different periods and also in different species under examination. In the follicle of hair-like feathers (bristles) of the neck in the ostrich, a single layer of flat to cubic cells is observed, in continuation with marginal plate cells (Fig. 4e). In the follicles of pennaceous feathers of quail, one to two layers of cubic cells are observed. In some large contour feathers of the chicken, two to four layers of cubic to polygonal epithelial cells are present (data not shown).
Ultrastructural and immunocytochemical observations
The ultrastructural examination of the pulp epithelium in zebrafinch that corresponds to that of Fig. 4a shows one to two epithelial layers that separate cells of the pulp from the cells of marginal plates of barb ridges (Fig. 5a). Numerous vesicles are present in these cells, and their basement membrane shows a waved surface. A continuous lamella densa is commonly present at the base of pulp cells of maturing barb ridges located from the ramogenic zone and above the exit of feathers from the follicle, within 0.5 mm after the exit from the epidermis. The lamella densa is also present beneath the pulp epithelium and the pulp in the more distal (apical) part of the feathers. The dense lamella follows the more or less undulating surface at the base of pulp cells in all chick and quail (Fig. 5b, c), but appears remarkably folded in the pulp epithelium of the zebrafinch (Fig. 6a). Dense organelles of secretory type or secondary lysosomes are sparse in these cells.
Ultrastructure of the pulp epithelium in zebrafinch (a), chick (b) and quail (c) wing feathers. a A layer of pseudostratified pulp epithelial cells is in continuation with cells of the marginal plate surrounding a ramus area of a barb ridge. Arrowheads indicate the basement membrane. Arrows points to some dense granules (non pigments) commonly seen in these cells (bar 2.5 μm). b High magnification detail of the basal folds of pulp cell (arrows) contacting fibroblasts of the pulp (bar 250 nm). c Less accentuated folds are also seen in the basal cytoplasm of pulp cells along the basement lamella (arrow). Arrowheads point to dense, lamellated, organelles (bar 250 nm). fi Cell elongation of pulp fibroblast, l lipid droplets, mc marginal plate cells, p pulp, pe pulp epithelial cells, rbr ramus cells of barb ridges
Ultrastructural detail of pulp cells of the zebrafinch (stages like in a of Fig. 5). a The basal part of pulp epithelial cells presents a folded plasma membrane supported by the dense lamella (arrows). Double arrowheads point to dense granules near the basememnt membrane (bar 0.5 μm). The inset (bar 250 nm) shows a periderm granule (arrow) within a pulp epithelial cell. b Other image showing only one cell of the pulp epithelium (asterisks) separates the ramus of a barb ridge from pulp cells. Note the extremely folded basement membrane (arrows) (bar 1 μm). cl Collagen fibrils (cross-sectioned), k keratin bundle, l lipid droplet, mi mitochondrion, p elongation of pulp fibroblast, pe pulp epithelium cell/cytoplasm, pg periderm granule, rm cells forming the ramus of a barb ridge, ve vesicles of endoplasmic reticulum
Numerous blood vessels are often seen near the folded basement membrane. The pale cytoplasm of epithelial pulp cells contains sparse ribosomes, pynocytotic or small smooth vesicles (100–200 nm large), and denser granules of variable dimensions and aspects that resemble those of secretory granules and/or lysosomes. On a few occasions also periderm granules are present in these cells (Fig. 6a, inset). The lamella densa also remains when barb ridges are mature and supportive cells among barb and barbule cells have largely disappeared (Fig. 6b). Lipid vesicles or intracellular lipid droplets not surrounded by a membrane are accumulated during degeneration in the cytoplasm of these cells, which occurs by a process of fat cell necrosis, the typical degenerative process described for other supportive cells within barb ridges (Alibardi 2005a).
In large regenerating feathers, long barb ridges are still forming at the base of the follicle (Fig. 7a), while in the external part of the feather, 2-3 mm above the epidermal surface, mature barbs are still surrounded by a thick corneous sheath (Fig. 7b). In the latter, most barb ridges have transformed into corneous barbs with a central, medullated ramus and connected with cornified barbule plates (arrowheads in Fig. 7b). The stratified pulp epithelium at this level shows the initial formation of a corneous layer made of thin corneocytes in contact with the cornified ramus (Fig. 7c, d). The detailed study using the electron microscope and immunogold shows that the accumulation of alpha-keratin material occurs in long bundles present in differentiating and pre-corneous pulp cells. The immunolabelling with the A1 antibody shows that gold particles are labelling the denser material present in keratin bundles accumulated in upper and pre-corneous cells. (Fig. 7e, f).
Light (a–d) and ultrastructural (e–f) images of specific areas of chicken wing feather with largely mature vanes. a Barb ridges are still forming in the ramogenic collar within the follicle. Arrowheads indicate barbule plates (bar 15 μm). b Outside the follicle, barb ridges are keratinized in both barbule plates (arrowheads) and in the ramus with its central pith (arrow). The double arrowhead points to flat keratinocytes anticipating the formation of a corneous layer in the pulp epithelium, still united to the degenerating marginal plates among barbs (bar 15 μm). c In a slightly upper level compared to the previous figure, the stratum corneum of the pulp epithelium is seen (arrows) (bar 10 μm). d A more distal cross section shows that the stratum corneum of the pulp epithelium (arrow) is separated from barbs here separated from each other as marginal plates have disappeared (bar 15 μm). e A1-immunogold-labelled keratin bundles (arrow) of upper spinosus cell of the pulp epithelium (bar 200 nm). f Detail of A1-immunolabelling of short and dense keratin bundles in transitional cells of pulp epithelium (bar 100 nm). a Axial plate, dk dense keratin bundle, k keratin bundle, p pulp, pe pulp epithelium, ra ramus area, s sheath, v barb vane ridge cells area within barb ridges
The ultrastructural analysis of the keratinizing epidermis shows the production of 14–20 layers of superficial corneocytes of the chick pulp epithelium (Fig. 8a). Basal and suprabasal cells contain sparse ribosomes and glycogen particles, while the ergastoplasm is poorly developed, and cisternae are smooth or show few attached ribosomes. Keratin filaments are sparse, and short keratin bundles are present. Nuclei become more eterochromatinic in the upper, pre-corneous layers and become fragmented among the corneous and lipid material in transitional and corneous cells.
Ultrastructural aspects of the cornification of pulp epithelium of chicken wing feather. a The scarse keratin bundles present in basal and suprabasal cells become more concentrated in transitional cells where also lipid vesicles increase in number and dimension. Lipids occupy large areas of flattening keratinocytes (arrowheads) (bar 2 μm). The inset shows the A1-immunogold labelling of corneocytes (bar 100 nm). b Keratin bundles of intermediate cells (double arrowheads) concetrate along the thickened, cornified cell membrane of transitional cells forming an electrondense rim (arrowheads). Irregular lamellae (arrows) are seen within the lipid core. The inset (bar 200 nm) shows the detail of a pre-corneous keratinocyte without any labelling for the beta-1 antibody (against beta-keratin) (bar 0.5 μm). A1 Immuno-reaction to the A1 antibody, c corneous layer, k keratin bundles, l lipid vesicle, n nucleus, t transitional (pre-corneous) cells
The corneocytes are immunoreactive for the A1 antibody for alpha-keratins (inset in Fig. 8a). Transitional keratinocytes also accumulate pale vesicles of lipid nature, or sections of the smooth endoplasmic reticulum, that occupy the central part of the flattening cells. Degenerating mitochondria are sometimes identified in these cells, and therefore part of the lipid material may also derive from their degeneration. The lipid material is sometimes secreted on the surface of these cells, but most of it remains inside the thin corneocytes and forms the central pale areas of corneocytes of the pulp epithelium (Fig. 8a, b). The scarce and short keratin bundles observed in basal and suprabasal layers of the pulp epithelium become prevalently concentrated and oriented along the cell perimeter of transitional cells. However, these filaments appear immuno-negative to the beta-1 antibody (inset of Fig. 8b). The plasma membrane of transitional cells forms a dense marginal layer (cornified cell envelope) that becomes indistinct in mature corneocytes from the dark keratin material that is also accumulated in these peripheral areas. No keratohyaline granules are present in suprabasal and transitional cells.
Discussion
Late anagen and pulp regression
The regression of the dermal papilla and of the pulp determines the inhibition of the production of barb ridges. The genetic control on the morphogenesis of feathers is directed to the formation of barb ridges and the differentiation of cornifying cells (barb/barbules) versus supportive cells (degenerating). Genes control the production of specific keratins and of associated proteins in barb/barbule cells and the synthesis of cell-cell recognition proteins to form specific and stable connections within barb ridges. Other genes activate the production of lipids and soft keratins in supportive cells. Both barb/barbule and supportive cells undergo terminal differentiation and die, but only the cornified ones remain as feathers.
The remaining germinal epithelium produces a lipid-stuffed layer above the pulp that probably limits the dehydration of the pulp during telogen (Lillie 1943; Lucas and Stettenheim 1972; Maderson and Alibardi 2000). Cells of the pulp epithelium are supportive cells that do not produce feather keratin, but only alpha-keratin filaments mixed with a large amount of lipids. The negative reactivity to beta-1 antibody confirms these keratinocytes only contain alpha-keratin. During anagen barb ridges are produced (Fig. 1A, A1); the cells come from the stratification of keratinocytes of the germinal or papillary collar (Fig. 1B). From the more external layers of the collar epithelium (blue and pink in Fig. 1), keratinocytes of the sheath are derived.
From the intermediate layer, indicated as subsheath (in green in Fig. 1), newly formed keratinocytes that accumulate feather keratin are produced from the thick epithelium of the collar and are displaced into a ramus and two barbule plates within barb ridges (Fig. 1C–C4) to form the final barbs. During barb ridge formation, the linear epithelium of the collar (Fig. 1 C1) is folded into discrete column of cells from which barbs are produced (Fig. 1 C2–C4). As barb ridges fuse with the rachidial ridge, they are moved upward. It is probably the combined growth of rachidial and merged barb ridges, which possess more cells than the three isolated elements, that determines the upward growth of the rachis and merged barb ridges.
Keratinocytes of the sub-sheath layers (green in Fig. 1) form barb and barbule cells after barb ridge formation during anagen. By the end of anagen, keratinocytes of the subsheath layers tend to stratify and form the calamus. The epithelium of the collar becomes linear, and barb ridges are no longer produced (Fig. 1A, A1 and E–E1). The wall of the collar produces some layers of hard keratinocytes containing feather keratin and that form the calamus (Fig. 1 C1, D). In the central part of the follicle, the pulp epithelium becomes circular in cross section, but in 3D the epithelium forms a series of cones (indicated as 1, 2 and 3 in Fig. 1 E2). The epithelium eventually becomes keratinized into a series of pulp cups (Fig. 1E; see Lucas and Stettenheim 1972; Maderson and Alibardi 2000).
Following feather moulting or after plucking, a lot of water can be lost through the follicle if no specific sealing layers are formed (Menon and Menon 2000). In moulting feathers, the loss of the old appendage occurs after a new, regenerating feather germ is present in the follicle, and its sheath is connected to the lowermost part of the calamus of the old feather. Following the detachment of the old feather, the cutaneous discontinuity is sealed through a layer of cornified pulp cells that form the pulp cups. This layer derives from the switch from keratinocytes producing hard corneous material containing sKAPs (beta-keratins) in the calamus to keratinocytes that do not produce sKAPs. Therefore, only alpha-keratin and lipids are synthesised in these cells and are used to form a barrier against water-loss. The A1 antibody has been shown to be mainly specific for alpha-keratins in different avian species (Alibardi and Toni 2008), but it also recognises a protein of 20 kDa, within the “beta-keratin” range. Therefore, it remains uncertain whether this antibody can be considered specific only for alpha-keratins.
Keratinization of pulp cups
The reversion of the germinal epithelium of the collar and barb ridges to pulp epithelium at the end of anagen is probably due to the de-coupling between cells of the dermal papilla and the epithelium. In fact, the retraction of dermal cells from the germinal epithelium probably determines a qualitative change in these cells, which become unable to produce feather keratins. These proteins have also been recently indicated as feather KAP proteins as they represent the functional analogous proteins of mammalian KAPs (Alibardi and Toni 2008). Only alpha-keratin and lipids are produced in pulp epithelial keratinocytes surrounding the pulp within the calamus. This modality of keratinization is typical of interfollicular and apterilae regions of bird epidermis, with some morphological similarities to mammalian and human epidermis (Menon and Menon 2000).
While mesenchymal elongation among barb ridges is present at the beginning of feather morphogenesis (Alibardi 2005, 2006, 2007c), the elongation regresses when cells change their activity by the end of anagen and turn into degenerating pulp cells. The cylindrical epithelium of marginal plates presents a linear basement membrane, while the pulp epithelium surrounding maturing barb ridges shows numerous folds of the basement membrane. When barb ridges disappear by the end of anagen, the epithelium of the follicle remains circular, but the lamella densa of the basement membrane becomes relaxed and forms folds. This aspect may be in relation with an intense exchange activity with blood vessels and cells of the pulp. It is also likely that the folding of the basement membrane of this epithelium simply reflects the shrinkage of barb ridges as they turn into barbs.
The pulp epithelium presents a variable degree of stratification, probably related to the size of the feather follicle and the dimension of feathers, more than specific histological differences in the four species of birds analysed. In fact, as the follicle varies its dimension in conjunction to that of the dermal papilla during anagen, also the epithelium contacting the pulp varies its extension and stratification. Cells of the pulp epithelium share general cytological featuers with those of the marginal plate and may also form an embryonic type of organelle, the periderm granule. The latter characterises supportive cells among barb/barbule cells, indicating that also pulp epithelial cells belong to the cell lineage of feather supportive cells. The adult epidermis among feathers is very rich in lipids in birds and can be very efficient as a barrier against water loss in xeric conditions (Menon and Menon 2000). This barrier could be weakened by the presence of feathers that represent a highly dispersing surface unless their corneous material is well lipidised. Like inter-barb/barbule cells, cells of the pulp epithelium are very lipogenic and produce a lipid barrier against water loss necessary to maintain hydrated cells of the underlying pulp (Lillie 1943; Lucas and Stettenheim 1972; Maderson and Alibardi 2000). The last pulp cup also covers and maintains cells of the dermal papilla alive before a new (unknown) stimulus reactivates the papilla for re-entering a new anagen phase for the regeneration of the feather (Fig. 1 E2).
References
Alibardi L (2002) Keratinization and lipogenesis in epidermal derivatives of the zebrafinch, Taeniopygia guttata castanotis (Aves, Passeriformes, Ploecidae) during embryonic development. J Morphol 251:294–308
Alibardi L (2005) Cell structure of developing barbs and barbules in downfeathers of the chick: central role of barb ridge morphogenesis for the evolution of feathers. J Submicrosc Cytol Pathol 37:19–41
Alibardi L (2006) Cells of embryonic and regenerating germinal layers within barb ridges: implication for the development, evolution and diversification of feathers. J Submicrosc Cytol Pathol 38:51–76
Alibardi L (2007a) Cell organization of barb ridges in regenerating feathers of the quail: implications of the elongation of barb ridges for the evolution and diversification of feathers. Acta Zool 88:101–117
Alibardi L (2007b) Keratinization of sheath and calamus cells in developing and regenerating feathers. Ann Anat 189:583–595
Alibardi L (2007c) Cell interactions in barb ridges of developing chick downfeather and the origin of feather branching. Ital J Zool 74:143–155
Alibardi L, Sawyer RH (2006) Cell structure of developing downfeathers in the zebrafinch with emphasis on barb ridge morphogenesis. J Anat 208:621–642
Alibardi L, Toni M (2008) Cytochemical and molecular characteristics of the process of cornification during feather morphogenesis. Prog Histochem Cytochem 43:1–72
Brush AH (1993) The origin of feathers: a novel approach. In: Farner D, King JA, Parker KC (eds) Avian Biol. IX. Academic Press, New York, pp 121–162
Chuong CM, Edelman GM (1985a) Expression of cell-adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J Cell Biol 101:1009–1026
Chuong CM, Edelman GM (1985b) Expression of cell-adhesion molecules in embryonic induction. II. Morphogenesis of adult feathers. J Cell Biol 101:1027–1043
Chuong CM, Widelitz RB (1999) Feather morphogenesis: a model of the formation of epithelial appendages. In: Chuong CM (ed) Molecular basis of epithelial appendage morphogenesis. Landes Bioscience, Georgtown, pp 57–73
Chuong CM, Wu P, Zhang FC, Xu X, Yu M, Widelitz RB, Jiang TX, Hou L (2003) Adaptation to the sky: defining the feather with integument fossils from mesozoic china and experimental evidence from molecular laboratories. J Exp Zool 298B:42–56
Gregg K, Rogers GE (1986) Feather keratin: composition, structure and biogenesis. In: Bereiter-Hahn J, Matoltsy AG, Sylvia-Richards K (eds) Biology of the integument, vol 2, Vertebrates. Springer, Berlin, pp 666–694
Lillie FR (1943) On the development of feathers. Biol Rev 17:247–266
Lucas AM, Stettenheim PR (1972) Growth of follicles and feathers. Color of feathers and integument. In “Avian anatomy. Integument”. Agriculture Handbook, vol 362. Chap. 7, US Department of Agriculture, Washington DC, pp 341–419
Maderson PFA, Alibardi L (2000) The development of the sauropsid integument: a contribution to the problem of the origin and evolution of feathers. Am Zool 40:513–529
Matulionis DH (1970) Morphology of the developing down feathers of chick embryos. A descriptive study at the ultrastructural level of differentiation and keratinization. Z Anat Entw Gesch 132:107–157
Menon GK, Menon J (2000) Avian epidermal lipids: functional considerations in relation to feathering. Am Zool 40:540–542
Prum RO (1999) Development and evolutionary origin of feathers. J Exp Zool 285:291–306
Prum RO, Williamson S (2001) Theory of the growth and evolution of feather shape. J Exp Zool 291:30–57
Rawles ME (1960) The integumentary system. In: Marshall AJ (ed) Biology and comparative physiology of birds, vol 1. Academic Press, New York & London, pp 189–240
Sawyer RH, Knapp LW (2003) Avian skin development and the evolutionary origin of feathers. J Exp Zool 298B:57–72
Sawyer RH, Glenn T, French JO, Mays B, Shames RB, Barnes GL, Rhodes W, Ishikawa Y (2000) The expression of beta (β) keratins in the epidermal appendages of reptiles and birds. Am Zool 40:530–539
Scala C, Cenacchi G, Ferrari C, Pasquinelli G, Preda P, Manara G (1992) A new acrylic resin formulation: a useful tool for histological, ultrastructural, and immunocytochemical investigation. J Histochem Cytochem 40:1799–1804
Spearmann RIC, Hardy JA (1985) Integument. In: King AS, McLelland J (eds) Form and function of birds, vol 3. Academic Press, London, pp 1–56
Yu M, Yue Z, Wu P, WU DY, Mayer JA, Medina M, Widelitz RB, Jiang TX, Chuong CM (2004) The developmental biology of feather follicle. Int J Dev Biol 48:181–191
Yue Z, Jiang TX, Widelitz RB, Chuong CM (2005) Mapping stem cell activities in the feather follicle. Nature 438:1026–1029
Acknowledgments
The study was partially supported by a 60% grant from the University of Bologna and largely by self-support. Dr. Mattia Toni’s skill with the Corel Draw 11 program in computer-generated figures is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alibardi, L. Cornification of the pulp epithelium and formation of pulp cups in downfeathers and regenerating feathers. Anat Sci Int 84, 269–279 (2009). https://doi.org/10.1007/s12565-009-0033-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12565-009-0033-2