Abstract
Arsenic toxicity in an aquatic environment is a major concern, and its elimination has become a global challenge. In the current study, histopathology, serum biomarkers and cytokine gene expression were comparatively examined in fish fed with a control diet or diets containing Chlorella vulgaris (Ch) after exposure to sodium arsenite (NaAsO2) in Nile tilapia (Oreochromis niloticus) with the aim of evaluating the protective role of Ch against arsenite-induced toxicity. Severe histopathological alterations were evident in fish exposed to 7 ppm (parts per million) arsenite for 21 days, compared to unexposed fish. Levels of serum biomarkers ALT, AST, ALP, urea and creatinine were increased, but the levels of Na+, total proteins, albumins and globulins were decreased. Moreover, the expression of all the cytokine genes examined, including IL-1β (7-fold), TNF-α (14-fold) and TGF-β1 (13-fold), were significantly upregulated after arsenite exposure. However, in fish fed with diets containing 5% or 10% Ch, the histopathological alterations in the gills, liver and head kidney were reduced, the biomarkers were stabilized, and the upregulation of cytokine gene expression was lowered, with the high Ch diet (10%) showing more prominent effects. These results suggest the protective and therapeutic roles of Ch as a feed supplement in Nile tilapia against arsenic induced toxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The contamination of aquatic ecosystems is one of the major threats worldwide affecting aquatic lives. Heavy metal pollution is one of these threats (Ahmed et al. 2013), where the exposure of heavy metals in living organisms leads to various deleterious health hazards such as immunotoxicity, genotoxicity, and nephrotoxicity (Cobbina et al. 2015). Arsenic (As) is one of the most hazardous heavy metals that is released in the aquatic environment through both geogenic and anthropogenic processes (ATSDR 2002). In aquatic habitats, As is biologically available to organisms, including fish, by the aid of several species of microorganisms (Duker et al. 2005; Gonzalez et al. 2006). Fish, in particular, are very sensitive to these pollutants in their environment, and are considered a good model for monitoring potential risks associated with them (Lakra and Nagpure 2009). Arsenite (As3+) and arsenate (As5+) are the predominant types present in the aquatic environment and are interconvertible through redox and methylation reactions. In fish, arsenic speciates into two oxidation states, methylated species, arseno-sugars and arsenolipids, that differ in toxicity, and their combinations in fish tissues result in several pathophysiological effects (Kavitha et al. 2010).
Fish are readily susceptible to As toxicity through continuous gill exposure and intake of As-contaminated food (Aruljothi 2014), resulting in accumulation in various tissues. Liver, as the organ responsible for xenobiotic detoxification, is negatively affected by heavy metal contaminated water, which reduces the integrity of hepatocytes and hampers metabolic processes and xenobiotic removal, with subsequent induction of oxidative stress (Guerriero et al. 2010). Reports suggest that chronic As exposure causes abnormal liver function, hepatomegaly, liver fibrosis and cirrhosis in different fish species (Mazumder 2005). Biochemical changes in tissues/organs after As exposure have been reported in freshwater fish fingerlings of Labeo rohita (Palaniappan and Vijayasundaram 2009), and in mammals as well (Tripathi and Kumar 2011).
Recently, studies have been focused on the innate immune gene disorders observed in organisms after xenobiotics exposure. In this regard, As exposure has been associated with immunosuppression with increased risk to pathogen invasion (Cobbina et al. 2015). Zebrafish exposed to Cd and Cr resulted in significant upregulation of TNF-α, IL-6 and IL-1β gene expression (Jin et al. 2015). Similar upregulation of gene expression was observed in NADPH oxidase and GPx in macrophages after 24-h exposure to Cu (Teles et al. 2011). Many previous studies have reported on the harmful impacts of low concentration heavy metals on development-related genes of aquatic organisms (McCollum et al. 2014; Soetaert et al. 2007; Thévenod 2009; Wang et al. 2012).
Histopathological analysis is considered to be a good tool for detecting direct effects of xenobiotics within target organs of fish in laboratory experiments (Capkin et al. 2009), that could induce a number of lesions/changes in different organs (Ahmed et al. 2013). Liver and gills are suitable organs for histological investigations in order to assess the effect of pollution (Figueiredo-Fernandes et al. 2007). Pathological alterations have been observed previously in the gills and liver tissues in Oreochromis mossambicus exposed to different concentrations of NaAsO2; lesions observed were in the form of epithelial hyperplasia, and there was epithelial lifting and edema, lamellar fusion, aneurism, desquamation and necrosis in gill tissue. The liver tissue showed focal lymphocytic and macrophage infiltration, congestion, vacuolization and shrinkage of hepatocytes, dilation of sinusoids, cloudy swelling, vacuolar degeneration, focal necrosis and nuclear hypertrophy (Ahmed et al. 2013).
The presence of As contaminating water in an aquatic environment is particularly serious in fish, affecting their survival and growth, and even resulting in extinction of entire fish populations in polluted reservoirs, in addition to the human health risks arising from the consumption of contaminated fish with high As content in their tissues (Avigliano et al. 2015). Thus, it is important to develop methods to counteract the toxic effects of heavy metals. In this regard, many physical and chemical methods have been considered, but all were inefficient (Suzuki et al. 2013; Torres-Perez et al. 2012). Microalgae are renowned for their potential role in a variety of applications as live feeds for different growth stages of many aquatic species such as bivalve mollusks, abalone, crustaceans, and some fish species. This is due to the great effects it has on body weight gain, increased triglyceride and protein deposition in muscle, enhanced disease resistance, lowering nitrogen output released into the environment, increased digestibility, physiological activity, starvation tolerance, and carcass quality (Kamat et al. 2000; Sirakov et al. 2015). Chlorella vulgaris (Ch), a freshwater microalgae, is rich with many bioactive components such as carotenoids, phycobilins, fatty acids, polysaccharides, vitamins, and sterols (Soontornchaiboon et al. 2012). Such bioactive components contribute to immunity-modulating, anti-cancer, and protective properties against hematopoiesis, hypertension, cataracts, and age-related diseases. Thus, Ch as GRAS (generally recognized as safe) is used in many medical treatments (Kumar et al. 2018).
The higher content of carotenoids in microalgae has shown not only antioxidative properties but also anti-inflammatory (Soontornchaiboon et al. 2012). Deleterious effects, such as immune suppression, oxidative stress and carcinogenicity, have been observed due to As3+ intoxication (Elia et al. 2018). Our previous work also revealed that As3+ intoxication suppressed innate immune responses and induced oxidative stress, and found that Ch supplementation had a protective role against As3+ intoxication (Zahran and Risha 2014). Therefore, the current study was undertaken to better understand the mechanisms underlying the As3+ intoxication through evaluating the histopathological alterations in tissues, cytokines expression analysis, and biochemical parameters, to gain more knowledge regarding the potential protective and therapeutic roles of Chlorella dietary supplementation to ameliorate As-induced toxicity in Nile tilapia Oreochromis niloticus.
Materials and methods
Chemicals
Sodium arsenite (NaAsO2) with 98% purity was obtained from MERCK (0082970, Art. 6287) and used without further purification for the experiment. A dried powder of Chlorella vulgaris (Ch) culture was purchased from the Institute of National Research Center, Cairo, Egypt.
Fish maintenance
One hundred and twenty Nile tilapia, (average body weight 90 g), were procured from a private fish farm in Ad-Dakahliya province, Egypt. They were acclimatized for 3 weeks in large tanks provided with adequate aeration and an underwater internal powered filter under laboratory conditions before they were exposed to As treatment. During that period, fish were fed ad libitum with a control basal diet (Table 1), at 25 ± 2 °C, and about 50% of the water was exchanged daily to maintain water quality. No clinical signs were ever observed during this period.
As exposure
The fish were exposed to a sub-lethal concentration of 7 ppm (parts per million) sodium arsenite for up to 21 days. This concentration equates to 1/10th of the median lethal concentration of 71.7 ppm based on a 96-h toxicological assay in Nile tilapia (Hwang and Tsai 1993). A fresh daily stock solution of NaAsO2 was prepared by dissolving the analytical grade NaAsO2 in double-distilled water, and then the desired concentration was prepared from the stock solution. Unexposed fish were maintained in separate tanks without As under identical conditions.
Preparation of experimental diets
A dried Ch powder was purchased from the Institute of National Research Center, Cairo, Egypt. The components of diets with supplementation of Ch powder are detailed in Table 1. All ingredients were mixed with oil and then with water, resulting in a stiff dough. Each diet was then extruded through a mincer. The resulting strands were shadow-dried, broken up, sieved into pellets, and stored in plastic bags at 4 °C until use.
Experimental design
Nile tilapia were distributed in the aquaria and triplicate tanks were assigned per dietary treatment group (total N = 120 fish). Four groups were assigned: a control group fed with the control diet without sodium arsenite in the water (Ch0), and groups with sodium arsenite (7 ppm) in the water that were fed with the control diet (Ch0+As), or diets supplemented with 5% (Ch5+As) or 10% (Ch10+As) Ch powder (Table 1). Fish were fed ad libitum twice daily in equal rations at 09.00 h and 16.00 h for 21 days. Water was changed daily at about 80% during the experimental period and the water quality (temperature 25 ± 2°C, dissolved oxygen (mg/l) 6.62 ± 0.10, and pH 7.25 ± 0.04) was maintained during the entire experiment. The fecal matter and other waste materials were siphoned off daily to reduce ammonia content in the water.
Sample collection
Three fish from each aquarium (9 fish /group) were sampled on days 7, 14 and 21. Each aquarium was sampled one at a time; the fish were sedated with a low dose of buffered tricaine, MS-222 (tricaine methanesulfonate, FINQUEL®, ARGENT) (30 mg/l tricaine + 60 mg/l sodium bicarbonate), and each fish was then euthanized one at a time in a separate container.
For determination of serum biochemical parameters, fish were bled from the caudal peduncle. Blood was transferred immediately to a plain centrifuge tube and centrifuged at 1700×g for 15 min at 4 °C. The serum was separated carefully and frozen at − 80 °C until use. For histopathology, the gills, liver and head kidney tissues were excised and fixed immediately in 10% neutral buffered formalin. For gene expression analysis, the liver tissue was collected at the end of the exposure period (21 days) and immediately placed in RNAlater (Sigma) and stored at − 20°C, until further use.
Semi-quantitative histopathological analysis
The gills, liver and head kidney were dehydrated in a graded series of ethanols after formalin fixation, cleared in xylene and embedded in paraffin. Sections (5 µm) were cut and stained with hematoxylin and eosin for the histopathological examination (Bancroft et al. 1996). Lesions were evaluated semi-quantitatively by ranking tissue lesion severity. Ranking from 0 to 3 depended on the degree of tissue alteration (DTA) as follows: (0) absent: structure of the tissue is normal with no lesion present, (1) lesions present in < 20% of each studied field, (2) lesions present in between 20 and 60% of each studied field, and (3) lesions present in > 60% of each studied field. This ranking was used according to Benli et al. (2008) to establish an overall assessment value of the histopathological lesions for each individual fish tissue.
Serum biochemical parameters
The activities of serum aspartate aminotranseferase (AST), alanine aminoranferase (ALT), and alkaline phosphatase (ALP) were estimated using commercial kits (Randox, UK). The concentration of serum total proteins, albumins and globulins were measured spectrophotometrically using test kits (Stanbio, USA). The serum sodium (Na+) and potassium (K+) levels were measured spectrophotometrically using test kits (Teco–Diagnostics, USA). The levels of serum urea and creatinine were measured using available kits (Diamond, Egypt and Human, Germany).
Extraction of total RNA
Total RNA was extracted from liver samples using an RNeasy kit according to manufacturer’s instructions (QIAGEN, Germany). Briefly, 100 mg of tissues was homogenized in 600 µl of the lysis buffer RLT (2-mercaptoethanol was added to buffer RLT before use) in a mortar. The lysate was centrifuged at 14,000 rpm for 2 min. Absolute ethanol (400 µl) was added to the cleared supernatant and mixed well by pipetting. The sample was transferred to an RNeasy spin column placed in a 2-ml collection tube and centrifuged for 1 min at 10,000 rpm. The filtrate was discarded and the RNeasy spin column was washed by adding 700 μl buffer RW1, then centrifuged for 1 min at 10,000 rpm. The washing step was repeated twice with 500 μl buffer RPE and centrifuged for 1 min at 10,000 rpm. The RNeasy spin column was transferred to a new tube and centrifuged for 1 min at full speed. The RNA was eluted by adding 40 μl RNase-free water directly to the spin column membrane. The column was incubated at room temperature for 1 min, then centrifuged at 8000 rpm for 1 min, and the RNA was stored at − 80 °C.
cDNA synthesis and real-time PCR analysis of gene expression
The total RNA was converted to cDNA using RevertAid™ Reverse Transcriptase (Fermentas), following the manufacturer’s instructions. Q-PCR was performed using SYBR Green PCR Master Mix (Fermentas, USA). Each reaction was performed in a 25-μl mixture, which contained 1 μl of 10 pmol/μl of each primer, 1 μl of template cDNA (conc. 50 ng), 12.5 μl of 2X SYBR Green PCR Master Mix and 9.5 μl of nuclease-free water. Samples were spun before being loaded in the rotor wells, and each sample was run in triplicate. The amplification program proceeded at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s; annealing at 60 °C for 30 s and extension at 72 °C for 30 s, using a Rotor-Gene 6000 cycler (QIAGEN, ABI System, USA). After the cycling protocol, the melting curves were obtained to assess the specificity. The expression of pro-inflammatory cytokines IL-1β, TNF-α and anti-inflammatory cytokine TGF-β1, as well as the housekeeping gene elongation factor-1α (EF-1α), were examined. The primers for real-time PCR are detailed in Table 2, with at least one primer of a pair designed to cross an intron so that genomic DNA could not be amplified under the PCR conditions used. The expression of each gene was first normalized to that of EF-1α, and presented as a fold change by calculating the average expression level of the arsenite exposed samples divided by that of the controls as described previously (Wangkahart et al. 2016).
Ethical statement
This study was conducted in accordance with strict guidelines for the use of laboratory animals. All experimental procedures were in compliance with the Animal Care and Use Guidelines at Mansoura University and approved by the local Administrative Panel on the Laboratory Animal Care Committee. All treatments were performed under tricaine anesthesia and were performed so as to minimize suffering and stress.
Statistical analysis
Data of serum biochemical parameters were subjected to one-way analysis of variance (ANOVA) using the SPSS computer software (SPSS version 17.0 for Windows). Serum biochemical parameters and gene expression levels were graphically presented using GraphPad Prism version 6 (GraphPad Software Inc.). Differences between means were assessed by Duncan’s multiple-range test and effects with a probability of P < 0.05 were considered significant. Scores of histopathological variables were tested by the chi-square test among all groups regardless of time.
Results
Ch dietary supplementation ameliorated histopathological alterations after arsenite exposure
The main histopathological changes in gills of fish exposed to 7 ppm sodium arsenite included epithelial hyperplasia, fusion of secondary lamellae, lifting up of the epithelium, and aggregations of inflammatory cells in gill filaments. Additional changes were inconsistently seen, such as edema, congestion, and focal hemorrhage at primary lamellae (Fig. 1a–d). Frequencies of histopathological lesions observed in gills were significant between all groups with no significance over time, (chi-square (χ2) test: χ2 = 99.9, 195.6, 163, and 94.9, respectively, df = 9, P < 0.0001). However, these frequencies of lesions were reduced greatly in the Ch10+As group (Table 3).
a–d Histopathology of sodium arsenite exposure in the gills of Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0+As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) after 21 days exposure to sodium arsenite. a Control gill filaments show normal histology. b Gill filaments of fish exposed to arsenic show primary filament hyperplasia and fusion of secondary lamellae (arrows). c Lifting up of the epithelium (arrowheads), and edema (arrows). d Leukocytic cells infiltration (arrows) and congestion (arrowhead) (H & E ×100)
Liver of the exposed group had hepatocyte hypertrophy, cytoplasmic vacuolation, nuclear pyknosis and karyorrhexis, focal lymphocytic and macrophage infiltration, peliosis hepatitis, and congestion and dilation of sinusoids. The pancreatic cells demonstrated vacuolization, decreased basophilia, shrinkage, and disorganization. Necrosis was evident in both hepatocytes and hepatopancreas (Figs. 2, 3 and 4). By day 21, the sinusoids almost regained their normal structure, and the compactness of the pancreatic tissue was found to reappear. The cytoplasm of the biliary epithelium was swollen and vacuolized. Similar histopathological changes were observed in groups Ch5+As and Ch10+As. DTA observed in livers of Nile tilapia fish was significant between all groups with no significance over time (chi-square test: χ 2 = 108.3, 112.5, 82.8, and 104, 157.9, respectively, df = 9, P < 0.0001) (Table 4). Similarly to gills, the frequencies of the highest score for the lesions detected in liver were reduced in the Ch10+As group (Table 4).
a–d Hepatocyte vacuolization in the liver of Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0+As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) after 21 days. a Control liver with score 0 shows normal histology. b Score 1 shows mild cytoplasmic vacuolization. c Score 2 shows moderate cytoplasmic vacuolization. d Score 3 shows severe cytoplasmic vacuolization with diffuse nuclear pyknosis (H & E ×200)
a–c Liver inflammation of Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) after 21 days. a Score 1 demonstrates mild leukocytic cells infiltration (arrow). b Score 2 demonstrates moderate leukocytic cells infiltration (arrows) c Score 3 demonstrates severe diffuse leukocytic cells infiltration (arrows) (H & E ×200)
a–c Histopathology of sodium arsenite exposure in the liver of Nile tilapia at day 14. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) after 14 days. a Focal area of necrosis (arrow), b Decreased basophilia, shrinkage and necrosis in hepatopancreas (arrow), c severe vacuolization of pancreatic cells (short thick arrow) and biliary epithelium (long thin arrow) (H & E ×200)
Head kidneys showed depletion of the hematopoietic precursors, necrosis in the hematopoietic elements, thickening of the basal endothelial membrane, disruption of tissue integrity, edema, and sinusoidal blood congestion upon As exposure. Degeneration and necrosis of melano-macrophage centers (MMC) were rarely seen (Fig. 5a–d). Similar lesions were found in treated groups (Ch5+As and Ch10+As) but to a lesser extent. In the same trend, DTA observed in head kidneys was significant between all groups with no significance over time, (chi-square test: χ2 = 87.9, 134.9, 27.6, respectively, df = 9, P < 0.0001). Similarly, the frequencies of the highest score for the lesions detected in head kidneys were reduced in the Ch10+As group (Table 5).
a–d Histopathology of sodium arsenite exposure in the head kidney of Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0+As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) after 21 days. a Control head kidney shows normal histology. b Depletion of the hematopoietic precursors, necrosis in the hematopoietic elements and disruption of tissue integrity (asterisk). c Thickening of the basal endothelial membrane (red arrowheads) and sinusoidal blood congestion (red arrows). d Edema (black arrows) (H & E ×200)
Ch dietary supplementation mitigated the upregulation of enzyme activity after arsenite exposure
Toxicant stress can lead to elevation of enzyme activities which are considered to be indicators of hepatic tissue damage and dysfunction, as previously described by Datta et al. 2007 and Ozmen et al. 2006, and verified in our experiment. There was no change of activities of AST, ALT and ALP in control fish during the time course fed with the control diet Ch0 (Fig. 6). Seven parts per million sodium arsenite exposure in fish fed with the diet Ch0+As significantly increased the plasma ALT and ALP activities at days 7, 14 and 21, and the AST activity at day 14 and 21. Five percent (Ch5+As) and 10% (Ch10+As) Ch supplementation significantly inhibited the arsenite-increased activities of all three enzymes (Fig. 6). The ALT activity in Ch5+As and Ch10+As fed fish wasn’t altered at day 7 and was lower than Ch0+As fed fish 14 days post arsenite exposure. Although the Ch5+As fed fish had a higher level of ALT activity at day 21, the Ch10+As fed fish had the same level of ALT activity as the control (Fig. 6a). The AST activity in 5% and 10% fed fish remained unchanged from 7 to 21 days except in 5% Ch fed fish at day 14 when the AST activity was higher than in control (Ch0) but lower than in Ch0+As fed fish (Fig. rb). The ALP activity in 5% and 10% Ch fed fish also remained unchanged at all time points tested (Fig. 6c).
a–c The effects of sodium arsenite exposure and diets on ALT, AST and ALP levels in Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) for 7, 14 and 21 days. The levels of plasma ALT, AST and ALP activities were measured. Data is expressed as the mean ± SEM of six fish. The levels between different groups are statistically significant (P < 0.05) where letters over the bars are different, as determined by one-way ANOVA
Ch dietary supplementation counteracted the down-regulation of plasma proteins after arsenite exposure
It had been previously demonstrated that toxicant stress negatively affects the plasma protein levels (Samuel et al. 2005). The fish exposed to As (Ch0+As) showed a significant reduction in plasma protein level at days 14 and 21. The albumin level was nominally decreased at days 7 and 14 while it was significantly lowered at day 21 in arsenite exposed fish. The globulin level was nominally decreased at days 7 and 21 but was significantly lowered at day 14 in the arsenite-exposed group. No changes of total protein, albumin and globulin levels were observed in arsenite exposed fish fed with Ch supplemented diets at both doses used (Fig. 7a–c).
a–c The effects of sodium arsenite exposure and diets on serum total protein levels in Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) for 7, 14 and 21 days. The levels of serum total protein, albumin and globulin were measured. Data is expressed as the mean ± SEM of six fish. The levels between different groups are statistically significant (P < 0.05) where letters over the bars are different, as determined by one-way ANOVA
Ch dietary supplementation counteracted the increased plasma urea and creatinine after arsenite exposure
Kidney biomarkers, such as plasma urea and creatinine, were significantly increased, as previously indicated, in response to other heavy metals that elucidate the toxicant action in impairing kidney function (Elgaml et al. 2015). Arsenite exposure significantly increased the plasma urea concentration from 7 to 21 days and creatinine concentration at 14 and 21 days in fish fed the control diet (Ch0). In contrast, both urea and creatinine concentrations remained unchanged in arsenite exposed fish fed with Ch supplemented diets at both doses used (Fig. 8a, b).
a–b The effects of sodium arsenite exposure and diets on serum urea and creatinine levels in Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) for 7, 14 and 21 days. The serum urea and creatinine levels were measured. Data is expressed as the mean ± SEM of six fish. The levels between different groups are statistically significant (P < 0.05) where letters over the bars are different, as determined by one-way ANOVA
Ch dietary supplementation counteracted the down-regulation of plasma electrolytes after arsenite exposure
Maintaining plasma electrolytes is important for many physiological functions and these electrolytes are very sensitive to environmental stressors and commonly modified upon pollutants exposure (Suvetha et al. 2010). As expected, the level of Na+ decreased significantly in arsenite exposed fish fed with the control diet (Ch0+As) from 7 to 21 days but remained unchanged when fed with Ch supplemented diets at both doses used (Fig. 9a). However, K+ levels showed no significant difference between different groups at the same time points (Fig. 9b).
a–b The effects of sodium arsenite exposure and diets on serum electrolytes levels in Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) for 7, 14 and 21 days. The levels of serum sodium and potassium were measured. Data is expressed as the mean ± SEM of six fish. The levels between different groups are statistically significant (P < 0.05) where letters over the bars are different, as determined by one-way ANOVA
Ch dietary supplementation counteracted the upregulation of cytokine gene expression after arsenite exposure
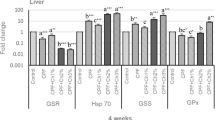
A heightened expression of pro-inflammatory cytokines, such as IL-1β and TNF-α, and anti-inflammatory cytokine, such as TGF-β1, indicates a disturbance of the immune system. The liver, by receiving blood both via the portal vein containing dietary antigens, toxins as well as pathogens from the gastro-intestinal tract, and via the hepatic artery containing antigens, pathogens and metastasizing cells from systemic blood circulation, represents a major immune organ of vertebrates (Möller et al. 2014). Hence, the arsenite and diet modulated cytokine gene expression was studied in this tissue at 21 days post arsenite exposure. Arsenite exposure significantly upregulated the expression of Nile tilapia IL-1β (7-fold), TNF-α (14-fold)), and TGF-β1 (13-fold) compared to the control diet Ch0 (Fig. 10). The heightened cytokine gene expression was reduced significantly in 10% Ch fed fish, with a lessened IL-1β expression also seen in 5% Ch fed fish.
The effects of sodium arsenite exposure and diets on gene expression of proinflammatory cytokines (IL-1β, TNF-α, and TGF-β1) determined by real-time PCR in liver of Nile tilapia. The fish were fed with the control diet (Ch0) or exposed to 7 ppm sodium arsenite and fed with the control diet (Ch0 + As), or with diets containing 5% Chlorella (Ch5+As) or 10% Chlorella (Ch10+As) for 21 days. Data is expressed as the mean ± SEM of six fish. The levels between different groups are statistically significant (P < 0.05) where letters over the bars are different, as determined by one-way ANOVA
Discussion
Great concern has been focused on heavy metals and other pollutants that can be harmful for ecosystems. Fish are very sensitive to such pollutants, leading to various deleterious effects upon entering their organs, due to unremitting exposure (Aruljothi 2014). Thus, fish are considered to be biological indicators for monitoring heavy metal contamination (Jancsó and Hermesz 2015). Heavy metal pollution warrants a new strategy to alleviate such adverse effects. In this respect, removal of toxic metals through biosorption, based on metal binding capabilities of algae and other aquatic plants have been adopted (Kamat et al. 2000).
In the current study, it was observed that As3+ exposure has deleterious effects on serum biochemical parameters of tilapia fish. The results clearly indicated that As3+ exposure significantly increased ALT, AST, and ALP levels and decreased total protein levels, indicating damage in liver tissue by As3+. However, in groups that were fed with Ch supplemented diets, these changes in ALT, AST, APL and protein levels were inhibited (P < 0.05), with the higher Ch diet showing more prominent effects on maintaining these biomarkers. Our findings of arsenite effects on liver biomarkers were in line with Lavanya et al. (2011) who found a significant increase in GOT (glutamic oxaloacetic transaminase) and decrease in plasma total protein after arsenic trioxide exposure. Additionally, Vetrivel et al. (2014) reported a decrease in total protein level and ALP activity in Clarias batrachus after arsenic trioxide exposure. Moreover, Fırat et al. (2011) reported significant increases in ALT, AST and ALP activities in response to Cu, and Pb after 21 days. Elgaml et al. (2015) reported a significant decrease in serum total protein in Nile tilapia exposed to lead acetate at a dose of 73.40 mg/l for 10 weeks. Lowered levels of the protein content in liver and muscle of Nile tilapia were also recorded upon exposure to aluminum sulphate (Al3+) (Correia et al. 2010). Previous studies (Harvey et al. 1994; Karan et al. 1998; Vaglio and Landriscina 1999) were also supportive of our results. These results could be attributed to the toxic effect of heavy metals on liver, which led to degenerative changes, and hypofunction of the liver. Liver damage caused cellular enzymes to be released from the cells or leakage of these enzymes from the liver into the blood (Fırat et al. 2011). ALP is involved in transphosphorylation reactions and mediates membrane transport; therefore, a higher level indicates dephosphorylation of biomolecules with a hazardous effect on the energy budget of the cell and subsequent ionic imbalance (Sharma et al. 2012). Hypoproteinemia reported in this study might arise either from kidney dysfunction with protein excretion in the urine, or due to inhibition of the hepatic protein synthesis that is responsible for reducing the synthesis of total proteins (Aly et al. 2015). The homeostatic effects of Ch supplemented diets on the liver biomarkers against the toxic effects of arsenite exposure might be due to the maintained level of membrane-bound enzymes and the activities of antioxidant enzymes near normal levels, confirming their effects as antioxidants and preventing enzyme leakage into the circulation (Elgaml et al. 2015). Besides, Ch is rich in carotenes and other bioactive components such as violaxanthin that exhibit strong antioxidant properties in oxidative stress and function as a chain breaking antioxidant in lipid peroxidation (Sharma et al. 2012; Soontornchaiboon et al. 2012).
Higher levels of kidney markers, urea and creatinine, were evident in the Ch0+As group compared to other groups, and are indicative of kidney dysfunction as a result of glomerular insufficiency (Abdel-Tawwab et al. 2007). Also, there was an increase in the production of reactive oxygen species (Elia et al. 2018; Zahran and Risha 2014) and kidney injury induced by the toxicant (Upasani and Balaraman 2003). Feed supplemented groups Ch5+As and Ch10+As exhibited an improvement in kidney function markers. This might be due to the breakdown of free radicals with subsequent formation of relatively unreactive radical species to protect the kidney tissue from peroxidative damage (Özkan-Yılmaz et al. 2014).
Ionic imbalance was observed in the present study, where Na+ levels were decreased (P < 0.05) in the Ch0+As group compared to other groups. Similar findings were reported by Özkan-Yılmaz et al. (2014) in Cyprinus carpio exposed to low pH 4.0 with low (normal water) calcium 6 mg/l and low pH 4.0 with added calcium 15 mg/l treatment for a period of 96 h. Kabilan et al. (2013) corroborated the same findings in Lates calcarifer exposed to HgCl2 and CdCl2 at concentrations of 3.5 ppm and 4.0 ppm and their combination at 3 ppm for 35 days. The same trend was reported by Fırat et al. (2011) in Nile tilapia after Cu 0.05 mg/l and Pb 0.05 mg/l exposure for 21 days. This could result from different toxicant actions on organs involved in osmoregulation, metabolism, active transport processes, or the endocrine system (Martinez and Cólus 2002). Pathological alterations can be induced by the toxicant in organs responsible for the exchange of ions between the fish and the surrounding water, or could be attributed to the reduction of Na+/K+-ATPase activity, which has a key role in whole body ion regulation (Fırat et al. 2011). Additionally, kidney dysfunction further contributed to ionoregulatory failure through different mechanisms, such as defects in ion reabsorption, proximal tubular cells and tubular dysfunction with hypocalcemia and hypophosphatemia (Patel et al. 2006). Additionally, disturbance in Ca2+ homeostasis may lead to disruption of the mechanisms of ion regulation (Moe 2008). Once again, the protective effects of a Ch supplemented diet were observed in regulation of the ionic balance owing to its biologically active components, especially carotenoids that revealed strong antioxidant properties in oxidative stress and function as a chain breaking antioxidant in lipid peroxidation, maintaining ionic regulation (Sharma et al. 2012; Soontornchaiboon et al. 2012; Özkan-Yılmaz et al. 2014).
Cytokines have important roles in the regulation of the fish immune response. Our results revealed upregulation of all analyzed cytokines, IL-1β, TNF-α, and TGF-β1 in the Ch0+As exposed group compared to the control group (Ch0). This is consistent with the findings of Teles et al. (2011), where upregulation of expression of IL-1β, TNF-α and IL-6 mRNA was seen in rainbow trout head kidney macrophages exposed to copper. Similar results were seen in increased expression of other immune-related genes (e.g., TGF-β), in the whole head kidney of Morone saxatilis (Geist et al. 2007) and Solea senegalensis (Prieto-Álamo et al. 2009) upon copper exposure in vivo. Upregulation of IL-1β expression in the present study was due to the system’s response to As3+ exposure. Similar IL-1β upregulation was noted against a low level of As (2 or 10 ppb) exposure (Nayak et al. 2007), and in acute promyelocytic leukemia cells (Jiang et al. 2003). In this study, TNF-α gene expression was significantly upregulated upon As3+ exposure as well. TNF-α is involved in many systemic inflammations and its upregulation is a response reflection of As3+ exposure. This result was similar to what had been found by Yu et al. (2002), who recorded TNF-α induction from mononuclear cells exposed to a low arsenic concentration, and 1 μM As led to a cytotoxic effect on T-cells. General upregulation of TNF- α, IL-6, and IL-1β was noted in the kidney of adult rats upon acrylamide (ACR) and aluminum (Al3+) exposure (Ghorbel et al. 2015).
The fish liver is especially relevant to the immune toxicity of water pollutants, as shown by liver histopathological alterations and changes of liver-related biochemical markers after arsenite exposure in this study and by others (Saïdi et al. 2013; Benhamed et al. 2016; Webster et al. 2017). Heavy metals exposure has the ability to alter the immune response in different ways. This could be attributed to many factors associated with the heavy metal mode of action. For example, As can modulate gene expression by modifying the cellular redox status which leads to an imbalance of pro/antioxidant species with subsequent tissue damage and the initiation of inflammation (Lantz and Hays 2006). Moreover, As can influence cytokines expression by affecting components of upstream signal transduction pathways. Kinases such as protein kinase C (PKC), mitogen activated protein kinases (MAPKs), and IκB kinase (IκK) can be modulated by As exposure. Activation of these kinases is known to activate the transcription factor NF-κB, which in turn controls cytokine expression (Cobbina et al. 2015). On the other hand, Ch contains a large quantity of carotenoids that act as antioxidants leading to the reduction of reactive oxygen species (ROS) under oxidative stress, in turn expressing a higher level of NO and improving endothelial function (Hamias et al. 2018; Zahran and Risha 2014). Furthermore, β-carotene can inhibit NF-κB activity and suppress the expression of pro-inflammatory genes; indicating that ROS and inflammatory gene expression are interconnected through the NF-κB signaling pathway (Hamias et al. 2018; Soontornchaiboon et al. 2012). These results suggested the potential protective and therapeutic roles of Ch supplemented diets in regulation of cellular redox status, attenuation of inflammation and improvement of endothelial function.
As induced severe histopathological changes in Nile tilapia gills, livers and head kidneys. Gills of Nile tilapia exposed to arsenic showed hyperplasia, congestion and lamellar fusion. These results were in accordance with other previous studies (Abdel-Tawwab et al. 2007; Simonato et al. 2008; van Heerden et al. 2004). Lesions reported in gills may affect oxygen consumption and disrupt osmoregulatory function. These alterations may be due to the increase in cellular metabolism leading to an imbalance of osmotic regulation by impairing ionic active transportation (Mazon et al. 2002) and may be attributed to the direct responses induced by the action of the irritants as well (Kan et al. 2012). As the main site of detoxification, liver is the most sensitive organ to xenobiotics (Crestani et al. 2007). In the present study, intense degenerative changes in liver were seen in the arsenite exposed group Ch0+As, whilst the degeneration was observed to a lesser extent after Ch supplementation, especially in the Ch10+As group. These results are inconsistent with what had been described by Camargo and Martinez (2007) in Prochilodus lineatus, subjected to in situ tests along the upper reaches of Cambé stream. Additionally, these induced alterations suggested metabolic damage owing to As3+ exposure (Pacheco and Santos 2002). Several pathological changes were induced in kidneys exposed to As3+, such as hematopoietic tissue necrosis and sinusoidal congestion. Similar results were observed in Astyanax altiparanae (Silva and Martinez 2007) and Oreochromis mossambicus (Ahmed et al. 2013). Similarly, histopathological alterations were reported in liver and gills of Odonthestes bonariensis inhabiting Chasicó Lake in the southwest of Buenos Aires Province, a lake known to have a high As concentration (Puntoriero et al. 2018). Our findings demonstrate that fish fed with a diet supplemented with Chlorella exhibited reduced histopathological alterations to some extent. Similar results were seen in Oreochromis niloticus fed T. laurifolia leaf extract and exposed to lead (II) nitrate (Palipoch et al. 2011). In the same context, cyclophosphamide administration in mice induced different pathological lesions in the form of cellular fibrosis, necrosis, and disordered cell arrangements, whereas supplementing Ch at 12 and 24% revealed normal cell appearance and minor fibrosis (Cheng et al. 2017). Consistently, our results showed that As3+ exposed fish showed much greater damage in comparison to groups fed Ch diets, especially the Ch10+As group, which showed the best reduction in the detected damage in the examined organs and decreased As3+ induced tissue alterations due to its anti-inflammatory properties and its ability to maintain healthy endothelial function.
Chlorella dietary supplementation has great benefits of alleviating such toxic effects induced by As3+ exposure, including stabilization of the biochemical parameters and cytokine gene expression, and the reduction of induced histopathological alteration. Microalgae such as Chlorella spp. have major uses in aquaculture, owing to their high contents of proteins, fatty acids of the ϖ3 and ϖ6 types, and many essential vitamins, minerals and antioxidant substances, in addition to its immunostimulating properties (Reyes-Becerril et al. 2013). In the same context, Chlorella had a preferable action in inhibiting lipid peroxidation in comparison to glutathione and had antioxidant properties (Bengwayan et al. 2010). Chlorella has a great role in immune response via augmentation of macrophage activity (Hasegawa et al. 1997; Liu et al. 2006). Similarly, other studies evaluated the benefits of using microalgae against xenobiotic exposure in common carp (Pugazhendy 2012), and in mice (Queiroz et al. 2003). Similar results were seen in previous studies emphasizing the role of using dietary Chlorella (Andrews et al. 2009; Mason 2001; Suhendrayatna et al. 1999). Together with other previously documented studies relating to the growth promoting abilities of Ch, we can suggest the possibility of combined use of microalgae for aquaculture water treatment and growth promotion. Furthermore, Ch has proved its anti-tumor and anti-inflammatory properties through improving healthy endothelial function leading to therapeutic control of induced inflammatory diseases. Thus, Ch as GRAS is consumed for medical treatment in humans as well (Hamias et al. 2018).
To conclude, our findings have clearly demonstrated the deleterious effects of As3+ exposure on Nile tilapia and the potential protective and therapeutic roles of Chlorella supplemented diets. The Ch diet supplementation reduced the histopathological alterations in different tissues, maintaining the serum biochemical parameters, and reduced the hepatic immune gene expression in Nile tilapia exposed to sodium arsenite. Therefore, our previous and current findings represent a comprehensive study of As3+ induced toxicity in Nile tilapia, and show a substantial role of Chlorella as a future biotech tool in the advancement of green innovation for a sustainable aquaculture industry.
References
Abdel-Tawwab M, Mousa MA, Ahmad MH, Sakr SF (2007) The use of calcium pre-exposure as a protective agent against environmental copper toxicity for juvenile Nile tilapia, Oreochromis niloticus (L.). Aquaculture 264:236–246
Ahmed MK, Habibullah-Al-Mamun M, Parvin E et al (2013) Arsenic induced toxicity and histopathological changes in gill and liver tissue of freshwater fish, tilapia (Oreochromis mossambicus). Exp Toxicol Pathol 65:903–909
Aly HA, El-Shitany NA, El-Beshbishy HA, Ashour OM (2015) Ameliorative effect of lycopene against 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin-induced rat liver microsomal toxicity: an in vitro study. Toxicol Ind Health 31:938–950
Andrews SR, Sahu NP, Pal AK, Kumar S (2009) Haematological modulation and growth of Labeo rohita fingerlings: effect of dietary mannan oligosaccharide, yeast extract, protein hydrolysate and chlorella. Aquac Res 41:61–69
Aruljothi B (2014) Effect of arsenic on lipid peroxidation and antioxidants system in fresh water fish, labeo rohita. Int J Mod Res Rev 2:15–19
ATSDR (2002) Toxicological profile for arsenic. agency for toxic substances and disease registry. Washington, DC, SUDHHS, PHS
Avigliano E, Schenone NF, Volpedo AV, Goessler W, Cirelli AF (2015) Heavy metals and trace elements in muscle of silverside (Odontesthes bonariensis) and water from different environments (Argentina): aquatic pollution and consumption effect approach. Sci Total Environ 506:102–108
Bancroft D, Stevens A, Turner R (1996) Theory and practice of histological techniques. Churchill Livingstone, New York
Bengwayan PT, Laygo JC, Pacio AE, Poyaoan JLZ, Rebugio JF, Yuson ALL (2010) A comparative study on the antioxidant property of Chlorella (Chlorella sp.) tablet and glutathione Tablet. Int Sci Res J 2:25–32
Benhamed S, Guardiola FA, Martínez S et al (2016) Exposure of the gilthead seabream (Sparus aurata) to sediments contaminated with heavy metals down-regulates the gene expression of stress biomarkers. Toxicol Rep 3:364–372
Benli AÇK, Köksal G, Özkul A (2008) Sublethal ammonia exposure of Nile tilapia (Oreochromis niloticus L.): effects on gill, liver and kidney histology. Chemosphere 72:1355–1358. https://doi.org/10.1016/j.chemosphere.2008.04.037
Camargo MM, Martinez CB (2007) Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop Ichthyol 5:327–336
Capkin E, Birincioglu S, Altinok I (2009) Histopathological changes in rainbow trout (Oncorhynchus mykiss) after exposure to sublethal composite nitrogen fertilizers. Ecotoxicol Environ Saf 72:1999–2004
Cheng D, Wan Z, Zhang X, Li J, Li H, Wang CJN (2017) Dietary Chlorella vulgaris ameliorates altered immunomodulatory functions in cyclophosphamide-induced immunosuppressive mice. Nutrients 9:708
Cobbina SJ et al (2015) A multivariate assessment of innate immune-related gene expressions due to exposure to low concentration individual and mixtures of four kinds of heavy metals on zebrafish (Danio rerio) embryos. Fish Shellfish Immunol 47:1032–1042
Correia TG, Narcizo AdM, Bianchini A, Moreira RG (2010) Aluminum as an endocrine disruptor in female Nile tilapia (Oreochromis niloticus). Comp Biochem Physiol C Toxicol Pharmacol 151:461–466
Crestani M et al (2007) Effect of clomazone herbicide on biochemical and histological aspects of silver catfish (Rhamdia quelen) and recovery pattern. Chemosphere 67:2305–2311
Datta S, Saha DR, Ghosh D, Majumdar T, Bhattacharya S, Mazumder S (2007) Sub-lethal concentration of arsenic interferes with the proliferation of hepatocytes and induces in vivo apoptosis in (Clarias batrachus) L. Comp Biochem Physiol C Toxicol Pharmacol 145:339–349
Duker AA, Carranza E, Hale M (2005) Arsenic geochemistry and health. Environ Int 31:631–641
Elgaml SA, Khalil R, Hashish EA, El-Murr A (2015) Protective effects of selenium and alpha-tocopherol against lead-induced hepatic and renal toxicity in Oreochromis Niloticus. J Aquac Res Dev 6:2
Elia AC et al (2018) A comparative study on subacute toxicity of arsenic trioxide and dimethylarsinic acid on antioxidant status in Crandell Rees feline kidney (CRFK), human hepatocellular carcinoma (PLC/PRF/5), and epithelioma papulosum cyprini (EPC) cell lines. J Toxicol Environ Health A 81:333–348
Figueiredo-Fernandes A, Ferreira-Cardoso JV, Garcia-Santos S, Monteiro SM, Carrola J, Matos P, Fontaínhas-Fernandes A (2007) Histopathological changes in liver and gill epithelium of Nile tilapia, Oreochromis niloticus, exposed to waterborne copper. Pesquisa Veterinária Brasileira 27:103–109
Fırat Ö, Cogun HY, Yüzereroğlu TA, Gök G, Fırat Ö, Kargin F, Kötemen Y (2011) A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem 37:657–666
Geist J, Werner I, Eder KJ, Leutenegger CM (2007) Comparisons of tissue-specific transcription of stress response genes with whole animal endpoints of adverse effect in striped bass (Morone saxatilis) following treatment with copper and esfenvalerate. Aquat Toxicol 85:28–39
Ghorbel I, Maktouf S, Fendri N, Jamoussi K, Ellouze Chaabouni S, Boudawara T, Zeghal N (2015) Coexposure to aluminum and acrylamide disturbs expression of metallothionein, proinflammatory cytokines and induces genotoxicity: biochemical and histopathological changes in the kidney of adult rats. Environ Toxicol 31:1044–1058
Gonzalez HO, Roling JA, Baldwin WS, Bain LJ (2006) Physiological changes and differential gene expression in mummichogs (Fundulus heteroclitus) exposed to arsenic. Aquat Toxicol 77:43–52
Guerriero G, Avino M, Zhou Q, Fugelstad J, Clergeot PH, Bulone V (2010) Chitin synthases from Saprolegnia are involved in tip growth and represent a potential target for anti-oomycete drugs. PLoS Pathog. https://doi.org/10.1371/journal.ppat.1001070
Hamias R, Wolak T, Huleihel M, Paran E, Levy-Ontman OJB (2018) Red alga polysaccharides attenuate angiotensin II-induced inflammation in coronary endothelial cells. J Biochem Biophys Res Commun 500:944–951
Harvey R, Kubena L, Elissalde M (1994) Influence of vitamin E on aflatoxicosis in growing swine. Am J Vet Res 55:572–577
Hasegawa T et al (1997) Effect of hot water extract of Chlorella vulgaris on cytokine expression patterns in mice with murine acquired immunodeficiency syndrome after infection with Listeria monocytogenes. Immunopharmacology 35:273–282
Hwang P, Tsai Y (1993) Effects of arsenic on osmoregulation in the tilapia Oreochromis mossambicus reared in seawater. Mar Biol 117:551–558
Jancsó Z, Hermesz E (2015) Impact of acute arsenic and cadmium exposure on the expression of two haeme oxygenase genes and other antioxidant markers in common carp (Cyprinus carpio). J Appl Toxicol 35:310–318
Jiang G et al (2003) Effect of arsenic trioxide on cytokine expression by acute promyelocytic leukemia cells. Chin Med J 116:1639–1643
Jin Y, Liu Z, Liu F, Ye Y, Peng T, Fu Z (2015) Embryonic exposure to cadmium (II) and chromium (VI) induce behavioral alterations, oxidative stress and immunotoxicity in zebrafish (Danio rerio). Neurotoxicol Teratol 48:9–17
Kabilan N et al (2013) The combined effects of mercury chloride and cadmium chloride metals on plasma electrolytes of a fish, Lates Calcarifer. Int J Anal Bioanal Chem 3:183–188
Kamat JP, Boloor KK, Devasagayam TP (2000) Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Mol Cell Biol Lipids 1487:113–127
Kan Y, Cengiz EI, Ugurlu P, Yanar M (2012) The protective role of vitamin E on gill and liver tissue histopathology and micronucleus frequencies in peripheral erythrocytes of Oreochromis niloticus exposed to deltamethrin. Environ Toxicol Pharmacol 34:170–179
Karan V, Vitorović S, Tutundžić V, Poleksić V (1998) Functional enzymes activity and gill histology of carp after copper sulfate exposure and recovery. Ecotoxicol Environ Saf 40:49–55
Kavitha C, Malarvizhi A, Senthil Kumaran S, Ramesh M (2010) Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem Toxicol 48:2848–2854. https://doi.org/10.1016/j.fct.2010.07.017
Kumar M, Jeon J, Choi J, Kim S-R (2018) Rapid and efficient genetic transformation of the green microalga Chlorella vulgaris. J Appl Phycol 30:1735–1745
Lakra WS, Nagpure NS (2009) Genotoxicological studies in fishes: a review. Indian J Anim Sci 79:93–97
Lantz RC, Hays AM (2006) Role of oxidative stress in arsenic-induced toxicity. Drug Metab Rev 38:791–804
Lavanya S, Ramesh M, Kavitha C, Malarvizhi A (2011) Hematological, biochemical and ionoregulatory responses of Indian major carp (Catla catla) during chronic sublethal exposure to inorganic arsenic. Chemosphere 82:977–985
Liu C, Leung M, Koon J, Zhu L, Hui Y, Yu B, Fung K (2006) Macrophage activation by polysaccharide biological response modifier isolated from Aloe vera L. var. i (Haw.) Berg. Int Immunopharmacol 6:1634–1641
Martinez C, Cólus I (2002) Biomarcadores em peixes neotropicais para o monitoramento da poluição aquática na bacia do rio Tibagi A bacia do Rio Tibagi Editora dos Editores, Londrina, PR, Brazil: 551–577
Mason R (2001) Chlorella and Spirulina: green supplements for balancing the body. Altern Complement Ther 7:161–165
Mazon A, Cerqueira C, Fernandes M (2002) Gill cellular changes induced by copper exposure in the South American tropical freshwater fish Prochilodus scrofa. Environ Res 88:52–63
Mazumder DG (2005) Effect of chronic intake of arsenic-contaminated water on liver. Toxicol Appl Pharmacol 206:169–175
McCollum CW, Hans C, Shah S, Merchant FA, Gustafsson J-Å, Bondesson M (2014) Embryonic exposure to sodium arsenite perturbs vascular development in zebrafish. Aquat Toxicol 152:152–163
Moe SM (2008) Disorders involving calcium, phosphorus, and magnesium. Prim Care Clin Office Pract 35:215–237
Möller A-M, Korytář T, Köllner B, Schmidt-Posthaus H, Segner H (2014) The teleostean liver as an immunological organ: intrahepatic immune cells (IHICs) in healthy and benzo [a] pyrene challenged rainbow trout (Oncorhynchus mykiss). Dev Comp Immunol 46:518–529
Nayak AS, Lage CR, Kim CH (2007) Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicol Sci 98:118–124
Özkan-Yılmaz F, Özlüer-Hunt A, Gündüz SG et al (2014) Effects of dietary selenium of organic form against lead toxicity on the antioxidant system in Cyprinus carpio. Fish Physiol Biochem 40:355–363
Ozmen M, Güngördü A, Kucukbay FZ, Güler RE (2006) Monitoring the effects of water pollution on Cyprinus carpio in Karakaya Dam Lake, Turkey. Ecotoxicology 15:157–169
Pacheco M, Santos M (2002) Naphthalene and β-naphthoflavone effects on (Anguilla anguilla) L. hepatic metabolism and erythrocytic nuclear abnormalities. Environ Int 28:285–293
Palaniappan PR, Vijayasundaram V (2009) The effect of arsenic exposure and the efficacy of DMSA on the proteins and lipids of the gill tissues of Labeo rohita. Food Chem Toxicol 47:1752–1759
Palipoch S, Jiraungkoorskul W, Tansatit T, Preyavichyapugdee N, Jaikua W, Kosai P (2011) Protective efficiency of Thunbergia laurifolia leaf extract against lead (II) nitrate-induced toxicity in Oreochromis niloticus. J Med Plants Res 5:719–728
Patel M, Rogers JT, Pane EF, Wood CM (2006) Renal responses to acute lead waterborne exposure in the freshwater rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 80:362–371
Prieto-Álamo M-J, Abril N, Osuna-Jiménez I, Pueyo C (2009) Solea senegalensis genes responding to lipopolysaccharide and copper sulphate challenges: large-scale identification by suppression subtractive hybridization and absolute quantification of transcriptional profiles by real-time RT-PCR. Aquat Toxicol 91:312–319
Pugazhendy K (2012) Protective role of spirulina on the variation of haematological parameter induced by herbicide atrazine in the fresh water fish Cyprinus carpio (Linn). Int J Pharm Biol Arch 3:249–254
Puntoriero ML, Cirelli AF, Volpedo AV (2018) Histopathological changes in liver and gills of Odontesthes bonariensis inhabiting a lake with high concentrations of arsenic and fluoride (Chasicó Lake, Buenos Aires province). J Revista Internacional de Contaminación Ambiental 34:69–77
Queiroz ML, Rodrigues AP, Bincoletto C, Figueirêdo CA, Malacrida S (2003) Protective effects of Chlorella vulgaris in lead-exposed mice infected with Listeria monocytogenes. Int Immunopharmacol 3:889–900
Reyes-Becerril M, Guardiola F, Rojas M, Ascencio-Valle F, Esteban MÁ (2013) Dietary administration of microalgae Navicula sp. affects immune status and gene expression of gilthead seabream (Sparus aurata). Fish Shellfish Immunol 35:883–889
Saïdi SA, Azaza MS, Windmolders P et al (2013) Cytotoxicity evaluation and antioxidant enzyme expression related to heavy metals found in tuna by-products meal: an in vitro study in human and rat liver cell lines. Exp Toxicol Pathol 65:1025–1033
Samuel S, Kathirvel R, Jayavelu T, Chinnakkannu P (2005) Protein oxidative damage in arsenic induced rat brain: influence of DL-α-lipoic acid. Toxicol Lett 155:27–34
Sharma KP, Upreti N, Sharma S, Sharma S (2012) Protective effect of Spirulina and tamarind fruit pulp diet supplement in fish (Gambusia affinis Baird & Girard) exposed to sublethal concentration of fluoride, aluminum and aluminum fluoride. Ind J Exp Biol 50:897–903
Silva AG, Martinez CB (2007) Morphological changes in the kidney of a fish living in an urban stream. Environ Toxicol Pharmacol 23:185–192
Simonato JD, Guedes CL, Martinez CB (2008) Biochemical, physiological, and histological changes in the neotropical fish Prochilodus lineatus exposed to diesel oil. Ecotoxicol Environ Saf 69:112–120
Sirakov I, Velichkova K, Stoyanova S, Staykov Y (2015) The importance of microalgae for aquaculture industry. Review. Int J Fish Aquatic Stud 2:81–84
Soetaert A, Vandenbrouck T, van der Ven K, Maras M, van Remortel P, Blust R, De Coen WM (2007) Molecular responses during cadmium-induced stress in Daphnia magna: integration of differential gene expression with higher-level effects. Aquat Toxicol 83:212–222
Soontornchaiboon W, Joo SS, Kim SM (2012) Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol Pharm Bull 35:1137–1144
Suhendrayatna Ohki A, Kuroiwa T, Maeda S (1999) Arsenic compounds in the freshwater green microalga Chlorella vulgaris after exposure to arsenite. Appl Organomet Chem 13:127–133
Suvetha L, Ramesh M, Saravanan M (2010) Influence of cypermethrin toxicity on ionic regulation and gill Na+/K+-ATPase activity of a freshwater teleost fish Cyprinus carpio. Environ Toxicol Pharmacol 29:44–49. https://doi.org/10.1016/j.etap.2009.09.005
Suzuki T, Moribe M, Okabe Y, Niinae M (2013) A mechanistic study of arsenate removal from artificially contaminated clay soils by electrokinetic remediation. J Hazard Mater 254:310–317
Teles M, Mackenzie S, Boltana S, Callol A, Tort L (2011) Gene expression and TNF-alpha secretion profile in rainbow trout macrophages following exposures to copper and bacterial lipopolysaccharide. Fish Shellfish Immunol 30:340–346
Thévenod F (2009) Cadmium and cellular signaling cascades: to be or not to be? Toxicol Appl Pharmacol 238:221–239
Torres-Perez J, Gerente C, Andres Y (2012) Conversion of agricultural residues into activated carbons for water purification: application to arsenate removal. J Environ Sci Health A 47:1173–1185
Tripathi S, Kumar A (2011) Effect of acute and chronic exposure of sodium arsenite (Na3AsO3) on total protein, albumin, and globulin in serum of Oryctolagus cuniculus L. Toxicol Environ Chem 93:307–313
Upasani C, Balaraman R (2003) Protective effect of Spirulina on lead induced deleterious changes in the lipid peroxidation and endogenous antioxidants in rats. Phytother Res 17:330–334
Vaglio A, Landriscina C (1999) Changes in liver enzyme activity in the teleost Sparus auratain response to cadmium intoxication. Ecotoxicol Environ Saf 43:111–116
van Heerden D, Vosloo A, Nikinmaa M (2004) Effects of short-term copper exposure on gill structure, metallothionein and hypoxia-inducible factor-1α (HIF-1α) levels in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 69:271–280
Vetrivel C, Pugazhendy K, Prabakaran S (2014) Protective effect of spirulina against the lead acetate induced ALP and ACP activity in the liver tissue of fresh water fish, Labeo rohita. Int J Modn Res Revs 2:226–228
Wang X et al (2012) Arsenic and chromium in drinking water promote tumorigenesis in a mouse colitis-associated colorectal cancer model and the potential mechanism is ROS-mediated Wnt/β-catenin signaling pathway. Toxicol Appl Pharmacol 262:11–21
Wangkahart E, Scott C, Secombes CJ, Wang T (2016) Re-examination of the rainbow trout (Oncorhynchus mykiss) immune response to flagellin: Yersinia ruckeri flagellin is a potent activator of acute phase proteins, anti-microbial peptides and pro-inflammatory cytokines in vitro. Dev Comp Immunol 57:75–87
Webster TMU, Williams TD, Katsiadaki I et al (2017) Hepatic transcriptional responses to copper in the three-spined stickleback are affected by their pollution exposure history. Aquat Toxicol 184:26–36
Yu H-S, Liao W-T, Chang K-L, Yu C-L, Chen G-S (2002) Arsenic induces tumor necrosis factor α; Release and tumor necrosis factor receptor 1 signaling in T helper cell apoptosis. J Invest Dermatol 119:812–819
Zahran E, Risha E (2014) Modulatory role of dietary Chlorella vulgaris powder against arsenic-induced immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 41:654–662
Acknowledgement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zahran, E., Awadin, W., Risha, E. et al. Dietary supplementation of Chlorella vulgaris ameliorates chronic sodium arsenite toxicity in Nile tilapia Oreochromis niloticus as revealed by histopathological, biochemical and immune gene expression analysis. Fish Sci 85, 199–215 (2019). https://doi.org/10.1007/s12562-018-1274-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-018-1274-6