Abstract
Blueberry and blueberry extracts are known for their health benefits and antimicrobial properties. Natural therapeutic or preventive options to decrease the incidences of foodborne viral illnesses are becoming popular and being researched. This study aimed to determine the antiviral effects of blueberry juice (BJ) and blueberry proanthocyanidins (BB-PAC, B-type PAC structurally different from A-type PAC found in cranberries) against the infectivity of hepatitis A virus (HAV) and human norovirus surrogates (feline calicivirus (FCV-F9) and murine norovirus (MNV-1)) at 37 °C over 24 h using standard plaque assays. Viruses at ~5 log PFU/ml were mixed with equal volumes of BJ (pH 2.8), neutralized BJ (pH 7.0), BB-PAC (1, 2, 4, and 10 mg/ml), malic acid (pH 3.0), or phosphate-buffered saline (pH 7.2) and incubated over 24 h at 37 °C. Each experiment was carried out in duplicate and replicated thrice. FCV-F9 titers were found to be reduced to undetectable levels with 1 and 2 mg/ml BB-PAC after 5 min, with 0.5 mg/ml BB-PAC after 1-h, and with BJ after 3-h. MNV-1 titers were reduced to undetectable levels after 3 h with 1, 2, and 5 mg/ml BB-PAC and after 6 h with BJ. HAV titers were reduced to undetectable levels after 30 min with 2 and 5 mg/ml BB-PAC, after 3 h with 1 mg/ml BB-PAC, and by ~2 log PFU/ml with BJ after 24-h. BB-PAC shows preventive potential against infection by the tested enteric viruses in a dose- and time-dependent manner, although further in vitro studies in model food systems and in vivo studies using animal models are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Foodborne pathogens in the United States lead to an estimated 9.4 million illnesses, 55,961 hospitalizations, and 1,351 deaths of which foodborne viruses such as human norovirus alone are responsible for 5.5 million illnesses (58 %) per year (Scallan et al. 2011). Recent reports indicate that this number has risen to 21 million illnesses per year (Hall et al. 2013). Foodborne viruses are now recognized all over the world as public health concerns and therefore effective natural alternatives to control their spread are being researched. This is important for the treatment or potential alleviation of illness symptoms and prevention of disease in the current absence of available vaccines for human noroviruses. The antiviral effects of grape seed extract (GSE), that has high phenolic content, against human norovirus surrogates, FCV-F9 and MNV-1, and hepatitis A virus (HAV) at 37 °C for 2 h has been reported (Su and D’Souza 2011). Cranberry juice that is rich in polyphenols and its proanthocyanidins (PAC) were also reported to cause reduction of human norovirus surrogates (Su, Howell, and D’Souza 2010a). However, cranberries are rich in PACs with A-type linkages (A-type PAC) that account for 51–91 % of total PACs (Blumberg et al. 2013) that differ from B-type PAC present in blueberries. B-type PACs can be converted to A-type PAC by radical oxidation (Kondo et al. 2000). The structural difference between A-type and B-type PAC is noteworthy, as it can influence their biological activity (Blumberg et al. 2013). The A-type PACs exhibit significantly greater inhibition of adhesion of P-fimbriated Escherichia coli to uroepithelial cells in vitro than the B-type PACs, which is the initial step in the development of urinary tract infection (UTI) (Howell et al. 1998). However, the effects of B-type PACs such as those found in blueberries against foodborne viruses need to be explored.
Blueberries are reported to contain around 88–261 mg of proanthocyanidin/100 g of edible portion as per the USDA database for flavonoid content (USDA Database for the proanthocyanidin Content of Selected Foods, August 2004). In addition to PACs, blueberries also contain other structurally related polyphenols, including anthocyanins and flavonoids (Huang et al. 2012). Blueberries and its polyphenols have been evaluated for their potential health benefits including their anticarcinogenic, neuroprotective, cardioprotective, antibacterial, and antiviral properties (Zafra-Stone et al. 2007; Heinonen 2007; Hou 2003; Basu et al. 2010; Elks et al. 2011; Joshi et al. 2014). Blueberry extracts at a concentration of 0.25 % are known to have antimicrobial activity and are reported to cause 50.5 % inhibition of Helicobacter pylori, the bacterium associated with ulcers (Chatterjee et al. 2004). Water and ethanol extracts of blueberries at 24 ppm were reported reduce Listeria monocytogenes by 5.90 log CFU/ml after 24 h at 37 °C in vitro (Park et al. 2011). Also, monomeric phenolics acids (0.4 g/L gallic acid) from blueberries were shown to cause 5 log CFU/ml reduction of E. coli O157:H7 along with cell-membrane damage after 24 h at 37 °C in vitro (Lacombe et al. 2013). Additionally, using the HCV replicon cell system, the methanol extract fraction of blueberry leaves (0.112–2200 μg/ml) was shown to inhibit hepatitis C (HCV) virus subgenomic expression after 72 h at 37 °C (Takeshita et al. 2009). Hydrolysable and galloylated tannin (0.03–0.1 μg/ml) was shown to inhibit the adsorption of herpes simplex virus to its hosts, African green monkey kidney cells and human adenocarcinoma cells at 37 °C (Fukuchi et al. 1989). Human norovirus surrogates such as feline calicivirus (FCV-F9) and murine norovirus (MNV-1) have been studied for survival in commercial blueberry juice (BJ) at 4 °C, where FCV-F9 was undetectable after 24 h from initial 5 log PFU/ml, whereas MNV-1 showed minimal reduction after 21 days at 4 °C (Horm et al., 2010).
Therefore, the objective of this study was to determine the effect of BJ and blueberry proanthocyanidins (BB-PAC) on the infectivity of HAV and human norovirus surrogates (FCV-F9 and MNV-1) at 37 °C over a period of up to 24 h using standardized plaque assays. The main rationale for this study was to understand the role of blueberry extracts and BJ in the prevention or alleviation of gastrointestinal disease caused by human enteric viruses, particularly human noroviruses and HAV. Thus, the study was carried out at 37 °C for over 24 h to determine the dose and time needed for enteric viral reduction to nondetection levels at body temperature.
Materials and Methods
Viruses and Cell Lines
Feline calicivirus (FCV-F9) and its host, Crandell Rees Feline Kidney (CRFK) cells were purchased from ATCC (ATCC identifying number VR-2057, Manassas, VA). Murine norovirus (MNV-1) was kindly gifted by Dr. Skip Virgin (Washington Univ., St. Louis, MO) and RAW 264.7 cells were obtained from the University of Tennessee at Knoxville. Hepatitis A virus (HAV; strain HM175) and fetal rhesus monkey kidney (FRhK4) cells were provided by our collaborator, Dr. Kalmia Kniel (University of Delaware). As described before, all three host cell lines were maintained using Dulbecco’s Modified Eagle’s Medium/Ham’s F-12 (DMEM-F12; HyClone Laboratories, Logan, UT) containing 10 % heat-inactivated fetal bovine serum (FBS, HyClone Laboratories) and 1× Anti–Anti (Antibiotic–Antimycotic, Invitrogen, Grand Island, NY) at 37 °C in an atmosphere containing 5 % CO2 (Su and D’Souza 2011; Joshi et al. 2015).
Propagation of Viruses
Previously described methods were used to prepare stocks of FCV-F9, MNV-1, and HAV, by inoculation of their respective host cell lines when 90 % confluent followed by incubation for 2, 6, and 8 days, respectively under 5 % CO2 at 37 °C (Su and D’Souza 2011). Briefly, the viruses were harvested by 1 to 3 freeze thaw cycles followed by centrifugation at 5,000×g for 10 min. The resulting supernatant was filtered through a 0.2 μm filter, aliquoted, stored at –80 °C until further use and titers of the recovered viral stocks were determined using standard reported plaque assays as described earlier (Su and D’Souza 2011; Joshi et al. 2015).
Isolation of PAC From Blueberries
Proanthocyanidins were isolated by Dr. Amy Howell (Marucci Center for Blueberry and Cranberry Research, Rutgers University, Chatsworth, NJ) from frozen highbush blueberry fruit (Vaccinium corymbosum L.) using solid-phase chromatography according to a well-established method for proanthocyanidin isolation (Howell et al. 2005). Briefly, blueberry fruit was homogenized with 70 % aqueous acetone, filtered, and the pulp discarded. The collected extract was concentrated under reduced pressure to remove acetone. The blueberry extract was suspended in water, applied to a preconditioned C-18 solid-phase chromatography column and washed with water to remove sugars, followed by acidified aqueous methanol to remove acids. The fats and waxes retained on the C-18 sorbent were discarded. The polyphenolic fraction containing anthocyanins, flavonol glycosides, and PAC (confirmed using reverse phase HPLC with diode array detection) was eluted with 100 % methanol and dried under reduced pressure. This fraction was suspended in 50 % ethanol, applied to a preconditioned Sephadex LH-20 column, which was washed with 50 % ethanol to remove low molecular weight anthocyanins and flavonol glycosides. PAC adsorbed to the LH-20 were eluted from the column with 70 % aqueous acetone, and monitored using diode array detection at 280 nm. The absence of absorption at 360–450 nm confirmed that anthocyanins and flavonol glycosides were removed. Acetone was removed under reduced pressure and the resulting purified proanthocyanidin extract freeze dried. Electrospray mass spectrometry, 13C NMR, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and acid catalyzed degradation with phloroglucinol have all been utilized to confirm the purity of PAC present in the extract obtained using this method (Howell et al. 2005).
Effect of BJ and BB-PAC on Host Cells Lines
Cytopathic effects of BB-PAC, BJ (pH 2.8) and NBJ (neutralized BJ; pH 7.0) treatments on the host cell lines were also determined. The maximum concentration of BB-PAC used to treat the virus directly in suspension was 5 mg/ml. However, after subsequent 10-fold dilution of the virus-treated mix in cell-culture media containing 10 % FBS to stop the reaction, the host cell lines are typically exposed to only this dilution of the treatment (with 0.5 mg/ml as the maximum concentration of BB-PAC) during the plaque assay to determine viral infectivity. Thus, each individual confluent host cell line in 6-well plates were exposed to 0.5 mg/ml BB-PAC and BJ and NBJ for 2 h at 37 °C and observed under an optical microscope (Fisher Scientific, Pittsburgh, PA; 120 VAC) to determine visible cytopathic effects or changes in cellular morphology. Ethanol at 0.5 % concentration (as this would be the maximum concentration the host would be exposed to after viral treatment with BB-PAC) did not show any visible effect on the host cells.
Antiviral Effect of BJ and BB-PAC
Commercial BJ (cocktail with grape and apple juice; preservatives listed include malic acid and sodium citrate; the polyphenol content is not provided, however literature states 88–261 mg proanthocyanidin/100 g edible blueberry) was purchased from local grocery stores and BB-PAC was fractionated by Dr. Amy Howell (as described above) and kindly gifted for this work. BB-PAC was dissolved in 10 % ethanol and filter sterilized through 0.2 micron filters to obtain a stock of 10 mg/ml. Equal volumes of each virus at a titer of ~5 log PFU/ml was individually mixed with BJ (pH 2.8), BB-PAC (1, 2, 4, and 10 mg/ml), malic acid (10 mM, pH 3.0), neutralized BJ (pH 7 using 4 M sodium hydroxide, NaOH), 10 % ethanol (control; as 10 mg/ml BB-PAC stock was prepared in 10 % ethanol and then subsequently diluted in sterile water to get lower working stock solutions) or phosphate-buffered saline (PBS; 7.2 as control), and incubated at 37 °C for 5 (0.08 h), 15 (0.25 h), 30 (0.5 h), 60 (1 h), 120 (2 h), 180 (3 h), 360 min (6 h), or 24 h. Treatments were stopped using initial serial dilutions of the treated virus with cell-culture media containing 10 % heat-inactivated FBS, followed by dilutions in cell-culture media containing 2 % FBS. The infectivity of viruses was evaluated using standard plaque assays as described before (D’Souza and Su 2010; Su and D’Souza 2011; Joshi et al. 2015). Each experiment was replicated thrice and assayed in duplicate.
Effect of BB-PAC on Viral Adsorption and Replication
To determine the effect of BB-PAC on viral replication, host CRFK, RAW 264.7, and FRhK4 cells in 6-well plates were first infected with FCV-F9, MNV-1, and HAV, respectively for 2 h, viruses were aspirated, followed by treatment with BB-PAC (0.5 mg/ml) for 20 min at 37 °C, the BB-PAC solution was aspirated and then overlaid with media as in previously reported plaque assays (Su and D’Souza 2011). This 0.5 mg/ml BB-PAC concentration was the highest amount that could be used directly on the host cell without causing any cytopathic effect on the host cells. This is the limitation of this assay as higher concentrations cannot be directly used to determine viral adsorption or replication inhibition by this assay. To determine effects on viral adsorption/binding, media was aspirated and the host cells were pretreated with 500 µl of BB-PAC (0.5 mg/ml) for 20 min, followed by aspiration of the unadsorbed BB-PAC and subsequent viral infection for 2 h at 37 °C, and aspiration of media. Cells were then overlaid with complete cell-culture media containing 0.75 or 1 % agarose. After incubation for 2 to 8 days at 37 °C under 5 % CO2, a second overlay containing neutral red was added followed by incubation at 37 °C under 5 % CO2 until visualization of plaques and plaques were counted and recorded as plaque-forming units (PFU/ml) (Su, Howell, and D’Souza 2010a; Su and D’Souza, 2011).
Statistical Analysis
Statistical analysis was carried out using ANOVA with SAS software (version 9.3, SAS Institute, Cary, NC, USA) and Tukey’s test with data obtained from the three replications for each individual treated virus and compared to control untreated virus assayed in duplicate as described in previous studies (Su and D’Souza, 2011; Su and D’Souza 2013; Su et al., 2010a; Su, Howell, and D’Souza 2010b; Joshi et al. 2015).
Results
Effect of BB-PAC and BJ on Host Cells Lines
BB-PAC at a concentration of 0.5 mg/ml was not found to be cytotoxic to the tested host cell lines. Undiluted BJ when added directly to the host cells had some cytopathic effects after 2 h as visualized under the inverted light microscope causing some sloughing/peeling of the cell monolayer. However, neutralized BJ (NBJ) did not show any morphological visual changes in the host cells and was hence used in further time of addition experiments. In the experiment involving direct treatment of viruses with BB-PAC, the treated viruses are first 10-fold serially diluted and then added to the host cells. Thus, the host cells are exposed to a maximum of 20-fold diluted BB-PAC and that did not result in any cytopathic effect on the host cells as observed under the light microscope.
Reduction of Viral Titers by BB-PAC and BJ
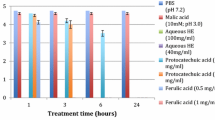
All three tested viruses were found to be susceptible to the BB-PAC treatment carried out at 37 °C. FCV-F9 at initial titers of ~5 log PFU/ml were reduced to undetectable levels (to <2 log PFU/ml per detection limit of the assay) after 5 min with 1, 2, and 5 mg/ml BB-PAC, and to undetectable levels (≥3 log PFU/ml reduction) after 60 min with 0.5 mg/ml BB-PAC. After 5 min, lower concentration of 0.5 mg/ml BB-PAC reduced FCV-F9 titers by only 1.33 log PFU/ml and after 30 min by 2.19 log PFU/ml (Table 1). Since, reduction to nondetectable levels occurred after 60 min with the lowest tested concentration of 0.5 mg/ml BB-PAC, further/longer BB-PAC treatment times of 3, 6, and 24 h for FCV-F9 were not carried out.
MNV-1 was less sensitive to BB-PAC treatments compared to FCV-F9, where MNV-1 at initial titers of ~5 log PFU/ml were reduced to undetectable levels only after 3 h with 1, 2, and 5 mg/ml of BB-PAC. Lower concentration of 0.5 mg/ml BB-PAC caused MNV-1 reductions of 1.47 log PFU/ml after 3 h and caused slightly higher reductions of 1.83 log PFU/ml after 6 h. (Table 2). Since, reduction of MNV-1 to nondetectable levels were not obtained with the lower 0.5 mg/ml BB-PAC concentration after even 3 h, further studies with BB-PAC treatment for MNV-1 until 6 h that mimic time for digestion at 37 °C was carried out.
HAV titers were reduced to undetectable levels after 30 min with 2 and 5 mg/ml BB-PAC, whereas 1 mg/ml BB-PAC reduced HAV titers by 1.71 log PFU/ml after 30 min and to undetectable levels only after 3 h (Table 3). Lower concentration of 0.5 mg/ml BB-PAC caused minimal reductions of 0.55, 0.79 and 0.69 log PFU/ml in HAV titers after 1, 3, and 6 h at 37 °C, respectively. This showed that increasing the time of treatment did not have improved effects against HAV for the lower concentration of 0.5 mg/BB-PAC.
Commercial BJ did not have much effect on reducing the viral titers after shorter contact times, but the effect did increase over time. Among the three tested viruses, FCV-F9 was the most sensitive, where BJ reduced FCV-F9 titers to undetectable levels after 3 h (Table 4). However, BJ did not have a significant effect on MNV-1 and HAV after 3 h, hence further treatment for longer times up to 24 h to mimic slow digestion were carried out, but were not needed for FCV-F9 that were reduced to nondetectable levels after 3 h with BJ. MNV-1 titers were reduced to undetectable levels only after 24 h with BJ while HAV was reduced by 1.86 log PFU/ml after 24 h with BJ (Table 5,6). Both, malic acid (pH 3.0, control) and NBJ (pH 7.0) did not have any significant effect in reducing the titers of all the three viruses, compared to non-neutralized BJ (pH 2.8) that showed ~2–3 log PFU/ml reduction after 24 h. Thus, the reduction cannot be attributed solely to pH effects, indicating that BJ bioactives contributed to the reduction. The actual levels of BB-PAC in the commercial BJ are not known, though 88–261 mg of proanthocyanidin/100 g of edible portion of blueberries are reported in literature (USDA database for proanthocyanidin Content of Selected Foods, August 2004). The possibility that the variety, season, and processing approaches could ultimately affect the levels of BB-PAC in prepared BJs cannot be ruled out, which could result in different bioactivity levels.
Effect of BB-PAC and BJ on Viral Adsorption and Viral Replication
Treatment of host cells with 0.5 mg/ml BB-PAC before viral infection reduced the titers of FCV-F9, MNV-1, and HAV by 0.63, 0.54, and 0.34 log PFU/ml, respectively (Table 7A). When the cells were treated with 0.5 mg/ml BB-PAC postviral infection, reductions of 0.38, 0.23, and 0.03 log PFU/ml were obtained for FCV-F9, MNV-1, and HAV, respectively (Table 7B). These results suggest that BB-PAC at 0.5 mg/ml plays a modest role in the prevention of virus attachment to the host and on viral adsorption rather than on inhibiting replication. Due to the assay limitation where higher BB-PAC concentrations showed cytopathic effects upon direct contact with the host cells, further studies with concentrations higher than 0.5 mg/ml BB-PAC could not be undertaken.
Discussion
BB-PAC and BJ were found to be effective in reducing the titers of all the three tested viruses. BB-PAC showed dose and time-dependence effects against the three tested viruses where higher concentration and longer time had the greatest antiviral effects especially for MNV-1 and HAV. Between the two tested human norovirus surrogates, FCV-F9 was found to be the more sensitive surrogate to treatments than MNV-1 and particularly sensitive to lower pH conditions (Cannon et al. 2006). This is not surprising as similar patterns are reported when comparing FCV-F9 and MNV-1 using chemical treatments such as trisodium phosphate (TSP) and plant-derived extracts such as grape seed extract, GSE (Su and D’Souza 2011; Su, Sangster, and D’Souza 2010c). Previous studies have also shown that MNV-1 is the sturdier of the surrogates when exposed to extreme heat and pH (Cannon et al. 2006). HAV was found to be moderately sensitive to BB-PAC treatment where complete reduction was achieved with 2 and 5 mg/ml BB-PAC after 30 min. HAV is known to be highly stable in the environment and can retain infectivity for long periods of time, and can survive in mineral water stored at room temperature for up to 300 days (Biziagos et al. 1988). It is also resistant to extreme acidic pH, where it was shown to survive in acidic marinade at pH 3.75 over 4 weeks and at pH 1.0 even after 5 h (Hewitt and Greening 2004; Scholz et al. 1989). Thus, the observed antiviral effect of BB-PAC against HAV is quite noteworthy.
Bioactive compounds such as gallic acid, epicatechin, tannins, and PAC found in berry fruits are known to be effective antimicrobial agents (Khurana et al. 2013; Bahadoran et al. 2013; Li et al. 2013). Polyphenols from fruits such as pomegranate, cranberry, grapes (seed), and other plant extracts have been shown to possess antiviral properties including against human norovirus surrogates (Su et al., 2010a; Su et al., 2010d; Oh et al. 2012). However, as mentioned in the introduction, the phenolic composition and amounts vary between the various fruit types, where blueberries contain mostly B-type PAC while cranberries mainly contain A-type PAC.
For comparison of effects, FCV-F9 was found to be undetectable after 1 day in BJ as well as in orange and pomegranate juice blend at 4 °C (Horm et al. 2012; Horm and D’Souza 2011). MNV-1 was reported to be completely reduced after 7 days in the orange and pomegranate juice blend, however only 1 log PFU/ml reduction of MNV-1 was observed in BJ at 4 °C (Horm et al. 2012; Horm and D’Souza, 2011). Black raspberry juice at 6 % was found to reduce MNV-1 plaque formation by 99 % after 1 h at 37 °C (Oh et al. 2012). However in the current study, BJ treatment needed longer contact time to achieve similar reduction, which could be attributed to the fact that black raspberry juice was freshly made by hand, and might have a higher concentration of polyphenols with different composition, whereas commercially available BJ was used in our study. This study aimed at primarily determining the effects of commercially available juices and BB-PAC from blueberries as potential preventive and therapeutic options against enteric viral infections. However, the effects of the food matrix, gastrointestinal conditions, immune system, and other factors need to be considered and results of feeding studies and appropriate regulatory approval need to be obtained before any recommendations can be made. Grape seed extract (GSE), cranberry PAC, and pomegranate polyphenols (PP) have been reported for their antiviral properties against FCV-F9 and MNV-1 (Su and D’Souza 2011; Su et al., 2010a, Su et al., 2010d). FCV-F9 and MNV-1 were reported to be reduced to undetectable levels and by 2.95 log PFU/ml, respectively with 0.6 mg/ml cranberry PAC after 1 h at RT (Su et al., 2010a; b). GSE at 0.5 and 1 mg/ml were found to reduce FCV-F9 titers to undetectable levels within 15 min at 37 °C and RT, and MNV-1 titers by ~1 log PFU/ml after 1 h at 37 °C and RT (Su and D’Souza 2011). FCV-F9 and MNV-1 were also reported to be sensitive to PP treatments at RT, where PP at 2 mg/ml decreased FCV-F9 titers to undetectable levels after 30 min and MNV-1 titers were reduced by 0.9 log PFU/ml after 60 min (Su et al., 2010d). In this study, BB-PAC at 2 mg/ml reduced FCV-F9 titers to undetectable levels within 5 min and MNV-1 by 1.65 log PFU/ml after 60 min at 37 °C. When comparing these polyphenol extracts for their antiviral activity against FCV-F9 and MNV-1, their effect follows the order of cranberry PAC > GSE > BB-PAC > PP.
In comparison to human norovirus surrogates, fewer studies are reported in literature on the antiviral effects of natural extracts against HAV. In a previous study, GSE at 1 mg/ml was shown to reduce HAV titers from initial 5 log PFU/ml by 2.89 log PFU/ml after 2 h at 37 °C (Su and D’Souza 2011). HAV titers at initial 5.74 log PFU/ml were also shown to be decreased by 0.66 log PFU/ml when treated with 10 μg/mL Korean red ginseng, and decreased by 0.37 log PFU/ml when treated with 10 μg/mL purified ginsenoside extract after 24 h at 37 °C (Lee et al. 2013). BB-PAC treatment, although at a higher concentration, was found to be effective in reducing HAV titers where 2 mg/ml BB-PAC caused reduction to undetectable levels within 30 min at 37 °C.
Neutralized BJ did not have any significant effect in reducing the titers of the tested viruses. As PACs are known to be stable under acidic conditions, increasing the pH can change the structure of PAC, thereby potentially changing its bioactivity. Moreover, the malic acid (pH 3.0) pH control by itself alone also did not significantly contribute to the viral titer reduction. It could be speculated that both the bioactive components of BJ and the acidic pH together contributed towards the antiviral activity. Bioactive components of BJ were not individually analyzed for antiviral activity in this study.
Extensive research is being carried out to study the mode of action of these polyphenols and their interaction with the viruses and their host cells. Researchers have proposed several theories as to the effects of plant polyphenols in vitro. Lingonberry PACs were reported to have an effect on host penetration by herpes simplex virus-2 (HSV-2) without much effect on viral attachment (Cheng et al. 2005). In another study with HSV-2, PAC from an African resurrection plant was found to inhibit both virus attachment and penetration (Gescher et al. 2011). Black raspberry juice was shown to be effective in reducing plaque formation of MNV-1 with pretreatment of host cells (effect on attachment) and after infection of host cells by treated viruses (Oh et al. 2012). However, these researchers showed that post treatment of cells after infection did not have any significant effect suggesting that the antiviral activity occurred at the point of host cell attachment or entry and not after infection or replication (Oh et al. 2012).
In this study, BB-PAC was shown to have limited effects in preventing viral adsorption to the host cells, with even lesser effects on viral replication (based on the limitation of the assay that cannot directly test higher concentrations of BB-PAC on the host cells compared to the concentrations ranging from 0.5 to 5 mg/ml BB-PAC used directly to inactivate the virus in suspension). The limited prevention of viral adsorption (relatively low amount of reduction) could be attributed to binding of the BB-PAC to the host cell receptors or alternately to the virus itself and causing disruption of the virus capsid. As minimal viral reduction was obtained after infection of the host cells (postinfection) with the BB-PAC treatment, it appears that BB-PAC does not play any significant role in inhibiting viral replication. These findings are in agreement with the study conducted with PE (persimmon extract) where no significant effect pre- or postinfection was reported and that the maximal antiviral activity was obtained by direct treatment of the virus particles (Ueda et al. 2013). Similar findings were reported for ginsenoside extract (5 μg/mL) treatment against HAV, where a very low reduction in titer was reported when the host cells were pretreated with the extract (Lee et al. 2013). Similar results with GSE on viral adsorption of FCV-F9, MNV-1, and HAV were previously reported, with less effect on replication (Su and D’Souza 2011). However, PAC from blueberry leaves was shown to have a pronounced effect on replication of the enveloped hepatitis C (HCV) virus by inhibiting expression of NS-3 gene (nonstructural gene-3), along with blocking of nuclear ribonucleoprotein essential for subgenomic replication (Takeshita et al. 2009). Thus, the mechanism of action seems to differ depending on the virus and the source and type of the PAC.
It is well recognized that the effectiveness of antimicrobials can decrease in the presence complex food matrices where the components of foods such as lipids, proteins, or other acidic or alkaline conditions may interfere with the antiviral properties. Hence, along with studying the effect of these blueberry polyphenols on the viral infectivity in vitro, it is also crucial to further investigate their effectiveness in presence of food matrices and under simulated gastric conditions for use as potential antiviral therapeutics. Some studies have been conducted with plant extracts (hibiscus) in milk and apple juice against E. coli and Staphylococcus aureus where antimicrobial activity was shown to decrease in foods with higher lipid and protein load (Higginbotham et al. 2014). A similar study was carried out using black tea and tea in milk against the oral pathogen Streptococcus mutans, where reduced antimicrobial activity of tea made in milk was reported (Abd Allah et al. 2012). This decrease in activity was attributed to the complex formation of milk proteins and tea polyphenols and subsequent decrease in the bioavailability of polyphenols. Therefore, future studies are aimed to determine the antiviral effects of BB-PAC in food systems such as milk (high in lipids) and apple juice, and also simulated gastric conditions. Furthermore, animal feeding studies with blueberries and BB-PAC using pre- and postchallenge should also be carried out to determine antiviral effects against enteric viruses.
Overall, this study determined the ability of BJ and BB-PAC to decrease human norovirus surrogate virus and HAV titers in a dose and time-dependent manner. Thus, they show potential as preventive or therapeutic options against viral gastrointestinal illness caused by these enteric viruses.
References
Abd Allah, A. A. M. I. I., Ab Allah, S. M., & Amin, M. A. (2012). Antimicrobial effect of tea and tea with milk beverages on oral Streptococcus mutans and Lactobacilli. World Applied Sciences Journal, 19, 1327–1334.
Bahadoran, Z., Mirmiran, P., & Azizi, F. (2013). Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. Journal of Diabetes and Metabolic Disorders, 12, 43.
Basu, A., Du, M., Leyva, M. J., Sanchez, K., Betts, N. M., Wu, M., et al. (2010). Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. The Journal of Nutrition, 140, 1582–1587.
Biziagos, E., Passagot, J., Crance, J. M., & Deloince, R. (1988). Long-term survival of hepatitis A virus and poliovirus type 1 in mineral water. Applied and Environmental Microbiology, 54, 2705–2710.
Blumberg, J. B., Camesano, T. A., Cassidy, A., Kris-Etherton, P., Howell, A., Manach, C., et al. (2013). Cranberries and their bioactive constituents in human health. Advances in Nutrition, 4, 618–632.
Cannon, J. L., Papafragkou, E., Park, G. W., Osborne, J., Jaykus, L. A., & Vinje, J. (2006). Surrogates for the study of norovirus stability and inactivation in the environment: a comparison of murine norovirus and feline calicivirus. Journal of Food Protection, 69, 2761–2765.
Chatterjee, A., Yasmin, T., Bagchi, D., & Stohs, S. J. (2004). Inhibition of Helicobacter pylori in vitro by various berry extracts, with enhanced susceptibility to clarithromycin. Molecular and Cellular Biochemistry, 265, 19–26.
Cheng, H. Y., Lin, T. C., Yang, C.-M., Shieh, D. E., & Lin, C.-C. (2005). In vitro anti-HSV-2 activity and mechanism of action of proanthocyanidin A-1 from Vaccinium vitis-idaea. Journal of the Science of Food and Agriculture, 85, 10–15.
D’Souza, D. H., & Su, X. (2010). Efficacy of chemical treatments against murine norovirus, feline calicivirus, and MS2 bacteriophage. Foodborne pathogens and disease, 7, 319–326.
Elks, C. M., Reed, S. D., Mariappan, N., Shukitt-Hale, B., Joseph, J. A., Ingram, D. K., & Francis, J. (2011). A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress. PLoS ONE, 6, e24028.
Fukuchi, K., Sakagami, H., Okuda, T., Hatano, T., Tanuma, S., Kitajima, K., et al. (1989). Inhibition of herpes simplex virus infection by tannins and related compounds. Antiviral Research, 11, 285–297.
Gescher, K., Kuhn, J., Lorentzen, E., Hafezi, W., Derksen, A., Deters, A., & Hensel, A. (2011). Proanthocyanidin-enriched extract from Myrothamnus flabellifolia Welw. exerts antiviral activity against herpes simplex virus type 1 by inhibition of viral adsorption and penetration. Journal of Ethnopharmacology, 134, 468–474.
Hall, A. J., Lopman, B. A., Payne, D. C., Patel, M. M., Gastanaduy, P. A., Vinje, J., & Parashar, U. D. (2013). Norovirus disease in the United States. Emerging Infectious Diseases, 19, 1198–1205.
Heinonen, M. (2007). Antioxidant activity and antimicrobial effect of berry phenolics–a finnish perspective. Molecular Nutrition & Food Research, 51, 684–691.
Hewitt, J., & Greening, G. E. (2004). Survival and persistence of norovirus, hepatitis A virus, and feline calicivirus in marinated mussels. Journal of Food Protection, 67, 1743–1750.
Higginbotham, K. L., Burris, K. P., Zivanovic, S., Davidson, P. M., & Stewart, C. N, Jr. (2014). Antimicrobial activity of Hibiscus sabdariffa aqueous extracts against Escherichia coli O157:H7 and Staphylococcus aureus in a microbiological medium and milk of various fat concentrations. Journal of Food Protection, 77, 262–268.
Horm, K. M., Davidson, P. M., Harte, F. M., & D’Souza, D. H. (2012). Survival and inactivation of human norovirus surrogates in blueberry juice by high-pressure homogenization. Foodborne pathogens and disease, 9, 974–979.
Horm, K. M., & D’Souza, D. H. (2011). Survival of human norovirus surrogates in milk, orange, and pomegranate juice, and juice blends at refrigeration (4 degrees C). Food Microbiology, 28, 1054–1061.
Hou, D. X. (2003). Potential mechanisms of cancer chemoprevention by anthocyanins. Current Molecular Medicine, 3, 149–159.
Howell, A. B., Reed, J. D., Krueger, C. G., Winterbottom, R., Cunningham, D. G., & Leahy, M. (2005). A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry, 66, 2281–2291.
Howell, A. B., Vorsa, N., Der Marderosian, A., & Foo, L. Y. (1998). Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. The New England Journal of Medicine, 339, 1085–1086.
Huang, W. Y., Zhang, H. C., Liu, W. X., & Li, C. Y. (2012). Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. Journal of Zhejiang University. Science. B, 13, 94–102.
Joshi, S. S., Dice, L., & D’Souza, D. H. (2015). Aqueous extracts of Hibiscus sabdariffa calyces decrease hepatitis A virus and human norovirus surrogate titers. Food and Environmental Virology, 7(4), 366–373. doi:10.1007/s12560-015-9209-1.
Joshi, S. S., Howell, A. B., & D’Souza, D. H. (2014). Cronobacter sakazakii reduction by blueberry proanthocyanidins. Food Microbiology, 39, 127–131.
Khurana, S., Venkataraman, K., Hollingsworth, A., Piche, M., & Tai, T. C. (2013). Polyphenols: benefits to the cardiovascular system in health and in aging. Nutrients, 5, 3779–3827.
Kondo, K. M., Kurihawa, K., Fukuhara, T., Tanaka, T., Suzuki, T., Miyata, N., & Toyoda, M. (2000). Conversion of procyanidin B-type (catechin dimer) to A-type: evidence for abstraction of C-2 hydrogen in catechin during radical oxidation. Tetrahedron Letters, 41, 485–488.
Lacombe, A., Tadepalli, S., Hwang, C. A., & Wu, V. C. (2013). Phytochemicals in lowbush wild blueberry inactivate Escherichia coli O157:H7 by damaging its cell membrane. Foodborne Pathogens and Disease, 10, 944–950.
Lee, M. H., Lee, B. H., Lee, S., & Choi, C. (2013). Reduction of hepatitis A virus on FRhK-4 cells treated with Korean red ginseng extract and ginsenosides. Journal of Food Science, 78, M1412–M1415.
Li, D., Baert, L., & Uyttendaele, M. (2013). Inactivation of food-borne viruses using natural biochemical substances. Food Microbiology, 35, 1–9.
Oh, M., Bae, S. Y., Lee, J. H., Cho, K. J., Kim, K. H., & Chung, M. S. (2012). Antiviral effects of black raspberry (Rubus coreanus) juice on foodborne viral surrogates. Foodborne Pathogens and Disease, 9, 915–921.
Park, Y. J., Biswas, R., Phillips, R. D., & Chen, J. (2011). Antibacterial activities of blueberry and muscadine phenolic extracts. Journal of Food Science, 76, M101–M105.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Diseases, 17, 7–15.
Scholz, E., Heinricy, U., & Flehmig, B. (1989). Acid stability of hepatitis A virus. The Journal of General Virology, 70(9), 2481–2485.
Su, X., & D’Souza, D. H. (2011). Grape seed extract for control of human enteric viruses. Applied and Environmental Microbiology, 77, 3982–3987.
Su, X., & D’Souza, D. H. (2013). Grape seed extract for foodborne virus reduction on produce. Food Microbiology, 34, 1–6.
Su, X., Howell, A. B., & D’Souza, D. H. (2010a). The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiology, 27, 535–540.
Su, X., Howell, A. B., & D’Souza, D. H. (2010b). Antiviral effects of cranberry juice and cranberry proanthocyanidins on foodborne viral surrogates–a time dependence study in vitro. Food Microbiology, 27, 985–991.
Su, X., Sangster, M. Y., & D’Souza, D. H. (2010c). In vitro effects of pomegranate juice and pomegranate polyphenols on foodborne viral surrogates. Foodborne Pathogens and Disease, 7, 1473–1479.
Takeshita, M., Ishida, Y., Akamatsu, E., Ohmori, Y., Sudoh, M., Uto, H., et al. (2009). Proanthocyanidin from blueberry leaves suppresses expression of subgenomic hepatitis C virus RNA. The Journal of biological chemistry, 284, 21165–21176.
Ueda, K., Kawabata, R., Irie, T., Nakai, Y., Tohya, Y., & Sakaguchi, T. (2013). Inactivation of pathogenic viruses by plant-derived tannins: strong effects of extracts from persimmon (Diospyros kaki) on a broad range of viruses. PLoS ONE, 8, e55343.
Zafra-Stone, S., Yasmin, T., Bagchi, M., Chatterjee, A., Vinson, J. A., & Bagchi, D. (2007). Berry anthocyanins as novel antioxidants in human health and disease prevention. Molecular Nutrition & Food Research, 51, 675–683.
Acknowledgments
The support provided by OceanSpray Cranberries Inc., and the University of Tennessee-Institute of Agriculture (TEN #00391) to carry out this work is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joshi, S.S., Howell, A.B. & D’Souza, D.H. Reduction of Enteric Viruses by Blueberry Juice and Blueberry Proanthocyanidins. Food Environ Virol 8, 235–243 (2016). https://doi.org/10.1007/s12560-016-9247-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-016-9247-3