Abstract
Na+/H+ antiporters are known to play a crucial role in pH and Na+ homeostasis. In the present study, we characterized the molecular structures and expression patterns of two Na+/H+ antiporters from the marine red alga Porphyra yezoensis (designated PySOS1 and PyNhaD). The full-length cDNAs of PySOS1 and PyNhaD were 5122 and 1804 bp, and contained open reading frames (ORFs) of 4773 and 1275 bp, respectively. The deduced amino acid sequences showed high similarity to SOS1 and NhaD from the higher plant Arabidopsis thaliana. PySOS1 and PyNhaD contained conserved sequences found in the cation–proton antiporter. Quantitative real-time PCR analysis revealed that both antiporter genes were expressed in both the gametophyte and sporophyte of P. yezoensis. In addition, mRNA expression of PySOS1 and PyNhaD was simultaneously upregulated by light irradiation, suggesting that coordinated activity between the two is important in pH and Na+ homeostasis under light conditions. Moreover, the expression levels of both genes were partially reduced by the photosynthetic inhibitors DCMU and DBMIB, suggesting that upregulation is linked to photosynthesis-related metabolism. These findings provide an initial step towards understanding Na+/H+ antiporters in marine red algae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Na+/H+ antiporters are found in all domains of life and have been shown to play important roles in cell homeostasis, including pH regulation, Na+ tolerance, and osmoregulation, and in vesicle trafficking and the control of the cell cycle and cell proliferation [1–3]. In land plants, three types of Na+/H+ antiporter have been identified. One SOS1-type Na+/H+ antiporter, AtSOS1, was initially identified as a gene locus required for salt tolerance in A. thaliana [4], and loss-of-function mutations in AtSOS1 were shown to result in extreme salt sensitivity and overaccumulation of Na+ in shoots under salt stress compared with the wild type [5]. AtSOS1 transcription is specifically upregulated upon NaCl stress [5] and AtSOS1-GFP fusion proteins localize at the plasma membrane [6]. The Nhx1-type Na+/H+ antiporter is located at the vacuolar membrane, and the expression of this gene is induced by salt and osmotic stress [7]. Moreover, transgenic plants that overexpress NHX1 are highly tolerant of salt stress [8]. NhaD-type Na+/H+ antiporters, which were first identified in Vibrio parahaemolyticus [9], are potential candidates for Na+ transport in plant organelles. In plants, NhaD transporters have been characterized in Populus euphratica [10] and Physcomitrella patens [11], and in P. patens they have been localized in chloroplasts [11]. However, while data on Na+/H+ antiporters in land plants have been accumulating, little is known about their counterparts in marine macroalgae.
The marine red alga Porphyra yezoensis has been proposed as a model organism for physiological and molecular biological studies of marine algae because of its biological and economical importance [12, 13]. This species inhabits the intertidal zone where the physiological environment rapidly changes with the turning tides, resulting in exposure to abiotic stresses such as salinity, pH, temperature, and light. To elucidate the molecular mechanisms regulating the responses to environmental stresses in marine red algae, we previously performed expressed sequence analysis (EST) analysis [14, 15] and developed transient gene expression systems in P. yezoensis [16–18].
In the P. yezoensis EST database (http://est.kazusa.or.jp/en/plant/porphyra/EST/index.html), partial cDNAs homologous to SOS1- and NhaD-type Na+/H+ antiporters (designed as PySOS1 and PyNhaD) were found by Barrero-Gil et al. [11, 19]; however, the full-length cDNAs have not yet been obtained. The aim of the present study, therefore, was to clone and characterize the full-length cDNAs of PySOS1 and PyNhaD to further our understanding of Na+/H+ antiporters in marine macroalgae. Subsequently, expression analyses of two Na+/H+ antiporter genes were carried out. To our knowledge, this is the first report to perform expression analyses of Na+/H+ antiporters in marine macroalgae.
Materials and methods
Algal material and abiotic stress treatment
The leafy gametophytes and filamentous sporophytes of P. yezoensis strain TU-1 were cultured as previously described [18]. The cultured algae were used to clone PySOS1 and PyNhaD, and for transcriptional analysis of the two genes under salt stress, acid stress, and the light–dark cycle. Salt and acid stresses were applied by treating vegetative gametophytes and sporophytes with enriched sealife (ESL) medium containing 0.3 M NaCl or adjusted to pH 6.0 through the addition of 6 N HCl under continuous light conditions. Light treatment was carried out by incubating at a light intensity of 80 μmol m−2 s−1. The light/dark cycle consisted of 10 h light and 14 h dark. 3-(3,4-Dichlorophenyl)-1,1-dimethylurea (DCMU) and 2,5-dibromo-3-methyl-6-isopropylbenzoquinone (DBMIB) treatments were performed from 30 min prior to light irradiation. Algal material was placed directly into liquid nitrogen and stored at −80 °C until it was used in rapid amplification of 5′ cDNA ends (5′RACE) and transcription analysis.
Cloning of full-length cDNAs encoding Na+/H+ antiporters from P. yezoensis
Based on the partial sequences of PySOS1 (EST accession no. AU192232) and PyNhaD (EST accession no. AV438891), cDNA 5′ ends were obtained by 5′RACE. Total RNA extraction from the gametophytes was carried out with an RNeasy plant mini kit (Qiagen, Hilden, Germany), and the resultant total RNA was further purified with an RNase-Free DNase Set (Qiagen). First-strand cDNA was synthesized with a SMART RACE cDNA amplification kit (Clontech, Mountain View, CA, USA) and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ instructions. The cDNA 5′ ends were amplified by polymerase chain reaction (PCR) with TaKaRa LA Taq (GC buffer type) (TaKaRa Bio, Shiga, Japan) with the primer SOS1-RACE-R1 (Table 1). The 5′ end of PyNhaD cDNA was also amplified by PCR in the same manner with the primer NhaD-RACE-R1. The PCR products were separated by electrophoresis with 1.0 % agarose gel, and the amplification products were excised and purified with a QIAquick gel extraction kit (Qiagen). Purified PCR products were cloned into the pT7Blue vector (Merck, Darmstadt, Germany), and nucleotide sequences were determined with an ABI 3130xl genetic analyzer (Applied Biosystems/Life Technologies, Carlsbad, CA, USA).
Transcript analysis of PySOS1 and PyNhaD
Total RNA extraction from gametophytes and sporophytes was carried out with an RNeasy plant mini kit (Qiagen), and the resultant total RNA was further purified with a TURBO DNA-free kit (Applied Biosystems/Life Technologies). Total RNA (0.5 μg per reaction) was used for first-strand cDNA synthesis with a PrimeScript II 1st strand cDNA Synthesis Kit (TaKaRa Bio). Real-time PCR was performed with an ABI Prism 7300 sequence detection system and software (Applied Biosystems/Life Technologies). All real-time PCR was performed under the following conditions: 30 s at 95 °C followed by 40 cycles of 5 s at 95 °C and 31 s at 60 °C. The gene-specific primers used for real-time PCR (SOS1-q-F/R, NhaD-q-F/R and 18SrRNA-q-F/R) are listed in Table 1. The specificity of the PCR products was confirmed by analyzing the dissociation curve at the end of each reaction (15 s at 95 °C, 1 min at 60 °C, and 15 s at 95 °C). The reaction mixture (20 μl) contained 10 μl SYBR Premix Ex Taq GC (TaKaRa Bio), 0.8 μl of each forward and reverse primer (5 μM), 0.4 μl of ROX reference dye, and 4 μl of cDNA template (100-fold dilution). The Py18SrRNA gene, whose transcriptional activity does not significantly fluctuate over the course of a whole day, was used as an internal control to normalize the amount of mRNA in each reaction. The mRNA amounts of the PySOS1 and PyNhaD genes were calculated based on a standard curve. The standard curve for each primer set was prepared by plotting serial cDNA dilution (1:10–1:105) against CT (threshold cycle). All experiments were conducted in triplicate.
Phylogenetic analysis of the Na+/H+ antiporters
A phylogenetic tree was constructed using the neighbor-joining method with MEGA version 5.0 (http://www.megasoftware.net). The amino acid sequences of the Na+/H+ antiporters were retrieved from the Genbank database.
In silico analyses of Na+/H+ antiporter
The BLAST program was used to identify homologous sequences in the GenBank database. Hydrophobicity profiles of PySOS1 and PyNhaD were analyzed using SOSUI (http://bp.nuap.nagoya-u.ac.jp/sosui/). WoLF PSORT (http://wolfpsort.org/) was used to predict the subcellular localization of PySOS1 and PyNhaD.
Results
Characterization of PySOS1 and PyNhaD
5′RACE was performed to obtain the full-length cDNAs of PySOS1 and PyNhaD. The full-length cDNA of PySOS1 was 5122 bp in length and contained an open reading frame (ORF) of 4773 bp, while that of PyNhaD was 1804 bp with an ORF of 1275 bp. PySOS1 and PyNhaD were shown to encode putative proteins of 1591 aa and 425 aa, respectively. The complete cDNA sequences and deduced amino acid sequences were deposited into GenBank under the accession numbers AB694755 and AB694756, respectively.
BLAST analysis revealed that the deduced amino acid sequences of PySOS1 and PyNhaD share 55 % similarity with SOS1 from A. thaliana and 76 % similarity with NhaD from A. thaliana, respectively. The hydrophobicity profiles of the amino acid sequences of PySOS1 and PyNhaD predicted 12 and 10 membrane-spanning regions, respectively. In addition, we used WoLF PSORT to predict the subcellular localization of PySOS1 and PyNhaD based on their amino acid sequences. PSORT predicted the localization of PySOS1 in the plasma membrane, but was unable to localize PyNhaD.
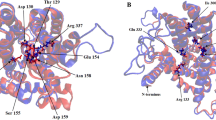
Several characteristic sequences have been identified in SOS1- and NhaD-type Na+/H+ antiporters. For example, Hamada et al. [20] reported that Asp 138 in the Na+/H+ antiporter from Synechocystis sp. PCC 6803 (that is highly homologous to AtSOS1 and named SynNhaP), which is involved in exchange activity, is conserved in the SOS1-type Na+/H+ antiporter. The conserved amino acid was also found in PySOS1 (Fig. 1). In NhaD-type Na+/H+ antiporters, Habibian et al. [21] reported that the residues Ser 150, Asp 154, Asn 155, Asn 189, Asp 199, Thr 201, Thr 202, Ser 389, Asn 394, Ser 428, and Ser 431 of NhaD from Vibrio cholera (named VcNhaD) are involved in the reaction of the cation–proton antiporter. These amino acids were also strongly conserved in PyNhaD (Fig. 2). Phylogenetic tree analysis revealed that PySOS1 and PyNhaD were located in SOS1-type and NhaD-type clusters, respectively (Fig. 3).
Structural characteristics of PySOS1. Alignment of the deduced amino acid sequences of SOS1-type Na+/H+ antiporters and SynNhaP. The sequences were aligned using ClustalW. The conserved Asp (Asp 138 in SynNhaP) is shown by an arrow. Black shading indicates 100 % conserved amino acid residues, dark gray indicates 80 % conservation, and light gray 60–80 % conservation. Bars represent gaps. Numbers correspond to amino acid positions from the first methionine residue. PySOS1, Porphyra yezoensis (AB694755); AtSOS1, Arabidopsis thaliana (NM_126259); PeSOS1, Populus euphratica (DQ517530); OsSOS1, Oryza sativa (AY785147); PpSOS1, Physcomitrella patens (AM707025); SynNhaP, Synechocystis sp. PCC 6803 (NP_441245). The amino acid sequences were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/)
Structural characteristics of PyNhaD. Alignment of the deduced amino acid sequences of NhaD-type Na+/H+ antiporters. The sequences were aligned using ClustalW. The amino acids involved in the reaction of the cation–proton antiport (VcNhaD) are shown by arrowheads. Black shading indicates 100 % conserved amino acid residues, dark gray indicates 80 % conservation, and light gray 60–80 % conservation. Bars represent gaps. Numbers correspond to amino acid positions from the first methionine residue. PyNhaD, Porphyra yezoensis (AB694756); AtNhD1, Arabidopsis thaliana (NP_566638); PeNhaD1, Populus euphratica (AJ561195); PpNhaD, Physcomitrella patens (AM491807); VcNhaD, Vibrio cholerae (AAG48354). The amino acid sequences were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/)
Phylogenetic tree of Na+/H+ antiporters. The phylogenetic tree was constructed based on the amino acid sequences of the ORF of Na+/H+ antiporters using the neighbor-joining method. Branch lengths indicate evolutionary distance, with a scale of 0.2. Species and accession numbers (in alphabetical order): AtNhD1, Arabidopsis thaliana; AtNhx1, A. thaliana (NP_198067); AtSOS1, A. thaliana; OsNhaD, O. sativa (BAD17583); OsNhx1, O. sativa (AB021878); OsSOS1, O. sativa; PeNhaD1, Populus euphratica; PeNhx1, P. euphratica (AJ853472); PeSOS1, P. euphratica; PpNhaD, P. patens; PpSOS1, P. patens; PySOS1, Porphyra yezoensis; PyNhaD, P. yezoensis; ScNha1, Saccharomyces cerevisiae (NP_013239); ScNhx1, S. cerevisiae (NP_010744); SynNhaP, Synechocystis sp. PCC 6803; VcNhaD, Vibrio cholerae; VpNhaD, Vibrio parahaemolyticus (BAA25994)
Expression patterns of PySOS1 and PyNhaD
P. yezoensis has a heteromorphic life cycle with a macroscopic leafy gametophyte and microscopic filamentous sporophyte. The expression patterns of PySOS1 and PyNhaD in gametophytes and sporophytes were therefore analyzed by real-time PCR. As shown in Fig. 4, the mRNA transcripts of the two Na+/H+ antiporters were expressed in both generations.
Expression of PySOS1 and PyNhaD transcripts in Porphyra yezoensis gametophytes and sporophytes. RNA prepared from gametophytes cultured at 15 °C (G) and sporophytes cultured at 15 °C (S) was used for quantitative real-time PCR. The 18S rRNA gene from P. yezoensis (Py18SrRNA) was used as an internal control. Results represent the relative expression compared to that of the PySOS1 gene in the gametophyte. Data are presented as the mean ± standard deviation (n = 3)
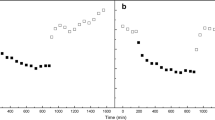
Next, we examined whether PySOS1 and PyNhaD were responsive to salt stress, acid stress (pH 6.0), and the light–dark cycle using the gametophyte. Expression patterns of the two genes in response to salt stress and acid stress (pH 6.0) did not fluctuate under continuous light conditions (data not shown). On the other hand, the transcript level of PySOS1 rapidly increased after 4 h of light, peaked at 2 h of dark, and subsequently decreased to its baseline level at 14 h of dark (Fig. 5). The expression pattern of PyNhaD was similar to that of PySOS1 (Fig. 5). The maximum expression levels of PySOS1 and PyNhaD were approximately 5.0-fold and 65.2-fold higher than the baseline, respectively. In addition, in the sporophyte, the expression patterns of both genes were similar to the gametophyte results (data not shown).
Expression of PySOS1 and PyNhaD transcripts in Porphyra yezoensis gametophytes under the light/dark cycle. Algal material was sampled every 4 h under a photoperiod of 10 h L:14 h D. The Py18SrRNA gene was used as an internal control. Results represent the relative expression compared to that at the start of the experimental culture period. Data are presented as the mean ± standard deviation (n = 3)
To elucidate whether photosynthesis affects the light-dependent accumulation of PySOS1 and PyNhaD transcripts, the effect of the photosynthetic inhibitors DCMU and DBMIB was investigated. We used 10 μM DCMU and 10 μM DBMIB, since it has been reported that 0.05–10 μM DCMU and 8–10 μM DBMIB effectively inhibited the photosynthetic activity of marine macroalgae [22, 23]. Figure 6 shows that the levels of the two transcripts were partially reduced by both inhibitors and that PyNhaD is more sensitive to the inhibitors than PySOS1.
Effect of DCMU and DBMIB on the expression of PySOS1 and PyNhaD in Porphyra yezoensis gametophytes. Algal material grown under a photoperiod of 10 h L:14 h D was sampled 8 h post light irradiation and then treated with 0.01 % DMSO (Control), 10 μM DCMU, or 10 μM DBMIB from 30 min prior to light irradiation. The Py18SrRNA gene was used as an internal control. Results represent the relative expression compared to that in 0.01 % DMSO (Control). Data are presented as the mean ± standard deviation (n = 3)
Discussion
In the present study, we cloned full-length cDNAs of two Na+/H+ antiporters, PySOS1 and PyNhaD. Both possessed conserved amino acid sequences found in Na+/H+ antiporters (Fig. 1), showing them to be functional proteins. Computational analysis suggests that PySOS1 localizes to the plasma membrane, similar to SOS1 in higher plants such as A. thaliana and P. euphratica [24], whereas the subcellular localization of PyNhaD could not be predicted. Previous subcellular localization analysis using GFP showed that NhaD from P. patens, PpNhaD1, localizes to the chloroplast [11], raising the possibility that PyNhaD does so too. As shown in Fig. 6, the effect of photosynthetic inhibitors on light-dependent accumulation of the PyNhaD transcripts was more pronounced than that of the PySOS1 transcript, indicating that PyNhaD expression is more closely related to chloroplasts than PySOS1 expression. However, this close relationship does not necessarily indicate the localization of PyNhaD in the chloroplasts. To confirm this, subcellular localization analysis of PyNhaD using GFP is now required.
SOS1- and NhaD-type Na+/H+ antiporters are known to be upregulated in response to salt stress [5, 24, 25]; however, transcripts of PySOS1 and PyNhaD were not upregulated in response to salt stress under continuous illumination (Fig. 5). The results raise the possibility that the amounts of the two gene transcripts reached their maximum values upon light irradiation, regardless of salt stress. Interestingly, it was reported in Porphyra leucosticta that an increase in the intracellular Na+ content was observed after the onset of light [26]. Also, in P. yezoensis cells, the Na+ content is predicted to increase under illumination with light. Thus, the simultaneous upregulation of PySOS1 and PyNhaD after light irradiation may play an important role in Na+ homeostasis under light conditions. However, we need to investigate PySOS1 and PyNhaD expression under salt stress in the continuous dark further.
In land plants and green algae, it has been shown that photosynthesis is responsible for the regulation of gene expression by light [27–29]. For example, light-dependent accumulation of superoxide dismutase genes from a liverwort was inhibited by both DCMU and DBMIB [30], which inhibits the flow of electrons from PSII to plastoquinone and the flow after plastoquinone by binding to the cytochrome b 6/f complex, respectively [31]. As shown in Fig. 6, levels of the PySOS1 and PyNhaD transcripts were partially reduced during incubation in the presence of DCMU and DBMIB under light irradiation, respectively, suggesting that their upregulation is linked to photosynthesis-related metabolism. However, little is known about the transcriptional regulatory mechanisms by light in red algae. Promoter analyses of PySOS1 and PyNhaD will therefore contribute to our understanding of the mechanisms regulated by light in red algae.
References
Brett CL, Donowitz M, Rao R (2005) Evolutionary origins of eukaryotic sodium/proton exchangers. Am J Physiol Cell Physiol 288:223–239
Mitsui K, Yasui H, Nakamura N, Kanazawa H (2005) Oligomerization of the Saccharomyces cerevisiae Na+/H+ antiporter Nha1p: implications for its antiporter activity. Biochim Biophys Acta 1720:125–136
Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011) The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell 23:224–239
Wu SJ, Ding L, Zhu JK (1996) SOS1, a genetic locus essential for salt tolerance and potassium acquisition. Plant Cell 8:617–627
Shi HZ, Ishitani M, Kim CS, Zhu JK (2000) The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc Natl Acad Sci USA 97:6896–6901
Shi HZ, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Yokoi S, Quintero FJ, Cubero B, Ruiz MT, Bressan RA, Hasegawa PM, Pardo JM (2002) Differential expression and function of Arabidopsis thaliana NHX Na+/H+ antiporters in the salt stress response. Plant J 30:529–539
Apse MP, Aharon GS, Snedden WA, Blumwald E (1999) Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 285:1256–1258
Nozaki K, Kuroda T, Mizushima T, Tsuchiya T (1998) A new Na+/H+ antiporter, NhaD, of Vibrio parahaemolyticus. Biochim Biophys Acta 1369:213–220
Ottow EA, Polle A, Brosche M, Kangasjarvi J, Dibrov P, Zorb C, Teichmann T (2005) Molecular characterization of PeNhaD1: the first member of the NhaD Na+/H+ antiporter family of plant origin. Plant Mol Biol 58:75–88
Barrero-Gil J, Rodriguez-Navarro A, Benito B (2007) Cloning of the PpNHAD1 transporter of Physcomitrella patens, a chloroplast transporter highly conserved in photosynthetic eukaryotic organisms. J Exp Bot 58:2839–2849
Saga N, Kitade Y (2002) Porphyra: a model plant in marine sciences. Fish Sci 68(Suppl):1075–1078
Waaland JR, Stiller JW, Cheney DP (2004) Macroalgal candidates for genomics. J Phycol 40:26–33
Asamizu E, Nakajima M, Kitade Y, Saga N, Nakamura Y, Tabata S (2003) Comparison of RNA expression profiles between the two generations of Porphyra yezoensis (Rhodophyta), based on expressed sequence tag frequency analysis. J Phycol 39:923–930
Nikaido I, Asamizu E, Nakajima M, Nakamura Y, Saga N, Tabata S (2000) Generation of 10,154 expressed sequence tags from a leafy gametophyte of a marine red alga, Porphyra yezoensis. DNA Res 7:223–227
Fukuda S, Mikami K, Uji T, Park E-J, Ohba T, Asada K, Kitade Y, Endo H, Kato I, Saga N (2008) Factor influencing efficiency of transient gene expression in the red macrophyte Porphrya yezoensis. Plant Sci 174: 329–339
Mikami K, Uji T, Li L, Takahashi M, Yasui H, Saga N (2009) Visualization of phosphoinositides via the development of the transient expression system of a cyan fluorescent protein in the red alga Porphyra yezoensis. Mar Biotechnol 11:563–569
Uji T, Takahashi M, Saga N, Mikami K (2010) Visualization of nuclear localization of transcription factors with cyan and green fluorescent proteins in the red alga Porphyra yezoensis. Mar Biotechnol 12:150–159
Barrero-Gil J, Garciadeblas B, Benito B (2005) Sodium, potassium-atpases in algae and oomycetes. J Bioenerg Biomembr 37:269–278
Hamada A, Hibino T, Nakamura T, Takabe T (2001) Na+/H+ antiporter from Synechocystis species PCC 6803, homologous to SOS1, contains an aspartic residue and long C-terminal tail important for the carrier activity. Plant Physiol 125:437–446
Habibian R, Dzioba J, Barrett J, Galperin MY, Loewen PC, Dibrov P (2005) Functional analysis of conserved polar residues in Vc-NhaD, Na+/H+ antiporter of Vibrio cholerae. J Biol Chem 280:39637–39643
Barros MP, Necchi O Jr, Colepicolo P, Pedersén M (2006) Kinetic study of the plastoquinone pool availability correlated with H2O2 release in seawater and antioxidant responses in the red alga Kappaphycus alvarezii exposed to single or combined high light, chilling and chemical stresses. Biochim Biophys Acta 1757:1520–1528
Hsu YT, Lee TM (2012) Modulation of gene expression of carotene biosynthesis-related protein by photosynthetic electron transport for the acclimation of intertidal macroalga Ulva fasciata to hypersalinity and excess light. Physiol Plant 144:225–237
Wu Y, Ding N, Zhao X, Zhao M, Chang Z, Liu J, Zhang L (2007) Molecular characterization of PeSOS1: the putative Na+/H+ antiporter of Populus euphratica. Plant Mol Biol 65:1–11
Kurz M, Brunig AN, Galinski EA (2006) NhaD type sodium/proton-antiporter of Halomonas elongata: a salt stress response mechanism in marine habitats? Saline Syst 2:10
Escassi L, Aguilera J, Figueroa FL, Fernández JA (2002) Potassium drives daily reversible thallus enlargement in the marine red alga Porphyra leucosticta (Rhodophyta). Planta 214:759–766
Maxwell DP, Laudenbach DE, Huner NPA (1995) Redox regulation of light-harvesting complex II and cab mRNA abundance in Dunaliella salina. Plant Physiol 109:787–795
Tullberg A, Alexciev K, Pfannschmidt T, Allen JF (2000) Photosynthetic electron flow regulates transcription of the psaB gene in pea (Pisum sativum L.) chloroplasts through the redox state of the plastoquinone pool. Plant Cell Physiol 41:1045–1054
Harashima S, Takano H, Ono K, Takio S (2004) Chalcone synthase-like gene in the liverwort, Marchantia paleacea var. diptera. Plant Cell Rep 23:167–173
Sakaguchi S, Fukuda T, Takano H, Ono K, Takio S (2004) Photosynthetic electron transport differentially regulates the expression of superoxide dismutase genes in liverwort, Marchantia paleacea var. diptera. Plant Cell Physiol 45:318–324
Fey V, Wagner R, Bräutigam K, Pfannschmidt T (2005) Photosynthetic redox control of nuclear gene expression. J Exp Bot 56:1491–1498
Acknowledgments
This study was supported, in part, by the Regional Innovation Cluster Program (Global Type) of the Ministry of Education, Culture, Sports, Science and Technology of Japan, awarded to N.S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uji, T., Monma, R., Mizuta, H. et al. Molecular characterization and expression analysis of two Na+/H+ antiporter genes in the marine red alga Porphyra yezoensis . Fish Sci 78, 985–991 (2012). https://doi.org/10.1007/s12562-012-0520-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-012-0520-6