Abstract
The activity of proteases may be lost during microquantification because of their nonspecific binding to tubes and pipette tips. In this study, the effects of blocking reagents (blockers) in dilutions of digestive proteases of yellowtail, Seriola quinqueradiata, were compared. EzBlock Chemi (EBC; protein free), Western BLoT Blocking Buffer (WBB; protein free), casein-based blocker, and bovine serum albumin were evaluated by the measurement of trypsin, chymotrypsin, and aminopeptidase activities using fluorogenic substrates. Protease activities were linear in extracts of pyloric caeca at dilution rates of up to at least 1/6400 with the four blockers. However, the activities of pancreatic enzymes differed, and the highest levels were observed with EBC and WBB. Extracts diluted with EBC and WBB were incubated for up to 24 h at 25 °C. Pancreatic enzyme activities increased with time in extracts diluted with EBC, but for those diluted with WBB there was no clear trend. The activities of chymotrypsin and aminopeptidase in whole-body extracts of individual yellowtail larvae extracted with EBC were significantly higher than in those extracted with 150 mM NaCl, suggesting that EBC inhibits the loss of enzyme activity that may otherwise occur during extraction. Thus, EBC is considered to be the most effective diluent for the microquantification of proteases amongst the four blockers tested.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteins are vital nutrients for the metabolism and growth of fish. Indeed, the activities of digestive proteases determine the efficiency of digestion and absorption of feed in fish. Many studies have characterized the digestive enzymes of fish both functionally and biochemically, and have analyzed their activities throughout various developmental stages from larval to juvenile fish (Ribeiroa et al. 1999; Zambonino Infante and Cahu 2001; Murashita et al. 2014; Zeytin et al. 2016). However, to measure enzyme activities in samples, dilution is needed to establish an appropriate range of concentrations. This range is determined by the sensitivity and dynamic range of the chosen equipment and system, the substrates used for the enzymes under consideration, and the measurement conditions, including temperature, pH, and types of buffer. In the measurement of low concentrations of peptides and proteins, bovine serum albumin (BSA), defatted milk, and other derivatives have been used as blocking reagents (blockers) in Western blotting and immunoassays. These blockers prevent the loss of proteins and peptides in the sample through their nonspecific binding to pipette tips and tube walls during handling, and are particularly useful for dilutions and preservation over time. BSA and casein have protein-stabilizing effects (Chang and Mahoney 1995; Kolena et al. 1999), but protein- or peptide-based blockers are not appropriate for the dilution or preservation of proteases and peptidases as these enzymes digest these types of blocker and may thus exhaust their catalytic activity as a consequence.
Protein-free blockers are presumed to be effective in preventing the loss of protease catalytic activity during dilution and preservation. Recently, several kinds of protein-free blockers have become commercially available for Western blotting and immunoassays. The exact composition of these blockers is not provided by the manufacturers, but they are assumed to be primarily based on chemically synthesized polymers, which are not digested by proteases. These blockers block the nonspecific binding of enzymes used in Western blotting and immunoassays. In addition, the catalytic activities of the enzymes used for detection, such as horseradish peroxidase and alkaline phosphatase, are not affected by these blockers, according to the information provided by the manufacturers. Therefore, it follows that the catalytic activity of proteases should not be exhausted when using these blockers, and nonspecific binding of the enzymes to pipette tips and tube walls should be inhibited by these blockers.
The catalytic activities of digestive proteases can be measured quantitatively at very low concentrations by using fluorogenic peptide substrates modified with 7-amino-4-methylcoumarilamide (MCA). The detection limit of chymotrypsin assays when using fluorogenic substrates is 1/20 that of the colorimetric assay using the p-nitroanilide complex, and the rate of hydrolysis shows linearity over at least a 100-fold range (Zimmerman et al. 1976). The catalytic activity of trypsin can be sufficiently measured even in a single fish larva by using substrates modified with MCA (Navarro-Guillén et al. 2017). These data suggest the possibility of performing a multiple protease assay by using different fluorogenic substrates with extracts of individual larvae in a multi-group experiment at a small scale, which is impossible with colorimetric assays. Therefore, catalytic activity assays using fluorogenic substrates are considered superior to colorimetric assays in terms of measurement performance. However, because of the high sensitivity of fluorogenic assays, the samples sometimes need to be diluted using buffers or solutions in order to avoid overshooting the range. These dilutions may not only induce the loss of protease activity but also affect the proportionality of the dilution rate and protease activity due to nonspecific binding during handling. When comparing protease activities between larva and juvenile, the dilution rate of the extracts is sometimes higher than several hundreds because of the large differences in body size. For adult fish, homogenates of milligrams of tissue from the digestive tract also need be more diluted for the measurement of the catalytic activities of enzymes in fluorogenic assays. Limited data exist for estimating the effects of dilution on the measurement of catalytic activities of fish proteases in fluorogenic assays. Thus dilution methods need to be evaluated for a combination of fluorogenic substrates for microquantification of the enzyme activities of fish.
The aims of this study were (1) to determine appropriate blockers and conditions for the dilution of samples for the measurement of the catalytic activity of digestive fish proteases in fluorogenic assays, and (2) to estimate the effects of the appropriate blockers in samples of fish larvae that contain proteases at very low concentrations.
Materials and methods
Reagents and buffers
The effects of four blocking reagents were estimated in this study. They included EzBlock Chemi (EBC; liquid based on TRIS buffer, pH 8.4; ATTO, Tokyo, Japan), Western BLoT Blocking Buffer (WBB; liquid, pH 8.2; Takara, Kusatsu, Japan), BlockAce (BA; liquid; DS Pharma Biomedical, Osaka, Japan), and BSA (crystal, fraction V; Wako, Osaka, Japan). EBC and WBB, which are protein free, are based on substances synthesized chemically; they are used in Western blotting with chemiluminescence detection. EBC is used after dilution with distilled water at a rate of 20% (v/v), according to the manufacturer’s instructions; WBB is used undiluted. BA is made from casein that contains preservative, and is used after dilution with the appropriate buffer at a rate of 25% (v/v). BA is used for Western blotting and immunoassays (Andoh 2007), and has a lower background than BSA in fish insulin immunoassays (data not shown). In this study, BA and BSA were diluted or dissolved with borate–KCl–NaOH buffer (BKS; modified Clark-Labs buffer, 50 mM borate, 50 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% NaN3, adjusted to pH 8.2 with 2 M NaOH); BSA was dissolved in BKS at a rate of 1%.
Three synthetic substrates modified with MCA were used for measuring catalytic enzyme activities in this study. Boc-Gln-Ala-Arg-MCA (Kawabataet al. 1988), Suc-Ala-Ala-Pro-Phe-MCA (Sawada et al. 1983), and Ala-MCA were purchased from the Peptide Institute (Osaka, Japan) and were used for measuring the catalytic activities of trypsin (EC 3.4.21.4), chymotrypsin (EC 3.4.21.1), and alanyl aminopeptidase (aminopeptidase; EC 3.4.11.2), respectively. These substrates produce a strongly fluorescent substance, 7-amino-4-methylcoumarin (AMC), after digestion. Protein concentration was measured using the Pierce 660-nm Protein Assay Reagent (Thermo Fisher Scientific). A multimode microplate reader (Spark 10 M; Tecan Trading, Switzerland) was used for measuring the absorbance after mixing the supernatant and reagent and incubating the mixture for 15 min at 30 °C. BSA was used as the standard, and was serially diluted with EBC from 2000 to 31.3 μg/ml.
Extraction of digestive enzymes
Pyloric caeca were removed from 2-year-old yellowtail, Seriola quinqueradiata (body weight 3 kg; grown at Goto Laboratory, Seikai National Fisheries Research Institute, Japan Fisheries Research and Education Agency), 2 h after they had been fed until satiety. Pyloric caeca were frozen by solid CO2 and stored at − 80 °C. The samples were lyophilized, and the fat tissue was removed manually with forceps before homogenization. An ice-cold tenfold volume of 150 mM NaCl (v/w) was added to the lyophilized pyloric caeca, which was then homogenized manually using the Biomasher-II polypropylene microhomogenizer (Nippi, Tokyo) or a Polytron homogenizer (PT-1200E; Kinematica, Lucerne, Switzerland) in a 5.0-ml tube (BIO-BIK ST-500; INA OPTICA, Osaka, Japan) placed in an ice-cold aluminum microtube block (TAITEC, BAL-8188 or AB-12–05; INA OPTICA) after incubation for 5 min for solution absorption. The extract was obtained by removing the precipitate and lipids from the homogenate by centrifugation at 15,000 g for 5 min at 0 °C.
Measuring catalytic activity of the enzymes
One hundred microliters of BKS buffer, 10 μl of 1 mM enzyme substrate in BKS buffer containing 10% dimethyl sulfoxide, and 10 μl of sample were dispensed into a 96-well microtiter plate (no. 267342 or 436110; Thermo Fisher Scientific, Waltham, MA). The catalytic activities of the enzymes were calculated by measuring the increase in fluorescence intensity per minute of the AMC produced during catalysis at fluorescence wavelengths of 380 nm for excitation and 485 nm for emission at 30 °C. A multimode microplate reader (Spark 10 M) was used to measure the increase in fluorescence intensity [relative fluorescence units (RFU)/min]. The contents of each well of the microtiter plate were measured at five different points, arranged crossways, at 400-μm intervals; the value for each well is the average of these five measurements.
Experimental conditions
Experiments 1, 2, and 3 were performed to fulfill the first aim of the experiment (to determine the appropriate blockers and conditions for the dilution of samples for the measurement of the catalytic activity of digestive fish proteases by fluorogenic assay), whereas experiment 4 was performed to fulfill the second one (to estimate the effects of the appropriate blockers in samples of fish larvae that contain proteases at very low concentrations).
Experiment 1
The linearity of the catalytic activities of the diluted enzymes was compared among blockers and buffers. Extracts of yellowtail pyloric caeca (14.3 mg) obtained using a polypropylene microhomogenizer were serially diluted with EBC, BA, BKS, and 150 mM NaCl at dilutions ranging from 1/200 to 1/25,600 under cooling conditions at 0 °C. Each dilution was mixed for 20 s; inversion mixing was performed using a vortex mixer (MS-3; IKA Werke, Staufen, Germany). The tubes and pipette tips used were as follows: 1.5-ml Eppendorf 3810X tubes (Eppendorf, Germany), 200-μl QSP 110-NEW pipette tips(Quality Scientific Plastics, CA) , and 10-μl Gilson D10 pipette tips (Gilson, WI) for . The catalytic activities of trypsin, chymotrypsin, and aminopeptidase were measured immediately after preparation of the extracts without preincubation, according to the protocol described in “Measuring catalytic activity of the enzymes.” All tubes, blockers, and buffers were precooled in ice.
Experiment 2
The linearity of the catalytic activities of diluted enzymes was compared among two protein-free blockers and two protein blockers. Yellowtail pyloric caeca (190 mg) were homogenized using a polytron homogenizer and extracts prepared from the homogenate. The extracts were diluted serially with EBC, WBB, BA, and BSA to give dilutions ranging from 1/200 to 1/6400, according to experiment 1. The catalytic activities of trypsin, chymotrypsin, and aminopeptidase were also measured according to experiment 1. The dilution methods and cooling conditions were the same as those in experiment 1.
Experiment 3
The effects on the catalytic activities of enzymes were compared between EBC and WBB. Extracts of yellowtail pyloric caeca (29.3 mg) obtained using a polypropylene microhomogenizer were diluted with EBC and WBB at a dilution of 1/200 under cooling conditions at 0 °C and incubated at 25 °C for 0, 4, 8, and 24 h in an aluminum block incubator (WSC-2620; ATTO). The catalytic activities of proteases were measured in triplicate. The activities are expressed as RFU/min and as a percentage of the average activity at 0 h.
Experiment 4
Catalytic activity was compared between EBC and 150 mM NaCl for extracts of yellowtail larvae. Yellowtail larvae were produced in the Goto Laboratory (Goto Islands, Nagasaki, Japan) of the Fisheries Research and Education Agency, and kept at 22 °C. Larvae were fed L-type rotifer-enhanced eicosapentaenoic acid and docosahexaenoic acid from 4 days post-hatching. Larvae at 7 days post-hatching (average total length: 5.3 mm) were frozen in 1.5-ml plastic tubes in an aluminum cooling block cooled at − 80 °C and stored at − 80 °C until use. Whole-body samples of nine larvae were thawed in an ice-cold aluminum block and homogenized individually in the block with 200 μl of EBC or 150 mM NaCl using a Biomasher-II; the precipitate was removed by centrifugation at 15,000 g at 0 °C for 5 min. Ten microliters of each supernatant was used for the extraction of yellowtail larvae samples without dilution. The catalytic activities of trypsin, chymotrypsin, and aminopeptidase were measured without preincubation according to experiment 1. Protein concentrations were measured in 20 μl of the supernatant with 100 μl of the Pierce 660-nm Protein Assay Reagent (Thermo Fisher Scientific).
Calculation
Data are presented as the average ± SEM of replicates. Student’s t-test was performed to compare protease activities of yellowtail larvae in experiment 4. The differences between groups were considered to be statistically significant at p < 0.05. Linearity between dilution rate and catalytic activity of each enzyme was evaluated by calculating correlation coefficients (r2) of the nonlinear regression curve fit on a log–log axis with weighting by 1/Y2, where Y is catalytic activity of the enzyme. All statistical calculations were performed using GraphPad Prism version 7.
Results
Figure 1 shows the relationships between the dilution rates with two blockers, buffer, and 150 mM NaCl of pyloric caeca extracts and their protease activities in experiment 1. Extracts with EBC and BA showed clear linearity in terms of trypsin (r2 > 0.998), chymotrypsin (r2 > 0.927), and aminopeptidase (r2 > 0.997) activities, with the exception of chymotrypsin activity at a dilution of 1/25,600 (regression line not shown). Only aminopeptidase activity showed linearity with BKS and 150 mM NaCl (r2 > 0.941); these activities were lower than those with EBC or BA. The activity of chymotrypsin in BA was also lower than that measured with EBC.
Linearity between the catalytic activity of the three proteases (trypsin, chymotrypsin, and aminopeptidase) and dilutions of pyloric caeca extracts with two blockers, buffer, and 150 mM NaCl. The extracts were diluted serially in the range from 1/200 to 1/25,600. RFU Relative fluorescence intensity, EBC EzBlock Chemi, BA BlockAce, BKS borate–KCl–NaOH buffer
In experiment 2, protein-free blockers and protein blockers were compared for pyloric caeca samples of yellowtail. There was clear linearity (r2 > 0.993) for all the blockers in terms of activities of the three proteases (Fig. 2); the slopes were close to − 1.000 and ranged from − 0.909 to − 1.336. Slopes for samples diluted in EBC ranged from − 0.948 to − 0.992, whereas for those diluted in WBB the range was from − 0.909 to − 1.078. However, the activities of the proteases differed among the blockers. Protease activities with EBC and WBB were identical for 1/200 to 1/6400 dilutions, but the activities of trypsin and chymotrypsin in BA and BSA were lower. For aminopeptidase, linearity and parallelism were identical among all the blockers.
Linearity between catalytic activity of the three proteases (trypsin, chymotrypsin, and aminopeptidase) and dilution rate of pyloric caeca extracts with four blockers. Extracts were diluted serially in a range from 1/200 to 1/6400. WBB Western BLoT buffer, BSA BKS buffer containing 1% (w/v) bovine serum albumin; for other abbreviations, see Fig. 1
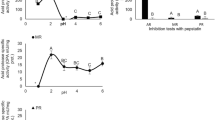
In experiment 3, the changes in protease activities during preservation were compared using pyloric caeca extracts diluted with EBC or WBB (Fig. 3). The activities of trypsin and chymotrypsin in EBC increased with time. The activity of trypsin in EBC increased from 81.7 ± 2.1 RFU/min at 0 h to 132.3 ± 2.5 RFU/min at 24 h. The activity of chymotrypsin increased from 50.6 ± 0.3 at 0 h to 136.9 ± 1.3 RFU/min at 24 h. These increases corresponded to changes of 162% and 271%, respectively, after 24 h. However, the activity of trypsin in WBB did not show a clear trend; its activity at 0 h was 60.1 ± 1.5 RFU/min. With EBC, trypsin activity was 90.8 ± 1.4 RFU/min at 4 h, which was significantly lower than its highest activity at 24 h with this blocker. The activity of chymotrypsin in WBB at 0 h was 38.4 ± 0.5 RFU/min; it increased to 81.3 ± 0.5 RFU/min at 8 h and then decreased. In EBC the activity of aminopeptidase at 0 h was 78.5 ± 0.5 RFU/min; its activity increased to 89.2 ± 0.8 RFU/min at 8 h, which corresponded to a 113% increase; its activity then decreased. The activity of aminopeptidase in WBB ranged from 71.3 ± 0.4 RFU/min at 0 h to 79.3 ± 0.1 RFU/min at 4 h, and decreased thereafter.
Changes in activity (relative RFU/min) of three proteases (trypsin, chymotrypsin, and aminopeptidase) diluted with EBC and WBB. Extracts of pyloric caeca of yellowtail were diluted at 1/200 with EBC and WBB and incubated for 0, 4, 8, and 24 h at 25 °C. Different letters indicate significant differences, bars indicate SEM. For abbreviations, see Figs. 1 and 2
The effect of EBC on the homogenates of yellowtail larvae was estimated in experiment 4 (Fig. 4). The activities of chymotrypsin and aminopeptidase were significantly higher in the extracts of larvae with EBC than in those extracted with 150 mM NaCl (p-values < 0.001). There was no significant difference between the activities of trypsin extracted with EBC and 150 mM NaCl (p 0.137).
Activities of three proteases (trypsin, chymotrypsin, and aminopeptidase) in extracts of whole yellowtail larvae homogenized with either EBC or 150 mM NaCl. Different letters indicate significant differences, bars indicate SEM. For abbreviations, see Fig. 1
Discussion
This study estimated the effect of protein-free blockers in dilutions of digestive proteases, including trypsin, chymotrypsin, and aminopeptidase, from yellowtail, one of the most important fish of the Japanese fishing industry. Trypsin and chymotrypsin are produced in pancreatic tissue surrounding the pyloric caeca (Hirji and Courtney 1982; Kurokawa and Suzuki 1995; Srivastava et al. 2002; Rungruangsak-Torrissen 2006), and aminopeptidase is produced in intestinal tissue (Kurokawa and Suzuki 1998; Zambonino Infante and Cahu 2001). Trypsin and chymotrypsin are considered major functional proteases in the fish digestive tract (Gamboa-Delgado et al. 2011; Conceição et al. 2011; Rønnestad et al. 2013). Aminopeptidase plays a role in the final digestion of peptides after the cleavage of substrate proteins by endopeptidases such as trypsin and chymotrypsin (Rønnestad et al. 2013). Aminopeptidase is also one of the major proteins of the intestinal microvillar membrane (Olsen et al. 1997). To our knowledge, this is the first report suggesting the importance of protein-free blockers for the microscale quantification of digestive proteases in fish.
The effects of the blockers were clear in terms of their inhibition of the loss of protease activity in diluted extracts of yellowtail pyloric caeca. Samples containing EBC and BA showed typical linearity between dilution rate and protease activity, with slopes of almost − 1.000 on log–log axis graphs, and parallelism for the three tested proteases. Extracts with BKS and 150 mM NaCl did not show linearity, and the protease activities in these were lost at higher dilution rates. These findings indicate that the addition of blockers is required for protease extract dilution in order to measure catalytic activities quantitatively at a microscale. Thus, the dilution of extracts with buffers without blockers should be avoided, especially at higher dilutions. In other words, dilution with a buffer that does not contain a blocker leads to underestimation of the catalytic activity of enzymes. This poses a problem in comparisons of protease activity among samples of experimental groups that are very different in terms of their physiology or individual body size, e.g., between larvae with digestive proteases that are activated by feeding and unfed larvae, and in the estimation of differences in the activity of individual proteases between larvae and juveniles or mature fish.
EBC enlarged the dynamic range of measurement for protease activities in adult yellowtail, particularly at lower dilutions. The lowest dilution for trypsin and aminopeptidase measurement was > 1/25,600. This high dilution corresponds to extracts of 40 ng of lyophilized pyloric caeca from yellowtail. The wide dynamic range and high sensitivity were actualized by the combination of fluorogenic substrates and EBC. Thus, EBC appears to be an appropriate blocker for the quantitative microscale measurement of digestive proteases. This enlargement of the dynamic range may be applicable to the measurement of protease activity in the feces of larvae when monitoring the state of enzyme secretion as a non-invasive technique, especially in the pancreas, as is also applied to human samples (Molinari et al. 2004). In fact, trypsin activity in the feces of a single eel larva can be sufficiently measured by using fluorogenic substrates (data not shown).
In experiment 2, dilutions with each of the four blockers showed linearity and parallelism with enzyme activities, although the activities of trypsin and chymotrypsin were highest with EBC and WBB. Linearity of activities of the three proteases in EBC and WBB was observed at dilution rates of at least 1/200 to 1/6400 (Fig. 2). These results suggest that, like EBC, WBB is also an appropriate blocker in the context of this study.
The activities of trypsin in BA and chymotrypsin in BA and BSA were lower than in EBC and WBB, although parallelism was observed at a dilution from 1/200 to 1/6400 in experiment 2 (Fig. 2). This suggests that trypsin and chymotrypsin lose activity with protein-based blockers. The activity of aminopeptidase with all four blockers was identical, suggesting that this enzyme is more stable, even with proteins and peptides, than trypsin and chymotrypsin.
The difference in loss of activity of aminopeptidase among the blockers and aqueous solutions was smaller than that observed for trypsin and chymotrypsin in experiment 1, suggesting that aminopeptidase does not bind more strongly to tubes and pipette tips during the dilution process than trypsin and chymotrypsin. One explanation for this might be that aminopeptidase is more hydrophilic than trypsin or chymotrypsin.
The most interesting findings in this study included not only the stabilizing effect of the protein-free blockers on the three proteases during the dilution process, but also the activation effect of EBC on trypsin and chymotrypsin during preservation. These findings suggest that the activation rates of the precursors of trypsin and chymotrypsin exceeded the activity loss rates of these proteases in EBC, at least until the 24-h point at 25 °C. Moreover, trypsin and chymotrypsin of yellowtail appear to be stable in EBC even at 25 °C. Trypsin and chymotrypsin activities had increased by 162% and 271%, respectively, at 24 h compared with those at 0 h in EBC, suggesting that more than half of these measured proteases were actually precursor molecules in the extracts of pyloric caeca. Trypsin and chymotrypsin are converted from precursors such as trypsinogen and chymotrypsinogen to the mature molecules; these precursors are activated by enteropeptidase (EC 3.4.21.9) and trypsin (Conceição et al. 2011; Gamba-Delgado et al. 2011). In contrast, aminopeptidase is not activated by enteropeptidase, and the increase in its activity was only 13% in EBC in experiment 3. These results suggest that EBC preserves the activation processes of trypsin and chymotrypsin, including the activity of enteropeptidase, in extracts of pyloric caeca. Extracts of pyloric caeca are expected to contain large amounts of several proteases, but EBC seems to preserve the activities of trypsin, chymotrypsin, aminopeptidase, and enteropeptidase by preventing their degradation by coexisting proteases.
The activation of trypsin and chymotrypsin occurred during the 24 h after homogenization in experiment 3; the temperature was set at optimum for the growth of yellowtail, 25 °C (Harada 1965). Thus, it appears that trypsin and chymotrypsin require more than several hours for activation from their respective precursors after their secretion from the pancreatic tissue into the intestines of fish. To date, the time required for activation after secretion has not been estimated for trypsin or chymotrypsin in fish. Although all the experiments in this study were performed in vitro, the estimates reported here are the first for fish. The period of time between conversion from precursors to the secretion of proteases from the pancreas is one of the most important factors determining the speed of digestion; EBC appears to be an effective reagent for the estimation of this period for trypsin and chymotrypsin.
In addition, EBC was effective for the extraction of the digestive proteases of yellowtail larvae in experiment 4. The activities of chymotrypsin and aminopeptidase extracted in EBC were significantly higher than those observed with 150 mM NaCl, suggesting preservation and an increase in the activities of the proteases by EBC in the whole-body extracts, as well as in protease extracts of pyloric caeca of adult yellowtail. There has recently been a drastic improvement in equipment used for fluorescent assays. The equipment used in this experiment enabled the measurement of protease activity in 10 μl of an extract obtained from 200 μl of homogenate of a single yellowtail larva at 7 days post-hatching. Ten microliters of an extract was sufficient for the measurement of trypsin, chymotrypsin, and aminopeptidase activities. EBC is an important reagent for the quantification of multiple protease activities using a number of fluorogenic substrates for a single fish larva extract. Genes of several types of trypsin and chymotrypsin are expressed in both larval and adult yellowtail (Yasuike et al. 2018). For example, at least two types of trypsin are expressed in the pyloric caeca of yellowtail, and their affinities for a fluorogenic substrate differ (data not shown). Multiple measurements of the catalytic activity of enzymes following dilution were carried out for the characterization and determination of ontogenic changes of digestive enzyme activities of yellowtail larvae in the present study.
This study showed that solutions containing a protein-free blocker were more effective for the quantification of the catalytic activity of protease in extracts of fish tissue at high dilutions than those containing protein-based blockers and those without blockers at all. In particular, digestive proteases in samples diluted with EBC showed good linearity, higher activity, and high activation after homogenization of the tissues of adult fish and larvae. Collectively, our findings indicate that, among the blockers compared in this study, EBC is the most appropriate as a diluting agent for digestive protease extracts. In conclusion, the application of EBC is considered to be of value for the preservation, and activation from precursors, of digestive enzymes measured by microquantification.
References
Andoh T (2007) Amino acids are more important insulinotropins than glucose in a teleost fish, barfin flounder (Verasper moseri). Gen Comp Endocrinol 151:308–317
Chang BS, Mahoney RR (1995) Enzyme thermostabilization by bovine serum albumin and other proteins: evidence for hydrophobic interactions. Biotechnol Appl Biochem 22:203–214
Conceição L, Aragão C, Rønnestad I (2011) Proteins. In: Joan H, John G (eds) Larval nutrition. Wiley, Hoboken, pp 83–116
Gamboa-Delgado J, Le Vay L, Fernández-Díaz C, Cañavate P, Ponce M, Zerolo R, Manchado M (2011) Effect of different diets on proteolytic enzyme activity, trypsinogen gene expression and dietary carbon assimilation in Senegalese sole (Solea senegalensis) larvae. Comp Biochem Physiol B Biochem Mol Biol 158:251–258
Harada T (1965) Studies on propagation of yellowtail (Seriola quinqueradiata T. & S.): with special reference to relationship between feeding and growth of fish reared in floating net craw. Mem Fac Agric Kinki Univ 1965:1–275
Hirji KN, Courtney WAM (1982) Leucine aminopeptidase activity in the digestive tract of perch, Perca fluviatilis L. J Fish Biol 21:615–622
Kawabata S, Miura T, Morita T, Kato H, Fujikawa K, Iwanaga S, Takada K, Kimura T, Sakakibara S (1988) Highly sensitive peptide-4-methylcoumaryl-7-amide substrates for blood-clotting proteases and trypsin. Eur J Biochem 172:17–25
Kolena J, Jezová M, Vranová J, Scsuková S (1999) Structure-stabilizing effect of albumin on rat ovarian LH/hCG receptors. Biochim Biophys Acta 1416:208–216
Kurokawa T, Suzuki T (1995) Structure of the exocrine pancreas of flounder (Paralichthys olivaceus): immunological localization of zymogen granules in the digestive tract using anti-trypsinogen antibody. J Fish Biol 46:292–301
Kurokawa T, Suzuki T (1998) Development of intestinal brush border aminopeptidase in the larval Japanese flounder Paralichthys olivaceus. Aquaculture 162:113–124
Molinari I, Souare K, Lamireau T, Fayon M, Lemieux C, Cassaigne A, Montaudon D (2004) Fecal chymotrypsin and elastase-1 determination on one single stool collected at random: diagnostic value for exocrine pancreatic status. Clin Biochem 37:758–763
Murashita K, Matsunari H, Kumon K, Tanaka Y, Shiozawa S, Furuita H, Oku H, Yamamoto T (2014) Characterization and ontogenetic development of digestive enzymes in Pacific bluefin tuna Thunnus orientalis larvae. Fish Physiol Biochem 40:1741–1755
Navarro-Guillén C, Rønnestad I, Jordal AO, Moyano FJ, Yúfera M (2017) Involvement of cholecystokinin (CCK) in the daily pattern of gastrointestinal regulation of Senegalese sole (Solea senegalensis) larvae reared under different feeding regimes. Comp Biochem Physiol A 203:126–132
Olsen J, Kokholm K, Norén O, Sjöström H (1997) Structure and expression of aminopeptidase N. In: Ansorge S, Langner J (eds) Cellular peptidases in immune functions and diseases. Springer, Berlin, pp 47–57
Ribeiroa L, Zambonino-Infante JL, Cahub C, Dinisa MT (1999) Development of digestive enzymes in larvae of Solea senegalensis, Kaup 1858. Aquaculture 179:465–473
Rønnestad I, Yúfera M, Ueberschär B, Ribeiro L, Sæle Ø, Boglione C (2013) Feeding behaviour and digestive physiology in larval fish: current knowledge, and gaps and bottlenecks in research. Rev Aquacult 5:S59–S98
Rungruangsak-Torrissen K, Moss R, Andresen LH, Berg A, Waagbø R (2006) Different expressions of trypsin and chymotrypsin in relation to growth in Atlantic salmon (Salmo salar L.). Fish Physiol Biochem 32:7–23
Sawada H, Yokosawa H, Hoshi M, Ishii S (1983) Ascidian sperm chymotrypsin-like enzyme; participation in fertilization. Experientia 39:377–378
Srivastava AS, Kurokawa T, Suzuki T (2002) mRNA expression of pancreatic enzyme precursors and estimation of protein digestibility in first feeding larvae of the Japanese flounder, Paralichthys olivaceus. Comp Biochem Physiol 132A:629–635
Yasuike M, Iwasaki Y, Nishiki I, Nakamura Y, Matsuura A, Yoshida K, Noda T, Andoh T, Fujiwara A (2018) The yellowtail (Seriola quinqueradiata) genome and transcriptome atlas of the digestive tract. DNA Res 25:547–560
Zambonino-Infante JL, Cahu CL (2001) Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol C 130:477–487
Zeytin S, Schulz C, Bernd Ueberschär B (2016) Diurnal patterns of tryptic enzyme activity under different feeding regimes in gilthead sea bream (Sparus aurata) larvae. Aquaculture 457:85–90
Zimmerman M, Yurewicz E, Patel G (1976) A new fluorogenic substrate for chymotrypsin. Anal Biochem 70:258–262
Acknowledgements
Yellowtails were kindly provided by T. Hotta, K. Yoshida, and Y. Fujinami from the Seikai National Fisheries Research Institute, Japan Fisheries Research and Education Agency, Japan. Ms. A. Ikimi and Y. Masuda helped with the sampling and measurements. This work was supported in part by funds from the Japan Science and Technology Agency.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Andoh, T. Dilution of digestive fish proteases with protein-free blocking reagents prevents loss of catalytic activity during microquantification. Fish Sci 86, 543–550 (2020). https://doi.org/10.1007/s12562-020-01422-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-020-01422-4