Abstract
The number of growth rings was counted on broken and burnt otoliths of eight Lethrinus spp. from two local populations: Lethrinus atkinsoni, L. harak, L. miniatus, L. nebulosus, L. obsoletus, L. ornatus, L. ravus, and L. rubrioperculatus, collected from 1985 to 1996 in waters off the Ryukyu Islands. Growth rings were revealed to be formed annually from about October to June by marginal analysis. In addition to obtaining the three parameters in von Bertalanffy growth equation and the maximum age in specimens, the relationship between age and both ovarian maturity rate and sex ratio (percentage female) was analyzed. The age at 50% of ovarian maturity was the lowest at 1–2 years old in L. harak, L. ravus, and L. rubrioperculatus and was the highest at approximately 4 years old in L. nebulosus. The age at which the sex ratio decreased to 50% due to sexual transition from female in protogynous hermaphrodite species was the lowest at 3–4 years old in the L. atkinsoni Okinawa population and was the highest at 7–8 years old in L. miniatus. The oldest maximum age for specimens was 26 years in L. nebulosus and the youngest maximum age was 12 years in L. ornatus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fish of genus Lethrinus have been utilized as significant food resources in areas from the western Pacific Ocean to the Indian Ocean because of their dominant catch amount [1]. The genus is a significant fish resource in the Ryukyu Islands, southwest of mainland Japan, as well. Because of their importance, the resources must be rationally utilized. For rational utilization of the fish resources, stock evaluation based on the age component in the catch is necessary. Accordingly, research to reveal the relationship between age and length, in other words “growth study,” is very important.
Growth studies of Lethrinus began in the mid-1960s [2] and the number has continued to increase [3–11]. During this period, many studies regarding age determination have revealed that using either a thin sectioned or broken and burnt otolith gives the best accuracy in all age groups, whereas ages determined from the whole otolith, scales, or other calcified tissues might result in a serious underestimation error, especially in older age groups [12–14]. In the case of Lethrinus species as well, the maximum ages determined from methods other than sectioned otoliths seem to be underestimates. For example, the oldest age determined from a sectioned otolith in Lethrinus nebulosus was 27 years [4], while that from a scale was 14 years [3], and those from vertebrae were 21 years [5] and 6 years [6]. Among the many growth studies in Lethrinus, those based on sectioned otoliths are limited [4, 8–11]. Almost all of the studies were limited to the southern hemisphere.
In Okinawa, the reproductive biology and sexual characteristics of eight commercially important Lethrinus species including two local populations were revealed based on body size [15–18]. The sexuality of the species is either protogynous hermaphroditism [16–20] or juvenile hermaphroditism [15, 17, 18], where sexual differentiation occurs before sexual maturation. As this trait is not species-dependent, the sexuality can differ among populations of the same species [17].
In the eight species, the main habitat of Lethrinus atkinsoni, L. harak, L. obsoletus, and L. ornatus is shallow coastal areas where different kinds of fishing gear are used for their capture, and that of L. miniatus, L. rubrioperculatus, and L. ravus is deeper offshore areas where mostly long-line fishing is utilized. The main habitat of L. nebulosus is wide and overlaps those of the other seven species. The abundance and the status of the stock differs from species to species [21–23]. In general, species inhabiting shallow coastal areas have suffered decline from various types of fishing gear, and species forming large schools have suffered from strong fishing pressure.
For stock management, stock analysis and stock simulation, which give us keys to choosing a management strategy, are employed [24–26]. These analyses are carried out based on age and age-related parameters, such as age at sexual maturation and sex ratio with age in the hermaphrodite species [27, 28]. Therefore, the age at sexual maturation and the age at sexual transition are to be clarified in parallel with the growth study.
According to earlier analyses [21–23], the rates of stock decreases in L. rubrioperculatus and in L. ravus in the waters of the Okinawa Island were relatively small, while the decline of L. miniatus in the same area was very large. The resilience of the species to fishing seems to be affected not only by the depth of the habitat, and whether or not it forms large schools, but also the species’ growth rate, longevity, age at sexual maturation, presence or absence of sexual transition, fertility, main habitat at juvenile stage, length of the period at buoyant fry, and so on. To compare and contrast biological parameters among species studied by the same methods by contrasting their stock status in the same area is meaningful. In addition, to elucidate similar and different biological parameters by comparing different populations of the same species is important for understanding the standardized ecological profile of the species. Developing a species profile is important for providing fundamental knowledge to areas where the resource is rationally to be utilized.
This research, using the same sources of specimens as earlier studies [16–18], reveals the growth and the maximum age using broken and burnt otoliths from two local populations of eight Lethrinus species: Lethrinus atkinsoni, L. harak, L. miniatus, L. nebulosus, L. obsoletus, L. ornatus, L. ravus, and L. rubrioperculatus. The timings of sexual transition and sexual maturation were re-analyzed based on age. By comparing the results among species within the Ryukyu Islands area, and within species among populations in the southern hemisphere, similar and different ecological profiles have been clarified.
Materials and methods
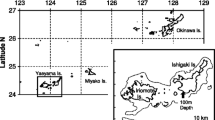
Sources of specimens
The scientific names in this study follow those used by Carpenter [29] and Carpenter and Randall [30]. Periods and locations of the samplings, the number of specimens of each of the eight species, and references describing reproductive and sexual characteristics using the same source of specimens are indicated in Table 1. Specimens of L. atkinsoni were sampled from both Okinawa and Yaeyama (Fig. 1), those of L. nebulosus, L. miniatus, L. rubrioperculatus, and L. ravus were sampled from Okinawa, and those of L. harak, L. obsoletus, and L. ornatus were sampled from Yaeyama. A total of 478 specimens of L. nebulosus, differing from those of Ebisawa [15], were newly collected from April 1994 to January 1996. Fork length (L f), standard length (L s), and body weight were measured to the nearest 0.1 cm and 1 g in each specimen. Gonads were examined histologically to determine the stage of development [15]. Otoliths were kept dry throughout this research.
Reading procedures
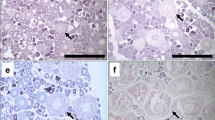
Each otolith was cut along the focus (Fig. 2a), burnt, and covered with oil [31], and then observed under reflected light with a microscope. On a sectioned burnt otolith, opaque bands and brownish translucent bands alternate (Fig. 2b). The brownish translucent band in the area of D1 is employed as the growth ring of the otolith. The number of growth rings was counted twice at separate times on the same otolith by the same reader, with the number at the first reading kept unknown at the second reading, which occurred 10–20 years after the first reading. Otoliths were digitally recorded (1,600 × 1,200 pixels, 24-bit color) at the time of the second reading with a PDMC-I.e Polaroid digital microscope camera. When the numbers of the two readings coincided, the number of growth rings on the otolith was determined. When the numbers did not coincide, two more additional readings by the same reader were carried out, with the previous numbers unknown to the reader. When the numbers of three readings among four agreed, the number of growth rings on the otolith was determined. In other cases, the otolith was excluded from the later analysis.
In order to determine the period and frequency of growth-ring formation, the following measurements were carried out using the otolith pictures. The outermost zone was judged to be a growth ring or not on the D1 area of the otolith. In some otoliths, the growth ring bifurcated and the opaque zone was formed at the extension of the original brownish band near the area of D1 (Fig. 2c). In this case, the condition of the outermost zone was impossible to determine. Therefore, for this type of bifurcated and reversed growth-ring otolith, areas D2, D3, and D4 were observed to determine the growth-ring numbers and the condition of the outermost zone. Widths of both the start of the outermost growth ring to the outer edge (X 0) and the start of the innermost growth ring to the end of the next opaque edge (X 1) were measured in the otolith picture (Fig. 2d). The marginal index was calculated as X 0 divided by X 1.
a Broken line of otolith. b Broken and burnt otolith of Lethrinus atkinsoni Yaeyama, sampled 25 April 1992, showing the 12th ring as the outermost. The specimen was female, had an L f of 31.1 cm, and weighed 649 g. Growth rings are shown with dots. c Broken and burnt otolith of Lethrinus nebulosus, sampled 21 May 1994, showing bifurcations of growth zones indicated by lines at area D1. Extensions of the original two growth zones were changed to opaque areas. The specimen was male, had an L f of 42.2 cm, and weighed 1,364 g. Growth rings are marked at areas D2 and D4. d Schematic diagram for the measurement of X0 and X1 in broken and burnt otoliths to calculate marginal index. Scale bars 1 mm
Determination of age for each individual
The birth month of the species was considered to be the start month of the spawning period (Table 1). The periodicity of growth-ring formation is described in the “Results” section; the growth rings are formed annually from about October to June. Hence, the birth months fall at the later period of the growth-ring formation. The integer part of the age is determined by the number of growth rings, and the order of birth and sampled months. The decimal part of the age is given by the fraction of a year from birth to sampled month.
Statistical analyses
Growth parameters in the von Bertalanffy growth equation are calculated using nonlinear regression (SPSS) for each age and L f of the specimens. Growth parameters are estimated combined with sex because most of the species are protogynous hermaphrodites. However, they are also estimated separately for each sex for juvenile hermaphroditic species, i.e., L. nebulosus [15], L. atkinsoni at Yaeyama [17], and L. obsoletus [18], where undifferentiated individuals are included as females and hermaphroditic individuals as males. Analysis of covariance [32] was conducted to examine whether the growth curves between sexes were significantly different.
Correspondence of sex and ovarian maturity with age
Mean L f and SD were calculated for each sex according to integer age group. Sex ratio (R S) was calculated as the number of females divided by the total number of females, hermaphrodites, and males in the same integer age group. Ovarian maturity rate (R OM) was calculated for each species from the histologically examined ovarian stages as the number of ovaries from the primary yolk globule stage to the ripe stage divided by the number of whole ovaries collected during the spawning period for each integer age group.
Results
Periods of growth-ring formation
To reveal the timing and periodicity of growth-ring formation, monthly changes in the rates of growth-ring-edged and opaque-band-edged otoliths are shown in Fig. 3. Though there were some temporal changes, the trends for the monthly changes were approximately the same in all eight species from two local populations. That is, almost all otoliths from January to April were growth-ring-edged ones. Opaque-band-edged otoliths began to appear from about May/June, and dominated in July to September/November. Growth-ring-edged otoliths began to appear in October and gradually increased. Thus, growth rings were determined to be formed once a year from October to June, i.e., autumn to spring.
Monthly changes in the rate of growth-ring-edged (filled square) and opaque-band-edged (open square) otoliths in a Lethrinus atkinsoni from Okinawa, b Lethrinus atkinsoni from Yaeyama, c Lethrinus harak, d Lethrinus miniatus, e Lethrinus nebulosus, f Lethrinus obsoletus, g Lethrinus ornatus, h Lethrinus ravus, and i Lethrinus rubrioperculatus
To show the relationship between the monthly changes of both the outermost condition and the marginal index of otoliths and the determination of age in each specimen, Fig. 4 for L. atkinsoni at Yaeyama is drawn as a representative of all eight species. The number of growth rings for groups with one to nine rings are drawn separately and those more than ten are combined as one group in Fig. 4. The changes in outermost conditions correspond to Fig. 3b.
Monthly changes in marginal index and outermost condition (filled circle growth ring, open circle outside of growth ring) in each age group from 1 to 9 and for 10 to 23 together in Lethrinus atkinsoni from Yaeyama. The crosses and lines indicate the average marginal index in each month. Arrows indicate the correspondence of the integer part of the age to the ring-number groups
The changes in the marginal indices of the otolith in each ring group were similar. Marginal indices increased gradually from the lowest in October at the first emergence of growth-ring-edged otoliths with average values of about 0.3 on both one- and two-ring groups, and to about 0.8 in the larger ring groups by the following September. In the area of D1 in the otolith, the number of growth rings is usually countable because of the alternate emergence of both growth rings and opaque bands, except for the type of otolith shown in Fig. 2c. The integer part of the age is given by the number of growth rings minus one for the specimens sampled before March, and by the total number of growth rings for those sampled after March (Fig. 4). All other specimens were aged in the same manner as that used for the L. atkinsoni Yaeyama population.
Growth parameters, mean size at age, sex ratio, and the maximum ages in specimens
The mean L f for each sex and the sex ratio are listed in Table 2, and plots of age and L f for all eight species are shown in Figs. 5 and 6. The mean L f at each age increased rapidly in the first few years, but had almost ceased by the latter half period in all eight species. The mean L f at each age in females was larger by the latter half period than that of males in L. atkinsoni and in L. harak, and it was smaller in L. miniatus and in L. ornatus.
The von Bertalanffy growth curves fitted to observed fork length (L f) and age of male (dotted line, filled triangle), female (broken line, open circle), and combined (bold line) in a Lethrinus atkinsoni from Yaeyama, b Lethrinus atkinsoni from Okinawa, c Lethrinus harak, d Lethrinus miniatus, e Lethrinus nebulosus, and f Lethrinus obsoletus
In the comparison of growth in juvenile hermaphroditic species, the results for both males and females were significantly different in L. atkinsoni Yaeyama (P < 0.01) and L. nebulosus (P < 0.05), whereas the differences were insignificant in L. obsoletus. All female specimens obtained were older than either zero or 1 year old, while all males were older than 2 years old except some specimens of 1-year-old males in L. nebulosus. The maximum ages of both males and females are summarized in Table 3. The species whose maximum ages in males or females exceeded more than 20 years of age were L. atkinsoni, L. miniatus, L. nebulosus, and L. obsoletus, and those less than 15 years of age were L. harak, L. ornatus, L. ravus, and L. rubrioperculatus.
Hermaphroditic individuals were obtained in the age range where obvious decreases in sex ratio (R s) were observed in most of the species. Beyond the range in which R s is obviously decreasing—between 1 (R s: 0.80) and 2 (R s: 0.62) years old—hermaphroditic specimens up to 4 years old were obtained in L. obsoletus. They were obtained only in the latter half of the period of decreasing R s in both L. ornatus and L. ravus, probably due to the relatively small number of specimens. Age when sex ratio had decreased to 50% (ARS50) was between 3 and 4 years old in L. atkinsoni Okinawa; about 4 years old in L. harak, L. ornatus, L. ravus, and L. rubrioperculatus; about 6 years old in L. miniatus; and 2 years old in L. nebulosus. ARS50 did not exist in L. atkinsoni Yaeyama or in L. obsoletus because sex ratios had not decreased to 50%. Growth parameters in the von Bertalanffy equations and the maximum age for specimens of the present research and those from previously reported studies are summarized in Table 3 and will compared below.
Ovarian maturity rate with age
The ovarian maturity rate in each age group (Table 4) indicates that only L. rubrioperculatus started to mature from 1 year old; the Okinawa and Yaeyama populations of L. atkinsoni, L. harak, L. obsoletus, L. ornatus, and L. ravus from 2 years old; and both L. minatus and L. nebulosus from 3 years old. Most females completed maturation in the following years. Therefore, age at 50% of ovarian maturity (AOM50) was not clear except for L. nebulosus (4 years old) and L. ornatus (2 years old).
Discussion
In the present study, a translucent zone was formed in the otoliths during autumn to spring. The number of translucent-margin L. miniatus otoliths at the Great Barrier Reef (GBR) has been shown to decrease from October to December [9]. The marginal index, taken by measuring the minimum value at the time of opaque band completion of the otolith, indicates constant growth from the minimum value in January/February to that in December in L. miniatus at the GBR [33]. Therefore, opaque-band formation (October to December: spring to early summer) in the GBR L. miniatus otolith approximately coincides with the annual cycles of the present results. Growth-ring formation of L. nebulosus and L. rubrioperculatus otoliths in New Caledonia (NC) [34] also coincides with the present results. However, in L. nebulosus of the northern GBR, marginal increment analysis of the whole otolith shows opaque zone formation during the winter period [35]. In many coral-reef species, opaque and translucent zones are formed during summer and winter, respectively [36]. There are some hypotheses regarding factors affecting growth-ring formation, including physiological change, growth-rate differences, and reproductive cycles, based on water temperature changes [36]. However, it is difficult to determine what factors control the timing of the different growth-ring formations in L. nebulosus in the northern GBR.
Growth was outstanding in the first few years, but was extremely slow in the remaining period in all eight species (Fig. 7). The longevities of L. miniatus and L. nebulosus, who belong to the large body-size group, were the longest at about 25 years, and that of the smallest species of L. ornatus was the shortest, approximately 12 years. However, the longevity of the third largest species of L. rubrioperculatus was 13 years, and those of the smaller species L. atkinsoni and L. obsoletus were approximately 25 and 20 years, respectively. Therefore, the longevity and the maximum body size of the species do not seem to be connected. AOM50 in L. nebulosus, the largest body-size species among juvenile hermaphrodites, was the highest, and that in L. miniatus, the largest body-size species among protogynous hermaphrodites was the second. Thus, larger body-size species were found to mature later in each sexual group.
Comparisons of growth curves in eight Lethrinus species from two local populations obtained in this study. Open diamonds indicate Lethrinus atkinsoni from Okinawa, open squares Lethrinus atkinsoni from Yaeyama, filled triangles Lethrinus harak, times signs Lethrinus miniatus, stars Lethrinus nebulosus, open circles Lethrinus obsoletus, plus signs Lethrinus ornatus, filled squares Lethrinus ravus, open triangles Lethrinus rubrioperculatus
In the protogynous hermaphrodite group, two types of sexual transition were observed. In one type, all females in the species changed to male; as such, no females appeared in the older age groups. In the other type, some portion of females in the species did not change sex and were present up to the oldest age group. All females in L. miniatus and in L. ravus changed to male, thus belonging to the former type. Though one female remained in the maximum age group in L. rubrioperculatus, that the second-oldest female was 6 years old suggests that a 13-year-old female might be the exceptional individual. Therefore, L. rubrioperculatus seems to belong to the former type. No female appeared in age classes older than 19 years in the Okinawa population of L. atkinsoni. However, considering the stable sex ratios at age classes higher than 7 years old and the small sample sizes in the higher age classes, females in the population seem to present in the oldest age class. Therefore, L. harak and the Okinawa population of L. atkinsoni belong to the latter type. The body sizes of old and sex-un-changed females in the latter type have a tendency to be larger than those of males in the same age groups. Categorization of L. ornatus to either of the two types was not possible due to the small sample sizes for the old age classes.
ARS50 of L. miniatus, which belongs to the large body-size group, was the highest, between 7 and 8 years of age. ARS50 of L. ornatus, the smallest species, and that of L. rubrioperculatus, the third largest body-size species, was 4 years old. ARS50 of the Okinawa population of L. atkinsoni, the fourth largest body-size species, was between 3 and 4 years of age. Except for the largest body-size species, ARS50 and the maximum body size of the species seem to be unrelated. In the juvenile hermaphrodite group, ARS50 existed only in L. nebulosus and not in L. obsoletus and the Yaeyama population of L. atkinsoni. Sex ratios in juvenile hermaphrodite species were stable after the age of sexual differentiation; as such, ARS50 seems to be meaningless for these species.
Thus far in the comparisons of biological parameters with age among different species in the Ryukyu Islands area, the longevities of Lethrinus species can be categorized into two groups of approximately 20–25 years and 12–15 years; the smaller body-size species do not always belong to the latter. AOM50 of larger body-size species were higher than those of smaller body-size species. In the protogynous hermaphroditic group, although ARS50 of the large body-size species was high, those of other species showed little relation to maximum body size of the species.
From the perspective of the usual habitat of the species, the complete disappearance of females in older age classes due to sexual transition was common in the offshore deeper-water group composed of L. miniatus, L. rubrioperculatus, and L. ravus. Due to the small body sizes, AOM50 were relatively low in the coastal shallow-water group composed of L. atkinsoni, L. harak, L. obsoletus, and L. ornatus. However, when L. nebulosus is included, those two common characteristics disappear because the species inhabits both coastal shallow and offshore deep areas, despite showing juvenile hermaphroditism with the highest AOM50. Thus, the biological characteristics related to age in the Lethrinus species appear to have less relation to the usual habitat of the species.
We compared our present results to the growth curves, the maximum age in specimens, ARS50, and AOM50 in the NC L. nebulosus, L. miniatus, L. atkinsoni, and L. rubrioperculatus [4, 37] and GBR (Brown and Sampton [9], GBR-BS; Sampton and Brown [38], GBR-SB; Williams et al. [39], GBR-W) and Norfolk Island (NI) [8] L. miniatus (Table 3). In L. atkinsoni in the NC population, although AOM50 was not indicated, the youngest mature female was 5 years old. Therefore, it appears that females in NC start to mature at a much later stage than do those in the two populations of the Ryukyu Islands. ARS50 probably does not exist because sex ratios are constant at about 80% in all length classes larger than 14 cm LS.
Because growth parameters of L. miniatus in the NC population are indicated separately by sex, Table 3 includes them separately and those of other areas combined. Extremely larger L∞ and smaller k were obtained in the NI population than those of other areas. The growth curve in the NI population shows relatively constant growth to the maximum age, whilst those of the other three areas attain near-maximum size at about age 10. However, the maximum size of about 66 cm LF in the specimens of NI is only slightly larger than those of the other three areas. The maximum ages in males were close to those at GBR-SB, NC, and Okinawa. The maximum ages of males were larger than those of females in all four populations, but the differences in age between males and females were large in NC and in Okinawa, but small in GBR and in NI. ARS50 is the largest between the 9 and 10–11 coupled age groups in NC, and is the smallest between 5 and 6 in the NI population.
AOM50 ranges from 2.1 years old in GBR-W to about 7 years old in GBR-SB. Age or size at ovarian maturation is affected by the definition of maturity employed. Williams et al. [39] concluded that the overestimation of age at maturity [38] was caused by the inadequate treatment of the “mature resting” and “immature” ovary. However, in all four studies, except that by Church [8], who did not indicate the age at maturity, ages of ovarian maturity were determined by using specimens collected only during their spawning periods. It is not plausible that such a difference in ages at maturity is caused only by the different treatment of the “mature resting” individuals. From this perspective, determining these parameters with age by the same methods in the species applied in this research is meaningful.
The maximum age in L. nebulosus found by Grandcourt et al. [11] is much smaller than those found in other studies. They concluded it that it was because of underestimation due to the absence of fish close to the maximum reported sizes in the region. Therefore, their study was excluded in the present discussion. In comparisons of L. nebulosus in the NC population, the maximum ages were very similar to the present results, while the AOM50 was not. If we consider the sizes of 50% ovarian maturation in NC being 51.3 cm Lf and 39.7 cm Lf in Okinawa [15], we see that both age and size for ovarian maturation in the species were considerably different between these areas.
L∞ in L. rubrioperculatus in the Okinawa population were larger than those in the NC population, however, the maximum ages in the two areas were not so different. ARS50 and AOM50 in Okinawa were slightly younger than those of NC.
In the studies of other NC Lethrinus species, only the maximum ages in L. harak and L. obsoletus were indicated. The maximum ages of L. harak in the NC population and in the Okinawa population were approximately the same. However, that of L. obsoletus at 14 years old in NC was much younger than that the 21 years old in Okinawa. This is probably due to the sample size of each specimen. In L. ravus and L. ornatus, which are species with smaller body sizes, ARS50 and AOM50 exhibited similar values, but there is no information available about a comparison with growth in other areas.
Upon comparison of the biological parameters for a particular species among the different areas, the maximum age of the specimens, which probably indicates the longevity of the species, exhibits similar values even in widely separated areas between the northern (Okinawa) and the southern (NC, GBR, NI) hemisphere, and their exploitation rates are probably different. In contrast, L∞ in the von Bertalanffy growth equation, or in other words “the maximum size” in the same species, differs from area to another. In addition, although ARS50 and AOM50 are often confined to a similar range, they occasionally differ considerably from area to area.
Stock status in Lethrinus species differs from species to species in the Ryukyu Islands area. These differences in status can be observed in severely decreased rates due to strong fishing pressures, as well as in the occurrence of different rates even in species suffering the same levels of fishing pressure in the same habitat [21–23]. For the rational use of these resources, careful evaluations of stock status and investigations of management strategy are necessary. Based on these analyses, some restrictive fisheries have been enacted for L. nebulosus [40] in the northwest area of Okinawa Island and for L. atkinsoni [41] in the Yaeyama Islands. Effective management strategies must be established through careful stock assessments and evaluations based on age structure for hermaphrodite species in tropical waters.
References
Carpenter KE, Allen GR (1989) FAO species catalogue, vol 9. Emperor fishes and large-eye breams of the world (family Lethrinidae). FAO Fisheries Synopsis 7:125
Toor HH (1964) Biology and fishery of the pig-face bream, Lethrinus lentjan Lacepede. III. Age and growth. Indian J Fish 11:597–620
Aldonov UK, Druzhinin AD (1978) Some data on scavengers (family Lethrinidae) from the Gulf of Aden region. J Ichthyol 18:527–535
Loubens G (1980) Biologie de quelques espèces de Poissons du lagon Néo-Calédonien. III. Croissance. Cah Indo-Pac 2:101–153
Edwards RRC, Bakhader A, Shaher S (1985) Growth, mortality, age composition and fisheries yields of fish from the Gulf of Aden. J Fish Biol 27:13–21
Kuo CL, Lee SS (1986) Age and growth of common porgy, Lethrinus nebulosus (Forsskal), in shelf waters off northern Australia. J Fish Soc Taiwan 13:22–31
Ezzat AA, El-Sayed AM, Al-Dossary NAM (1992) Growth of fish Lethrinus nebulosus from the Arabian Gulf waters off Damman (Saudi Arabia). Indian J Mar Sci 21:284–286
Church AD (1995) Ecology of the Norfolk Island domestic fishery. PhD Dissertation, University of New South Wales, New South Wales
Brown IW, Sumpton WD (1998) Age, growth and mortality of redthroat emperor Lethrinus miniatus (Pisces: Lethrinidae) from the southern Great Barrier Reef. Bull Mar Sci 62:905–917
Williams AJ, Davies CR, Mapstone BD, Russ GR (2003) Scales of spatial variation in demography of a large coral reef fish: an exception to the typical model? Fish Bull 101:673–683
Grandcourt EM, Al-Abdessalaam TZ, Al-Shamsi AT, Francis F (2006) Biology and assessment of the painted sweetlips (Diagramma pictum (Thumberg, 1792)) and the spangled emperor (Lethrinus nebulosus (Forsskål, 1775)) in the southern Arabian Gulf. Fish Bull 104:75–88
Beamish RJ, Chilton DE (1982) Preliminary evaluation of a method to determine the age of Sablefish (Anoplopoma fimbria). Can J Fish Aquat Sci 39:277–287
Beamish RJ, McFarlane GA (1987) Current trends in age determination methodology. In: Summerfelt RC, Hall GE (eds) Age and growth of fish. Iowa State University Press, Ames, pp 15–42
Reñones O, Piñeiro C, Mas X, Goñi R (2007) Age and growth of the dusky grouper Epinephelus marginatus (Lowe 1834) in an exploited population of the western Mediterranean Sea. J Fish Biol 71:346–362
Ebisawa A (1990) Reproductive biology of Lethrinus nebulosus (Pisces: Lethrinidae) around the Okinawan waters. Nippon Suisan Gakkaishi 56:1941–1954
Ebisawa A (1997) Some aspects of reproduction and sexuality in the spotcheek emperor, Lethrinus rubrioperculatus, in waters off the Ryukyu Islands. Ichthyol Res 44:201–212
Ebisawa A (1999) Reproductive and sexual characteristics in the Pacific yellowtail emperor, Lethrinus atkinsoni, in waters off the Ryukyu Islands. Ichthyol Res 46:341–358
Ebisawa A (2006) Reproductive and sexual characteristics in five Lethrinus species in waters off the Ryukyu Islands. Ichthyol Res 53:269–280
Young PC, Martine RB (1982) Evidence for protogynous hermaphroditism in some lethrinid fishes. J Fish Biol 21:475–484
Bean K, Mapstone BD, Davies CR, Murchie CD, Williams AJ (2003) Gonad development and evidence of protogyny in the red-throat emperor on the Great Barrier Reef. J Fish Biol 62:299–310
Ebisawa A (1995) Hooakakuchibi no seibutsujyouhou to sigenjyoutai. (Biological parameters and stock condition of spotcheek emperor Lethrinus rubrioperculatus). Committee for Promoting Agriculture, Forestry, and Fishery Research in Okinawa Prefecture
Ebisawa A (1995) Amifuefuki no seibtsujyouhou to sigenjyoutai. (Biological parameters and stock condition of drab emperor Lethrinus sp). Committee for Promoting Agriculture, Forestry, and Fishery Research in Okinawa Prefecture
Ebisawa A (1995) Amamifuefuki no seibutsujyouhou to sigenjyoutai (Biological parameters and stock condition of trumpet emperor Lethrinus miniatus). Committee for Promoting Agriculture, Forestry, and Fishery Research in Okinawa Prefecture
Obata Y, Takimoto A, Iwamoto A, Kitada S (2007) A simulation model for enhancement and management strategies of fisheries resources: a case study of Japanese Spanish mackerel Scomberomorus nipconius in the eastern Seto Inland Sea. Nippon Suisan Gakkaishi 73:43–50
Ebisawa A (2001) Hamafuefuki no sigenkanri (Stock management of Spangled emperor, Lethrinus nebulosus). Annu Rep Okinawa Fish Exp Sta Fiscal 1999 61:81–86
Ebisawa A (2001) Ryukyu rettou kaiiki ni okeru Sujiara no shigen jyoutai (Stock evaluations of Coral trout Plectropomus leopardus at the waters off the Ryukyu Islands). Annu Rep Okinawa Fish Exp Sta Fiscal 1999 61:73–80
Bannerot S, Fox WW, Powers JE (1987) Reproductive strategies and the management of snapper and groupers in the Gulf of Mexico and Caribbean. In: Polovina J, Ralston S (eds) Tropical snapper and groupers: biology and fisheries management. Westview Press, Colorado
Ebisawa A (2004) Yaeyama kaiiki ni okeru Isofuefuki no sigen kannri kouka. (Stock management effect of Pacific yellowtail emperor, Lethrinus atkinsoni fishery at the waters off Yaeyama Islands). Annu Rep Okinawa Fish Exp Sta Fiscal 2002 64:115–122
Carpenter KE (2001) Lethrinidae. Emperor (emperor snappers). In: Carpenter KE, Niem VH. (eds). FAO species identification guide for fisheries purposes. The living marine resources of the western central Pacific, vol 5. Bony fishes part 3 (Menidae to Pomacentrida). FAO, Rome, pp 3004–3050
Carpenter KE, Randall JE (2003) Lethrinus ravus, a new species of emperor fish (Perciformes: Lethrinidae) from the western Pacific and eastern Indian oceans. Zootaxa 240:1–8
Christensen JM (1964) Burning of otoliths, a technique for age determination of soles and other fish. J Cons Int Explor Mer 29:73–81
Snedecor GW, Cochran WG (1980) Statistical methods. Iowa State University Press, Iowa
Williams AJ, Davies CR, Mapstone BD (2005) Variation in the periodicity and timing of increment formation in red throat emperor (Lethrinus nimiatus) otoliths. Mar Freshwater Res 5:529–538
Loubens G (1978) Biologie de quelque espèces de poisson du lagon néo-calédonien. I. Détermination de 1’ âge (otolithométrie). Cah ORSTOM Sér Océanogr 16:263–283
McPherson G, Squire L, O’Brien J (1988) Demersal reef fish project 1984–1985. Age and growth of four important reef fish species. Report to Great Barrier Reef Marin Park Authority, Townsville
Fowler AJ (1995) Annulus formation in otolith of coral reef fish–a review. In: Secor DH, Dean JM, Campana SE (eds) Recent development in fish otolith research. University of South Carolina Press, Columbia, pp 45–63
Loubens G (1980) Biologie de quelques espèces de Poissons du lagon Néo-Calédonien. II. Sexualité et reproduction. Cah Indo-Pac 2:41–72
Sampton W, Brown I (2004) Reproductive biology of the red throat emperor Lethrinus miniatus (Pisces: Lethrinidae) from the southern Great Barrier Reef, Australia. Bull Mar Sci 74:423–432
Williams AJ, Davies CR, Mapstone BD (2006) Regional patterns in reproductive biology of Lethrinus miniatus on the Great Barrier Reef. Mar Freshwater Res 57:403–414
Ebisawa A (2007) Okinawa-shima hokubu kaiiki ni settei sareta Hamafuefuki hogoku no hogo-kouka. (Effects of MPA placed for the young stage of spangled emperor, Lethrinus nebulosus at the waters off northwest Okinawa Island). Annu Rep Okinawa Fish Ocean Res Cent Fiscal 2006 68:107–119
Ebisawa A (2002) Dentou-moguri no sigen kanri. (Stock managements of night-spire-fishing). Annu Rep Okinawa Fish Exp Sta Fiscal 2000 62:106–115
Acknowledgments
A.E. greatly appreciates the directors and co-workers of ONPFORC who have encouraged his long-term research into Lethrinus. The authors thank M. Kulbicki for the significant information about the references by G. Loubens. This research was partly supported by both “The Research Project of Fisheries Resources within Japan’s 200-Mile Zone” conducted by Japan Fisheries Agencies and the “Nagura Bay Sanctuary Research Survey.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ebisawa, A., Ozawa, T. Life-history traits of eight Lethrinus species from two local populations in waters off the Ryukyu Islands. Fish Sci 75, 553–566 (2009). https://doi.org/10.1007/s12562-009-0061-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-009-0061-9