Abstract
This study investigated the effects of dietary fatty acids and water salinity on fatty acid composition and expression of the fatty acid desaturase (fads)-like and elongase (elovl)-like genes of red sea bream Pagrus major. Sequence and phylogenetic analyses revealed that the fads-like and elovl-like genes isolated in this study encoded fads6 and elovl5, respectively. There was no significant difference in growth performance or proximate composition of fish fed diets formulated with 100% fish oil (FO) (FO diet) or with 67% of FO substituted with canola oil (VO diet). Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) contents were significantly higher in liver of fish reared at 15 and 20 ppt than those at 33 ppt. The liver fatty acid desaturase gene transcript was detected by reverse-transcription polymerase chain reaction (RT-PCR) in liver of fish fed the VO diet at 15 ppt, but not at either 20 or 33 ppt. The elovl5 gene was clearly expressed in liver for all treatments. These results suggest that red sea bream could synthesize EPA and DHA from their precursors in liver and that EPA and DHA biosynthesis potency was stimulated at lower salinity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
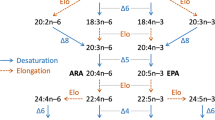
Increasing aquaculture production is inevitable to support the global requirements of protein and essential fatty acids for human consumption [1]. Consequently, use of fish feed has also been increasing. Fish oil (FO) is the major lipid source of fish feed. FO contains high amounts of n-3 highly unsaturated fatty acids (HUFAs) such as eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3). EPA and DHA are essential for normal growth and survival of marine fish [2]. FO is the major and indispensable source of n-3 HUFA, because vegetable oils do not contain a large amount of n-3 HUFA. However, rapid increase in global aquafeed production has resulted in limitation of market availability and increasing market value of fish oil. The imminent inability of global fish oil supply to support the sustained expansion of aquaculture production has boosted research effort to develop alternatives to fish oil, especially for diets of salmonids [3]. The development of other lipid sources that can replace FO is thereby an urgent subject for the aquaculture industry. The only sustainable alternatives to FO are vegetable oils, which are rich in polyunsaturated fatty acids (PUFAs) such as linoleate (18:2n-6) and linolenate (LNA, 18:3n-3) but devoid of n-3 HUFAs [4].
Freshwater fish species possess the capacity to synthesize EPA and DHA from LNA through a pathway involving desaturation and elongation of their respective precursors [5, 6]. However, marine fish cannot synthesize n-3 HUFA (EPA and DHA) in vivo because of either lacking or limited activities of Δ6 and Δ5 desaturase and elongase, which are involved in EPA and DHA biosynthesis [7, 8]. HUFA biosynthetic activity has also been shown to be under environmental regulation in Atlantic salmon Salmo salar, being increased during the period of parr–smolt transformation with peak activities around seawater transfer [9, 10]. However, feeding an FO-based diet during the parr–smolt transformation period was shown to attenuate HUFA biosynthetic activity and eventually reduced its activity during seawater transfer [9, 10], suggesting that HUFA biosynthesis in Atlantic salmon is regulated both nutritionally and by environmental salinity [11]. However, there are no reports on the effect of environmental salinity on HUFA biosynthesis potency in marine fish.
Therefore, the present study aims to clone red sea bream Pagrus major fatty acid desaturase (fads) and elongase (elovl) genes and to examine the effect of dietary fatty acids and salinity on fatty acid composition and fads and elovl gene expression.
Materials and methods
Tissue collection
Red sea bream used for molecular cloning and sequencing of fatty acid desaturase and elongase was collected offshore of the Tateyama Field Station of Tokyo University of Marine Science and Technology (Chiba, Japan) by hook and line. The weight of the fish was 180 g, and the total length was 20 cm. Tissue was sampled from different organs, such as the brain, gills, muscle, liver, kidney, spleen, stomach, and intestine. These tissues were stored at −80°C until total RNA isolation.
Total RNA isolation and cDNA synthesis
Total RNA was isolated from liver of red sea bream using Isogen reagent (Wako Pure Chemical Industries Co., Osaka, Japan) according to the manufacturer’s instructions. Five micrograms of total RNA was used for first-strand complementary DNA (cDNA) synthesis. Reverse transcription (RT) was performed using the GeneRacer™ kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions.
Partial cloning of red sea bream fads-like cDNA
RT-PCR was performed to amplify the cDNA fragment from liver of red sea bream using fads degenerate primers, which were designed on the basis of the highly conserved areas of fads6 cDNA from Atlantic salmon (AY458652), turbot Psetta maxima (AY546094.1), Atlantic cod Gadus morhua (DQ054840), gilthead sea bream Sparus aurata (AY055749), and rainbow trout Oncorhynchus mykiss (AF301910). The degenerate primer sequences for the forward des-degene FD1 and reverse des-degene RV1 were used for the first amplification, and nested PCR was performed using the des-degene FV1 primer and des-degene RV2 primer (Table 1). The PCR conditions are presented in Table 2. The resultant PCR products were ligated into a pGEM®-T easy vector (Promega Co., Madison, WI, USA) and transferred into E. coli JM109 (TaKaRa Co, Tokyo, Japan) competent cells. Purified cDNA was sequenced using an ABI PRISM® 3100-Avant genetic analyzer (Life Technologies, Inc., Carlsbad, CA, USA) system profile with the Big Dye Terminator V 3.1. cycle sequencing kit (Life Technologies, Inc., Carlsbad, CA, USA) according to the manufacturer’s protocol.
Cloning of red sea bream elovl-like cDNA
The elovl-like gene degenerate primers were designed according to the highly conserved regions of elovl5a cDNA of Atlantic salmon (GenBank accession no. AY170327), turbot elongase (AF465520), Atlantic cod elovl5 (AY660881), gilthead sea bream elongase (AY660879), Nile tilapia Oreochromis niloticus elongase (AY170326), rainbow trout elongase (AY605100.1), and zebrafish Danio rerio elongase (AF532782). The first amplification was performed using the Elo-degene FD1 primer and Elo-degene RV1 primer (Table 1). Nested PCR was performed using the Elo-degene FD2 primer and Elo-degene RV1 primer (Table 1). The PCR conditions are presented in Table 2. The resultant PCR products were cloned and sequenced using the protocol described in “Partial cloning of red sea bream fads-like cDNA.”
Rapid amplification of cDNA 3′ and 5′ ends (RACE) was done using the gene-specific primers. The primers were designed according to the partial sequence of the red sea bream elovl-like cDNA derived from a subcloned fragment (Table 1). The RACE PCR conditions are presented in Table 2. The nested PCR product of 3′ RACE was purified, cloned into the pGEM-T Easy vector, and sequenced as already described. Using a GeneRacer kit (Invitrogen, Carlsbad, CA, USA), 5′ RACE was also done, according to the manufacturer’s protocol. The products were also cloned and sequenced as already described.
Experimental diets
Two diets were formulated for the feeding experiment. Diets with 100% FO and with 67% of FO replaced by canola oil were designated as the FO diet and VO diet, respectively. It was shown that supplementation of 70% canola oil provided adequate quantities of essential fatty acid (EFA), especially EPA, DHA, and likely arachidonic acid (AA), for good growth and health of red sea bream [12]. We therefore decided to use the same formulation for the experimental diet. The ingredients and formulation of the experimental diets are presented in Table 3. The ingredients were well mixed and pelleted using a laboratory pellet mill (AEZ12M; Hiraga-Seisakusho, Kobe, Japan). The pellet was dried using a vacuum freeze-drier (RLE-206; Kyowa Vacuum Engineering, Tokyo, Japan) and stored at −20°C until use.
Experimental conditions and feeding trial
Twenty fish with average body weight of 51.1 ± 2.1 g were randomly distributed in each of twelve 60-l glass aquaria with two replications. Juvenile red sea bream were purchased from Kinki University, Wakayama, Japan, and acclimated to the experimental condition by feeding commercial feed (Nippon Formula Feed Manufacturing Co., Yokohama, Japan) for 3 weeks before initiation of the feeding experiment. The feeding trial was done in three closed recirculating systems maintained three different salinities (15, 20, and 33 ppt) for 12 weeks. Artificial seawater was prepared using salt (Sea Life®; Marine Tech, Tokyo, Japan), and salinity levels were adjusted by using a gravimeter. We set 15 ppt as the lowest salinity in the present experiment because the lowest salinity tolerance limit of red sea bream was 12.2 ppt [13]. The artificial seawater used for the recirculatory system was dechlorinated using sodium thiosulfate pentahydrate (Na2S2O3·5H2O), and the system was maintained with a biofilter and an ultraviolet irradiation lamp (REI-SEA Co., Tokyo, Japan) for disinfection. In each circularity system, 4 tanks were used to feed the FO or VO diet in duplicate. The water flow rate was adjusted to 700–800 ml/min. The water renewal rate in the system was 30% every week. Water quality parameters such as temperature, pH, and salinity were monitored everyday. The ammonia level was checked every 15 days. The average daily water temperature was 21.0–23.0°C, and the pH was 6.9–7.2. The photoperiod was maintained as 12 h light and 12 h darkness in a day. The fish were fed three times a day and 6 days a week until near satiation. During the experimental period, the fish were weighed after 5 weeks and the supplied diet pellet size was then adjusted.
Proximate and fatty acid composition analyses
At the onset and end of the experiment, 3 fish from each tank were randomly selected for chemical analyses of whole body and liver. For proximate composition and fatty acid analysis, fish samples were collected after 40 h fasting. Whole-body (except liver) samples were minced using a centrifugal mill (ZM 200; Retsch GmbH, Hann, Germany) fitted with a 0.25-mm screen. The homogenate was collected and kept at −30°C until analysis. A liver sample was collected and manually ground, then stored at −30°C for lipid and fatty acid analysis. The proximate composition analysis was determined by the standard method [14]. Duplicate analyses were conducted for each sample. Crude protein was analyzed using a Kjeltec Auto Sampler System 2400 analyzer (Foss Japan Co., Tokyo, Japan). Crude protein content was calculated by multiplying the nitrogen content by 6.25. Crude lipids were extracted from the diets, whole body, and liver using chloroform/methanol (2:1, v/v) [15]. Crude lipids were trans-methylated by boron trifluoride in methanol [16]. The fatty acid methyl esters were separated using a gas liquid chromatograph (GC-17B; Shimadzu, Kyoto, Japan) equipped with a hydrogen flame ionization detector and a 30 m × 0.25 mm × 0.25 μm capillary column (VF-23 ms; Agilent Technologies, St. Clara, CA, USA). The carrier gas was helium. The temperatures of the injector and detector were 250°C and 260°C, respectively. The column temperature was initially held at 170°C for 5 min, followed by an increase at the rate of 3°C/min to 210°C, then by an increase at the rate of 10°C/min to a final temperature of 230°C. Peaks of individual fatty acids were identified by comparison with FO standard.
Gene expression analysis
After 12 weeks of the experiment, the livers of three fish were collected for gene expression analysis after 12 h fasting. The isolation of the total RNA and cDNA synthesis procedure has already been described. The general RT-PCR for fads6 and elovl5 were performed with a primer designed from the partial cDNA sequences, and the GenBank accession numbers are HQ423164 and HQ415605, respectively. The forward and reverse primer sequences were Rsb fads6 F and Rsb fads6 R, respectively, for fads6, and Rsb elovl5 F and Rsb elovl5 R, respectively, for elovl5 (Table 1). The sequence of red sea bream β-actin was obtained from GenBank (accession no. AB036756). The forward and reverse primers for red sea bream β-actin were Rsb β-actin F and Rsb β-actin R (Table 1). The size of the PCR product for fads6, elovl5, and β-actin were 152, 156, and 156 bp, respectively. The RT-PCR conditions are presented in Table 2. The PCR products were separated by electrophoresis on a 1.5% agarose gel.
Evaluation of growth performance and statistical analyses
The experimental fish were anesthetized using 2-phenoxyethanol (Sigma Co., St. Louis, MO, USA) at 0.2 ml/l and weighed individually after removal of excess surface moisture. At the onset and end of the experiment, all fish from each tank were weighted individually for growth measurements. The effect of the dietary treatment on the growth performance of the fish was assessed using the following formulae: (1) weight gain (WG) (g) = [final mean wet weight (FW) (g) − initial mean wet weight (IW) (g)], (2) specific growth rate (SGR) = 100 × [ln(final mean weight) − ln(initial mean weight)]/days fed, and (3) feed conversion ratio (FCR) = feed intake (dry matter)/fish weight gain. Data for proximate and fatty acid composition of diets, initial body weight, final body weight, weight gain, FCR, SGR, and proximate composition of whole body of the fish after 12 weeks were analyzed by one-way analysis of variance (ANOVA) (Systat 8.0, SPSS 16.0; SPSS, Chicago, IL, USA). Interaction of dietary fatty acid and salinity was tested by two-way ANOVA, followed by Tukey’s multiple tests (Systat 8.0, SPSS 16.0; SPSS, Chicago, IL, USA). Differences were considered significant for P < 0.05.
Results
Sequence analysis of red sea bream fads-like cDNA
The fads-like cDNA was partially cloned from liver of red sea bream, and the length of the nucleotide sequence was 950 bp and 316 amino acids (accession no. HQ423164). The amino acid sequence of the red sea bream fads-like gene includes the characteristics of fads and contains two motifs that are typical for members of the membrane fads superfamily, which includes two histidine boxes and an N-terminal cytochrome b 5 domain containing the heme-binding motif, H-P-G-G. Hydropathy analysis also revealed that the red sea bream fads protein contained two transmembrane regions (Fig. 1). The desaturase amino acid sequence exhibited 69% sequence identity to Atlantic salmon Δ6 fatty acyl desaturase sequence (NP_001165752.1), 72% identity to Atlantic cod Δ6 fatty acyl desaturase (AAY46796.1), 78% identity to gilthead sea bream putative Δ6 desaturase (AAL17639.1), and 82% sequence identity to Asian sea bass Lates calcarifer fatty acyl Δ6 desaturase (ACS91458.1) (Fig. 1a). Phylogenetic analysis by the neighbor-joining method showed that the red sea bream fatty acid desaturase-like genes were clustered in the marine fish fads6 groups (Fig. 2a). The above nucleotide and amino acid sequence characteristics suggested that the cloned gene is Δ6 fads cDNA.
Alignment of amino acid sequences of fads-like and elovl-like genes of red sea bream and other known teleost species. a Alignment of amino acid sequence of fads-like gene of red sea bream and four other known teleost species. Identical residues are indicated by asterisks. Motifs highly conserved among fatty acid desaturase, the cytochrome b5 domains, are marked by a solid line, and two histidine domains are boxed. Transmembrane regions are marked by broken lines. b Alignment of amino acid sequences of red sea bream elovl-like and four other known elovl5 genes. Identical residues are marked by asterisks. Motifs highly conserved among elovl from a wide range of organisms, i.e., KxxExxDT, the extended histidine box QxxFLHxYHH, the tyrosine box NxxxHxxMYxYY, and TxxQxxQ, are boxed. Transmembrane regions are marked by broken lines
a The phylogenetic relationship between amino acid sequences of the red sea bream Pagrus major fads-like genes and other known fads. The online software bio-edit sequence alignment editor (accessed September 3, 2010) was used to translate the nucleotide sequence. The characterization of both genes was performed using National Centre for Biotechnology Information (NCBI; http://blast.ncbi.nlm.nih.gov/ Blast.cgi; accessed September 3, 2010) system, and the most conserved domains in the deduced amino acid sequence of fatty acid desaturase and elongase were identified by conserved domain database (CDD) search at the NCBI (accessed September 3, 2010). The fatty acid desaturase and elongase amino acid sequences were detected and compared with the other marine groups using the online multiple alignment program CLUSTALW version 1.83 (http://clustalw.ddbj.nig.ac.jp/; accessed September 3, 2010). Finally, phylogenetic reconstruction of the sequences was carried out using neighbor-joining MEGA 4.0.2 software (accessed September 3, 2010). The GenBank accession numbers of the aligned amino acid sequences of red sea bream fads-like are as follows: Atlantic cod fads6 (AAY46796.1), gilthead sea bream putative fads6 (AAL17639.1), Asian sea bass fads6 (ACS91458.1), cobia fads6 (ACJ65149.1), Nile tilapia fads6 (AB069727), cherry salmon Oncorhynchus masoufadsform 1 (AB070444) and fadsform 2 (AB074149), Atlantic salmon fads5 (AF478472), nibe croaker fads6 (GQ996729.1), zebrafish Danio rerio fads5/6 (AF309556.1), mouse Mus musculusfads5 (AB072976) and fads6 (AF126798), and human Homo sapiensfads5 (AF199596) and fads6 (AF126799). b. Phylogenetic relationship between amino acid sequences of the red sea bream elovl-like gene and other known elovl genes. The GenBank accession numbers of the aligned amino acid sequences of red sea bream elovl-like are as follows: Atlantic salmon elovl5 (NP_001130024.1), nibe croaker elongase (ACR47973.1), gilthead sea bream elovl5 (AAT81404.1), cobia elovl5 (ACJ65150.1), Atlantic salmon elovl2 (ACI62500.1) and elovl5b (ACI62499.1), cherry salmon elongase (DQ067616), Asian sea bass elovl5 (GQ214180.1), nibe croaker (FJ952143.1), human elovl2 (NP_060240.3) and elovl5 (AAH67123.2), rat Rattus norvegicuselovl5 (NP_599209.1), mouse elovl2 (NP_062296.1), and zebrafish elovl2 gene (AAI29269.1) and elovl5 (NP_956747.1)
Sequence analysis of red sea bream elovl-like cDNA
The 1457-bp open reading frame (ORF) and 294 amino acids (GenBank accession no. HQ415605) were obtained from the red sea bream liver cDNA (Fig. 1b). The amino acid sequence of Pagrus major elovl-like protein has 79% identity to Atlantic salmon elovl5 (NP_001130024.1), 91% identity to nibe croaker Nibea miskaruii polyunsaturated fatty acid elongase (ACR47973.1), 96% identity to gilthead sea bream elongase (AAT81404.1), and 92% sequence identity to cobia Rachycentron canadum elovl5 (ACJ65150.1). The cobia PUFA elovl5 has been functionally characterized by heterologous expression in yeast [17]. The amino acid sequence of the red sea bream elovl-like protein also contained two motifs that are typical for members of the microsomal elovl superfamily. It includes a histidine box (HxxHH) and a putative lysine retention signal in the endoplasmic reticulum membrane. The highly conserved motifs for the elovl cloned to date were KxxExxDT, the extended histidine box QxxFLHxYHH, the tyrosine box NxxxHxxMYxYY, and TxxQxxQ (Fig. 1b) [18]. Hydropathy analysis revealed that the red sea bream elovl-like protein contained multiple transmembrane regions (Fig. 1b). Finally, a phylogenetic analysis using the neighbor-joining method showed that the red sea bream elovl-like gene is clustered in the marine fish elovl5 group (Fig. 2b).
Growth and feed performance
The results for overall growth performance, SGR, FCR, and survival after 12 weeks of feeding are presented in Table 4. There were no significant differences in any of the parameters for growth performance after 12 weeks (P > 0.05). The proximate composition of the fish also did not show any significant difference among the treatments (Table 5, P > 0.05).
Fatty acid composition
The lipid level was about 15% in both experimental diets (Table 6). The FO diets contained 5.9% total n-6 fatty acids, predominantly 18:2n-6 (5.2%), and 19.5% total n-3 fatty acids, predominantly the n-3 HUFA (17.1%), DHA (8.4%) and EPA (7.4%) (Table 7). Inclusion of canola oil resulted in increased percentages of 18:3n-3, 18:2n-6, and 18:1n-(9+7) with concomitant decrease in the proportion of n-3 HUFA. In the diet formulated with 67% VO, the levels of 18:3n-3, 18:2n-6, and 18:1n-(9+7) had increased to 62% of total fatty acids, whereas EPA and DHA were only 9% (Table 7). After 12 weeks, the whole-body lipid content was approximately 10% in all groups (Table 5). Approximately 10% DHA and 5% EPA were found in fish fed the FO diet in all the salinity groups. However, the fish fed the VO diet exhibited graded increased percentages of 18:3n-3 and 18:2n-6, and decreased proportions of n-3 HUFA, predominantly 7% DHA and 2.5% EPA for all salinities (Table 8). No difference was observed in the whole-body fatty acid composition of fish fed the FO diet for all salinities (Table 8). Fish fed the VO diet for all salinities showed similar fatty acid compositions. Statistical analysis revealed that the effect of dietary fatty acids on the fatty acid content in crude lipid from whole body was significant, but not for salinity (Table 9). There was no interaction effect of dietary fatty acids and salinity on fatty acid content in the fish, except for the EPA content (Table 9).
The n-3 HUFA content in liver of fish fed the FO diet at 20 and 15 ppt was higher than that at 33 ppt (Table 10). The n-3 HUFA contents increased in liver of fish fed the VO diets at 20 and 15 ppt salinity compared with those reared at 33 ppt salinity (Table 10). Total PUFA content in liver of fish fed the FO diet at 15 ppt salinity (29.5%) was approximately 1.6-fold higher than at 33 ppt salinity (18.5%) (Table 10). The n-3 HUFAs in liver of fish fed the FO diet were significantly higher at 15 and 20 ppt salinities (DHA 13.2–13.8%, EPA 5.8–6.1%) than at 33 ppt (DHA 8.2%, EPA 3.5%) (Table 10). Total PUFA content in liver of fish fed the VO diet was higher at 15 (26.9%) and 20 ppt (26.3%) salinities than at 33 ppt salinity (23.1%). Among the thirteen examined fatty acids, twelve and five fatty acids were significantly affected by dietary fatty acids and salinity, respectively (P < 0.05, Table 11). It was also observed that there is an interactive effect of dietary fatty acids and salinity on six fatty acids in the liver (Table 11).
Expression of fads6 and elovl5 genes
A similar expression level in liver was observed for elovl5 in all examined fish (Fig. 3). On the other hand, fads6 was not detected in liver at 33 or 20 ppt, irrespective of the dietary treatment, except for one weak positive in the FO group at 33 ppt (Fig. 3). However, clear expression of fads6 in liver was observed in fish fed the VO diet at 15 ppt, but not in fish fed the FO diet at the same salinity (Fig. 3).
Discussion
The fads-like and elovl-like cDNA were cloned from red sea bream. The sequence homology, predicted topology, and consensus sequences of the deduced red sea bream fads-like protein sequence strongly agreed with the fads predicted structural traits. fads6 has been cloned in freshwater and marine fish including Atlantic salmon, gilthead sea bream, common carp Cyprinus carpio, cobia, turbot, nibe croaker, and Atlantic cod [17, 19–23]. However, fads5 cDNA has not been identified in marine fish to date [17, 19–23]. Only a single fads gene has been identified in marine fish, the puffer fish Fugu rubripes, and Tetraodon nigroviridis. Phylogenetic analysis by the neighbor-joining method showed that the red sea bream fads-like gene is closely related to the marine fish fads6 group. Therefore, it was concluded that the identified red sea bream fads-like gene is fads6.
The red sea bream elovl-like gene has a peptide with a 485-amino-acid sequence that showed high homology with different marine species such as nibe croaker, gilthead sea bream, and cobia. The amino acid sequence of the red sea bream elovl-like cDNA included the characteristic sequences of elovl. Phylogenetic analysis showed that the red sea bream elovl-like gene is closely related to the elovl5 group. It was thereby concluded that the identified red sea bream elovl-like gene is elovl5.
Two diets formulated with 100% or 33% FO induced no adverse effects on growth performance, whole-body proximate composition, SGR or FCR of red sea bream. These results are consistent with previous studies showing the viability of partial replacement of less than 70% of dietary FO with canola oil in red sea bream [12]. The minimal level of 1.2% n-3 HUFA was sufficient for normal growth of juvenile red sea bream, when dietary lipid was 15% [24]. It was also found that about 0.75% n-3 HUFA and 0.03% AA was just adequate or marginal for rearing red sea bream [12]. It seems that the n-3 HUFA contents in the two diets meet the requirements of red sea bream.
In liver, DHA and EPA levels were significantly higher for both dietary treatments in the 15 and 20 ppt salinities. Red sea bream may have limited ability to convert α-linolenic acids into n-3 HUFAs, but this capacity was higher at lower salinity. Low salinity enhanced the percentage of some PUFAs such as 22:5n-3, 22:6n-3, and 20:4n-6 in sea mullet Mugil cephalus fry [25]. Similar findings were made in Atlantic salmon and rabbit fish Siganus canaliculatus [10, 26]. Therefore, it is suggested that lower water salinity enhances n-3 HUFA biosynthesis capability from short-chain n-3 PUFAs in red sea bream. Although 1.3–2.0-fold higher n-3 HUFA levels were detected in fish reared at 15 and 20 ppt, fatty acid desaturase expression was not detected in fish at normal salinity except for that fed the VO diet. This seemed to imply that the stimulation of fads6 expression at lower salinity occurs under the detection level.
It was observed that the interaction of dietary fatty acid and lower salinity stimulated fatty acid desaturation and elongation (Table 11). It was also demonstrated that fatty acid desaturase expression in liver was enhanced only in fish fed the VO diet at 15 ppt. Furthermore, fatty acid analysis revealed higher 20:5n-3 and 22:6n-3 in liver of fish fed the VO diet at 15 ppt. Because production of 22:6n-3 is mediated by Δ6 desaturase, it seems that lower salinity stimulates Δ6 desaturase expression in liver of fish fed the VO diet. Several reports have suggested the correlation of salinity changes and fatty acid composition changes of fish. The 22:6n-3 in phospholipids decreased in some teleosts upon exposure to higher salinity [27, 28]. It was suggested that n-3 fatty acid plays a role in ion regulation in gill [29, 30]. The detailed mechanism is unknown, but modulation of cell membrane fluidity by altering fatty acid composition seems to play a role in the osmotic response to environmental salinity changes. However, the total n-3 HUFA contents in the whole body of fish fed the VO diet at 15 ppt did not differ from the other groups. Salinity changes were also suggested to affect fatty acid transport. A substantial increase in plasma fatty acid during salinity changes suggested mobilization of fatty acid in fish body [31]. It was postulated that 20:5n-3, 22:6n-3, and total n-3 HUFA produced in the liver is quickly delivered and consumed in peripheral tissue, eventually resulting in a similar level of these n-3 HUFAs in the fish body among all the treatments.
Negative feedback of fads and elovl was reported when lower dietary n-3 HUFA stimulated fads and elovl expression in marine fish species such as nibe croaker, Atlantic salmon, and gilthead sea bream [11, 19, 23]. On the contrary, this study showed only higher expression of desaturase but not elongase in fish fed the VO diet, which contains lower 20:5n-3 and 22:6n-3 levels, at normal salinity. Expression of Δ6 desaturase genes in rabbit fish Siganus canaliculatus was always significantly greater in fish fed vegetable oil-based diet compared with fish fed FO-based diet [26]. Expression of the Δ6 desaturase gene peaked just prior to seawater transfer and was low in seawater [26]. This could be explained by interspecies differences in response to dietary fatty acids. It was reported that the capacity for conversion of 18:3n-3 to n-3 HUFA differs among several marine species [32, 33]. Similarly, it was reported that black sea bream Acanthopagrus schlegelii and European sea bass Dicentrarchus labrax are capable of converting linoleic acid to AA [34, 35]. This study shows that increasing levels of liver DHA and EPA at lower salinity of 15 ppt, and the promoting action of Δ6 desaturase gene expression on α-linolenic acid/linoleic acid, are stronger in red sea bream fed canola oil-enriched feed at 15 ppt. Further studies are required to clarify the mechanism of regulation of desaturase gene expression by dietary nutrition and salinity in red sea bream.
Both dietary fatty acids and salinity affect n-3 HUFA biosynthesis and are involved in regulation of fads6 gene expression in red sea bream. Red sea bream may synthesize n-3 HUFAs from vegetable oil at different salinities, and this capacity is stronger at low than at normal salinity. Dietary fatty acids and low salinity may stimulate fads6 gene activity and enhance DHA synthesis in the liver of red sea bream. Combined manipulation of dietary fatty acid and environmental salinity can potentially enhance the endogenous n-3 HUFA biosynthesis pathway in marine fish and may help to reduce use of fish oil in marine fish diets.
References
Kriton G (2010) Ethical issues in aquaculture production. J Agric Environ Ethics 23:345–370
Takeuchi T (2001) A review of feed development for early life stages of marine finfish in Japan. Aquaculture 200:203–222
Turchini GM, Torstensen BE, Ng WK (2009) Fish oil replacement in fin fish nutrition. Rev Aquac 1:10–57
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Academic, San Diego, pp 181–257
Tocher DR, Fonseca-Madrigal J, Bell JG, Dick JR, Henderson RJ, Sargent JR (2002) Effects of diets containing linseed oil on fatty acid desaturation and oxidation in hepatocytes and intestinal enterocytes in Atlantic salmon (Salmo salar). Fish Physiol Biochem 26:157–170
Zheng X, Tocher DR, Dickson CA, Bell JG, Teale AJ (2004) Effects of diets containing vegetable oil on expression of genes involved in highly unsaturated fatty acid biosynthesis in liver of Atlantic salmon (Salmo salar). Aquaculture 236:467–483
Watanabe T, Thongrod S, Takeuchi T, Satoh S, Kubota SS, Fujimaki Y, Cho CY (1989) Effect of dietary n-6 and n-3 fatty acids on growth, fatty acid composition and histological changes of white fish Coregonus lavaretus maraena. Nippon Suisan Gakkaishi 55:1977–1982
Ostrowski AC, Divakaran S (1990) Survival and bioconversion of n-3 fatty acids during early development of dolphin (Coryphaena hippurus) larvae fed oil-enriched rotifers. Aquaculture 89:273–285
Bell JG, Tocher DR, Farndale BM, Cox DI, McKinney RW, Sargent JR (1997) The effect of dietary lipid on polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr–smolt transformation. Lipids 32:515–525
Tocher DR, Bell JG, Henderson RJ, McGhee F, Mitchell D, Morris PC (2000) The effect of dietary linseed and rapeseed oils on polyunsaturated fatty acid metabolism in Atlantic salmon (Salmo salar) undergoing parr–smolt transformation. Fish Physiol Biochem 23:59–73
Zheng X, Torstensen BE, Tocher DR, Dick JR, Henderson RJ, Bell JG (2005) Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar). Biochim Biophys Acta 1734:13–24
Huang SSY, Oo AN, Higgs DA, Brauner CJ, Satoh S (2007) Effect of dietary canola oil level on the growth performance and fatty acid composition of juvenile red sea bream, Pagrus major. Aquaculture 271:420–431
Tamura M (1949) The influence of the changes of external medium upon the fishes X. The survival time, changes of body weight and the water contents of some organs of marine fish which are living in diluted sea water. Suisangakuzasshi 54:48–71 (in Japanese with English abstract)
Takeuchi T (1988) Laboratory work—chemical evaluation of dietary nutrients. In: Watanabe T (ed) Fish nutrition and mariculture. Kanagawa International Fisheries Training Centre, Japan International Cooperation Agency, Kanagawa, pp 179–226
Folch J, Lees M, Stanley SGH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Christie WW (1973) Lipid analysis. Pergamon Press, Oxford
Zheng X, Ding Z, Xu Y, Monroig O, Morais S, Tocher DR (2009) Physiological roles of fatty acyl desaturases and elongases in marine fish: characterization of cDNAs of fatty acyl ∆6 desaturase and elovl5 elongase of cobia (Rachycentron canadum). Aquaculture 290:122–131
Meyer A, Kirsch H, Domergue F, Abbadi A, Sperling P, Bauer J, Cirpus P, Zank TK, Moreau H, Roscoe TJ, Za¨hringer U, Heinz E (2004) Novel fatty acid elongases and their use for the reconstitution of docosahexaenoic acid biosynthesis. J Lipid Res 45:1899–1909
Yamamoto Y, Kabeya N, Takeuchi Y, Alimuddin, Haga Y, Satoh S, Takeuchi T, Yoshizaki G (2010) Cloning and nutritional regulation of polyunsaturated fatty acid desaturase and elongase of a marine teleost, the nibe croaker Nibea mitsukurii. Fish Sci 76: 463–472
Zheng X, Seiliez I, Hastings N, Tocher DR, Panserat S, Dickson CA, Bergot P, Teale AJ (2004) Characterization and comparison of fatty acyl ∆6 desaturase cDNAs from freshwater and marine teleost fish species. Comp Biochem Physiol 139B:269–279
Zheng X, Tocher DR, Dickson CA, Bell JG, Teale AJ (2005) Highly unsaturated fatty acid synthesis in vertebrates: new insights with the cloning and characterization of a ∆6 desaturase of Atlantic salmon. Lipids 40:13–24
Tocher DR, Zheng X, Schlechtriem C, Hastings N, Dick JR, Teale AJ (2006) Highly unsaturated fatty acid synthesis in marine fish: cloning, functional characterization, and nutritional regulation of fatty acyl ∆6 desaturase of Atlantic cod (Gadus morhua L.). Lipids 41:1003–1016
Seiliez I, Panserat S, Corraze G, Kaushik S, Bergot P (2003) Cloning and nutritional regulation of a Δ6-desaturase-like enzyme in the marine teleost gilthead sea bream (Sparus aurata). Comp Biochem Physiol 135B:449–460
Takeuchi T, Shiina Y, Watanabe T (1992) Suitable levels of n-3 highly unsaturated fatty acids in diet for fingerlings of red sea bream. Nippon Suisan Gakkaishi 58:509–514
Kheriji S, EI Cafi M, Masmudi W, Castall JD, Romdhane MS (2003) Salinity and temperature effects on the lipid composition of Mullet Sea fry (Mugil cephalus, Linne, 1758). Aquac Int 11:571–582
Li Y, Hu C, Zheng Y, Xia X, Xu W, Wang S, Chen W, Sun Z, Huang J (2008) The effects of dietary fatty acids on liver fatty acid composition and ∆6 desaturase expression differ with ambient salinities in Siganus canaliculatus. Comp Biochem Physiol 151B:183–190
Takeuchi T, Kang S-J, Watanabe T (1989) Effects of environmental salinity on lipid classes and fatty acid composition in gills of Atlantic salmon. Nippon Suisan Gakkaishi 55:1395–1405
Cordier M, Brichon G, Weber J-M, Zwingelstein G (2002) Changes in the fatty acid composition of phospholipids in tissues of farmed sea bass (Dicentrarchus labrax) during an annual cycle. Roles of environmental temperatures and salinity. Comp Biochem Physiol 133B:281–288
Di Costanzo G, Duportail G, Florents A, Leray C (1983) The brush border membrane of trout intestine: influence of its lipid composition on ion permiability, enzyme activity and membrane fluidity. Mol Physiol 4:279–290
Martinez-Alvarez RM, Sanz A, Garcia-Gallego M, Domezain A, Domezain J, Carmona R, del Valle Ostos-Garrido M, Morales AE (2005) Adaptive branchial mechanisms in the sturgeon Acipenser naccarii during acclimation to saltwater. Comp Biochem Physiol 141A:183–190
Jarvis PL, Ballantyne JS (2003) Metabolic responses to salinity acclimation in juvenile shortnose sturgeon Acipenser brevirostrum. Aquaculture 219:891–909
Kanazawa A, Teshima S, Ono K (1979) Relationship between essential fatty acid requirements of aquatic animals and the capacity for bioconversion of linolenic acid to highly unsaturated fatty acids. Comp Biochem Physiol 63B:295–298
Bell JG, Tocher DR, MacDonald FM, Sargent JR (1994) Effects of diets rich in linoleic (18:2n-6) and alpha-linolenic (18:3n-3) acids on the growth lipid class and fatty acid compositions and eicosanoid production in juvenile turbot (Scophthalmus maximus L.). Fish Physiol Biochem 13:105–118
Ji W (1999) The influence of different fat sources in feed on the growth rate of juvenile and fatty acid composition of body fat of black sea bream (Sparus Macrocephalus). Mar Fish Res 20:69–74
Asturiano JF, Sorbera LA, Zanuy S, Ramos J, Navarro JC, Bromage N (2001) Reproductive performance in male European sea bass (Dicentrarchus labrax L.) fed two PUFA-enriched experimental diets: a comparison with males fed a wet diet. Aquaculture 194:173–190
Acknowledgments
This study was financially supported by a Grant-in-Aid for Exploratory Research (19658080) to S.S. from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarker, M.AA., Yamamoto, Y., Haga, Y. et al. Influences of low salinity and dietary fatty acids on fatty acid composition and fatty acid desaturase and elongase expression in red sea bream Pagrus major . Fish Sci 77, 385–396 (2011). https://doi.org/10.1007/s12562-011-0342-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-011-0342-y