Abstract
Naturally occurring plant-derived flavonoids are reported to have antibacterial, antiviral, and pharmacological activities. The objectives of this study were to determine the antiviral effects of four flavonoids (myricetin, l-epicatechin, tangeretin, and naringenin) on the infectivity of food borne norovirus surrogates after 2 h at 37 °C. The lab-culturable surrogates, feline calicivirus (FCV-F9) at titers of ~7 log10 PFU/ml (high titer) or ~5 log10 PFU/ml (low titer) and murine norovirus (MNV-1) at ~5 log10 PFU/ml, were mixed with equal volumes of myricetin, l-epicatechin, tangeretin, or naringenin at concentrations of 0.5 or 1 mM, and incubated for 2 h at 37 °C. Treatments of viruses were neutralized in cell culture medium containing 10 % heat-inactivated fetal bovine serum, serially diluted, and plaque assayed. Each treatment was replicated thrice and assayed in duplicate. FCV-F9 (low titer) was not found to be reduced by tangeretin or naringenin, but was reduced to undetectable levels by myricetin at both concentrations. Low titer FCV-F9 was also decreased by 1.40 log10 PFU/ml with l-epicatechin at 0.5 mM. FCV-F9 at high titers was decreased by 3.17 and 0.72 log10 PFU/ml with myricetin and l-epicatechin at 0.5 mM, and 1.73 log10 PFU/ml with myricetin at 0.25 mM, respectively. However, MNV-1 showed no significant inactivation by the four tested treatments. The antiviral effects of the tested flavonoids are dependent on the virus type, titer, and dose. Further research will focus on understanding the antiviral mechanism of myricetin and l-epicatechin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human noroviruses are now recognized as the leading causes of viral gastroenteritis outbreaks worldwide (Mead et al. 1999; Siebenga et al. 2009; Scallan et al. 2011). They have low infectious doses of 10–100 viral particles and are found to be resistant to environmental degradation and chemical inactivation (Cheesbrough et al. 2000; Kuusi et al. 2002). Transmission of human noroviruses has been shown to be via person-to person, food-borne, and water-borne routes. Human norovirus infections are typically self-limiting. However, newly emergent strains are known to be highly virulent and can be life-threatening to the elderly and immuno-compromised (Siebenga et al. 2009). Currently, no effective medication is available to prevent these infections. It therefore remains challenging and interesting to explore natural remedies for their treatment and prevention.
Various naturally occurring substances are known to have antimicrobial properties, among which plant flavonoids seem to be promising antivirals (Cushnie and Lamb 2005; Jassim and Naji 2003). Flavonoids are plant pigments that are composed of a large group of polyphenolic compounds which are characterized by a benzo-y-pyrone structure. Flavonoids are widely present in edible plants, such as fruits, tea leaves, vegetables, and medicinal herbs (El Gharras 2009). They are known to have antioxidant, antimicrobial, anticancer, anti-inflammatory activities, to list just a few of their associated health benefits (Yao et al. 2004). Research on the use of natural antimicrobials and naturally occurring flavonoids is becoming increasingly popular in the food industry. Their antiviral activities have been demonstrated against human immuno-deficiency virus-1, herpes simplex virus, influenza A virus, human rotavirus, etc. (Cushnie and Lamb 2005; Jassim and Naji 2003). Recently, the extracts from cranberry and pomegranates were reported to have antiviral activities against the human norovirus surrogates, feline calicivirus (FCV-F9), murine norovirus (MNV-1), and bacteriophage MS2 (Su et al. 2010a, b).
Along these lines of natural therapeutics and natural antivirals, the effect of flavonoids that naturally occur in citrus fruits, such as myricetin, l-epicatechin, tangeretin, and naringenin (El Gharras 2009), were examined against human norovirus surrogates in this study. Each of these four flavonoids at concentrations of 0.5 and 1 mM was mixed with equal volumes of FCV-F9 at titers of ~7 log10 PFU/ml (high titer) or ~5 log10 PFU/ml (low titer), or MNV-1 at ~5 log10 PFU/ml, and incubated at 37 °C for 2 h. The viral titers were evaluated using standard plaque assays, and differences between treated and untreated viral titers were determined. The effects of these flavonoids on the replication and binding of FCV-F9 and MNV-1 to their respective host cell lines were also evaluated.
Materials and Methods
Viruses, Hosts and Cell Lines
FCV-F9 and Crandell Reese Feline Kidney (CRFK) cells were purchased from ATCC (Manassas, VA, USA). MNV-1 was provided as a gift by Dr. Skip Virgin (Washington University, St. Louis, MO, USA), and RAW 264.7 cells were obtained from the University of Tennessee at Knoxville (Knoxville, TN, USA).
Propagation of Viruses
CRFK and RAW 264.7 cells were grown in cell culture flasks at 37 °C in a CO2 incubator with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) and 1X Antibiotic–Antimycotic solution. Confluent CRFK and RAW 264.7 cells were inoculated with FCV-F9 and MNV-1 respectively, and incubated until > 90 % cell lysis. Then, the media containing infected cells were freeze-thawed thrice for MNV-1 and once for FCV-F9 and centrifuged at room temperature for 10 min at 5,000 x g. Finally, the supernatants were passed through 0.2 μm membrane filters, aliquoted, and stored at -80 °C until use.
Infectious Plaque Assays
For MNV-1, the plaque assay, as previously described, was followed with minor modifications (D’Souza and Su 2010; Wobus et al. 2004). Confluent RAW 264.7 cells were inoculated with serial dilutions of viral samples and incubated for 2 h at 37 °C in a CO2 incubator. Then, inocula were aspirated and cells were overlaid with 2 ml of complete DMEM medium containing 0.75 % agarose. Plates were incubated for 3 days at 37 °C and the cells were stained with neutral red. Plaques were counted 3 h post staining at 37 °C.
FCV-F9 plaque assays were similar to MNV-1 plaque assays as described earlier (D’Souza et al. 2006). Serial dilutions of FCV-F9 in DMEM containing 2 % fetal bovine serum were added to confluent CRFK cells and incubated for 2 h at 37 °C in a CO2 incubator. After aspiration of media, cells were then overlaid with complete DMEM medium containing 0.75 % agarose. Plates were incubated for 2 days at 37 °C and overlaid with a second overlay media containing neutral red. Plaques were counted after overnight incubation at 37 °C.
Antiviral Effects of Myricetin, l-Epicatechin, Tangeretin, and Naringenin
Myricetin, l-epicatechin, tangeretin, and naringenin were commercially obtained from Fisher Scientific (Pittsburgh, USA) and prepared by dissolving in 200 proof ethanol to a concentration of 5 mM, filter-sterilized and further diluted aseptically in sterile distilled deionized water to 1 mM (which is equivalent to 0.032 % (w/v) for myricetin, 0.029 % (w/v) for l-epicatechin, 0.037 % (w/v) for tangeretin, and 0.027 % (w/v) for naringenin). Myricetin, l-epicatechin, tangeretin, and naringenin at 1 mM were then diluted in 20 % ethanol to make 0.5 mM solution (0.016 % (w/v) for myricetin, 0.015 % (w/v) for l-epicatechin, 0.019 % (w/v) for tangeretin, and 0.014 % (w/v) for naringenin). FCV-F9 at titers of ~7 log10 PFU/ml (high titer) or ~5 log10 PFU/ml (low titer) and MNV-1 at ~5 log10 PFU/ml were prepared in phosphate buffered saline (PBS, pH 7.2) and mixed with equal volumes of myricetin, l-epicatechin, tangeretin, or naringenin at concentrations of 0.5 mM and 1 mM (to reach final concentrations of 0.25 and 0.5 mM), and incubated for 2 h at 37 °C. The viruses treated with sterile distilled deionized water containing 20 % of ethanol were used as controls. Treatments were neutralized in cell culture medium containing 10 % heat-inactivated fetal bovine serum (FBS). The organic components in fetal bovine serum containing cell culture medium are known to neutralize chemical treatments. All treatments were run in triplicates. The infectivity of viruses was evaluated in duplicates using standard plaque assays.
To study if the tested four flavonoids have an effect on viral adsorption and viral replication of FCV-F9 and MNV-1, confluent CRFK and RAW 264.7 cells were treated with PBS, myricetin, l-epicatechin, tangeretin, and naringenin at 0.25 and 0.5 mM (in PBS) for 1 h before (adsorption effects) or post-viral (replication effects) infection. The viral infectivity before and after treatment was determined by plaque assays, as described above.
Cytotoxicity Effect of Myricetin, l-Epicatechin, Tangeretin, and Naringenin on CRFK and RAW 264.7
Myricetin, l-epicatechin, tangeretin, and naringenin at concentrations of 0.2–1.0 mM were each added to individual wells of confluent CRFK or RAW 264.7 cells in 6-well plates and incubated for 2 h. The solution was then aspirated, and the cells were overlaid with complete DMEM containing 0.75 % agar and incubated further for 2–3 days. Cytopathic effects were determined by both visual inspection under the optical light microscope and neutral red staining.
Statistical Analysis
Statistical analysis was carried out using ANOVA (Regal 1986) with SAS software (version 9.2, SAS Institute, Cary, NC, USA) and Tukey’s test (Ramachandran 1956) on a completely randomized design with six sets of data for each treatment condition.
Results and Discussion
The effects of four naturally occurring plant flavonoids, myricetin, l-epicatechin, tangeretin, and naringenin (at final concentrations of 0.5 and 0.25 mM after mixing), on human norovirus surrogates, FCV-F9 and MNV-1, at 37 °C for 2 h using standard plaque assays are summarized in Table 1.
Of the four tested flavonoids, myricetin was found to be the most effective against both high titer (~7 log10 PFU/ml) and low titer (~5 log10 PFU/ml) FCV-F9. FCV-F9 at low titer was reduced to undetectable levels after treatment for 2 h with myricetin at both concentrations of 0.25 and 0.5 mM. High titer FCV-F9 was reduced by 1.73 and 3.17 log10 PFU/ml with 0.25 and 0.5 mM myricetin, respectively. On the other hand, l-epicatechin at 0.25 and 0.5 mM reduced high titer FCV-F9 by merely 0.18 and 0.72 log10 PFU/ml; and low titer FCV-F9 by 0.33 and 1.40 log10 PFU/ml, respectively. However, reductions of ≤ 1 log10 do not have any significant/practical applications for the food industry. Tangeretin and naringenin at both tested concentrations of 0.25 and 0.5 mM did not cause any significant inactivation of both high and low titer FCV-F9 (p > 0.05).
Myricetin, l-epicatechin, tangeretin, and naringenin at 0.25 mM did not show any measurable inactivation of low titer MNV-1 after 2 h incubation at 37 °C. However, at 0.5 mM concentration, only myricetin and l-epicatechin showed negligible low titer MNV-1 reduction of 0.22 log10PFU/ml and 0.27 log10 PFU/ml, respectively. Tangeretin and naringenin even at 0.5 mM did not show any measurable effect against low titer MNV-1. As the antiviral effects of the four tested flavonoids on low titer MNV-1 were found to be minimum, their effect against high titer MNV-1 was not explored any further.
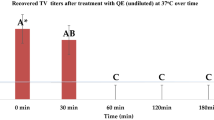
The effect of myricetin, l-epicatechin, tangeretin, and naringenin at two concentrations (0.25 and 0.5 mM) on the viral adsorption and replication of FCV-F9 and MNV-1 was also studied. However, since the four flavonoids at 0.5 mM appeared to cause cytotoxic effects on the CRFK cells as determined by visual inspection and neutral red staining, treatments with 0.25 mM flavonoids were carried out and the results are reported in Fig. 1.
To determine if the four flavonoids have any effect on viral adsorption, myricetin, l-epicatechin, tangeretin, and naringenin at 0.25 mM were each added to confluent CRFK cells or RAW 264.7 cells and incubated for 1 h, before infection with FCV-F9 and MNV-1 for 2 h. Among the four flavonoids, 0.25 mM myricetin showed insignificant minor measurable effects (0.2 log10 PFU/ml) on the adsorption/binding of FCV-F9 to the host cells. No measurable effect of the four flavonoids was observed against adsorption/binding of MNV-1 to the RAW 264.7 host cells (Fig. 1 a).
Treating the cells with 0.25 mM myricetin, l-epicatechin, tangeretin, and naringenin post viral infection was carried out to obtain information regarding their effects on viral replication. As shown in Fig. 1 b, all the four flavonoids did not show any significant effect on the replication of FCV-F9 or MNV-1.
Flavonoids from various sources have been shown to have antiviral effects against viruses that cause gastroenteritis. Proanthocyanidins (PAC) extracted from cranberries at 0.03 % were found to decrease titers of human norovirus surrogates, FCV-F9 and MNV-1, as well as MS2 and ϕ-X174 bacteriophages by 5.0, 2.8, 0.8, and 3.7 log10 PFU/ml, respectively, from initial viral titers of ~5 log10 PFU/ml after 1 h incubation at room temperature (Su et al. 2010a). Grape seed extract at 0.025 % was shown to decrease titers of FCV-F9, MNV-1, hepatitis A virus, and bacteriophage MS2 by 5.0, 1.5, 1.9, and 1.4 log10 PFU/ml, respectively, from initial viral titers of ~5 log10 PFU/ml after 2 h incubation at 37 °C (Su and D’Souza 2011). Thus, at similar concentrations (~0.03 %), cranberry extract and grape seed extract showed greater antiviral activities against human norovirus surrogates than myricetin, l-epicatechin, tangeretin, and naringenin. Flavonoids also have been reported to have antiviral activity against rotaviruses (Debiaggi et al. 1990; Kwon et al. 2010; Mukoyama et al. 1991), adenoviruses (Chiang et al. 2003; Debiaggi et al. 1990; Matias et al. 2010), and enteroviruses (Mukoyama et al. 1991; Tait et al. 2006).
Flavonoids are classified into at least ten chemical groups: among which flavones (have three -OH groups at the C4′, C5, and C7 positions), flavonols (have one OH group at the C3 positions), flavanones (have a single bond between the C2 and C3 positions in the C-ring) are the most common in vegetarian (plant-based) diets (Lyu et al. 2005). The antiviral activity of flavonoids has been shown to be related to their chemical structure. Liu et al. (2008) studied the structure–activity relationship of 25 flavonoids against influenza virus and found that myricetin had moderate effects, while naringenin and epicatechin had only weak effects against influenza A and B viruses. The order of potency for neuraminidase inhibition followed the order of flavon(ol)es > flavanon(ol)es and flavan(ol)es. On the other hand, a study on antiherpetic activities of 18 flavonoids concluded that flavanols and flavonols are more effective in reducing the infectivity of herpes simplex virus than flavones (Lyu et al. 2005). The results of this study showed that the antiviral activity against FCV-F9 followed the order of Myricetin (Flavonols) > l-Epicatechin (Flavanol) > Naringenin (Flavanone), which is somewhat closer in agreement with the results obtained by Liu et al. (2008).
The study of the antiviral mechanisms of flavonoids against Moloney murine leukemia virus (Chu et al. 1992) showed that flavonols and flavanonols had greater inhibitory effects on Moloney murine leukemia virus reverse transcriptase activity than flavones and flavanones. These researchers concluded that the presence of free hydroxyl groups at positions 3 and 4′ enhanced the reverse transcriptase inhibitory activity. Other proposed antiviral mechanisms of flavonoids include inhibition of viral polymerase, binding of viral nucleic acid or viral capsid proteins, and inhibition of viral replication (Cheng and Wong 1996; Cody et al. 1986). The results reported in this current study showed that myricetin had minor/negligible effects on viral adsorption, and that all four flavonoids did not have any effect on the replication of FCV-F9 and MNV-1.
As the demand for natural preventive measures increases, further research needs to be undertaken to determine alternative therapeutics or prophylactics that have protective effects on human health and disease prevention. The screening of natural flavonoids for health benefits that have both antioxidant and antimicrobial properties needs further in-depth studies that could lead to a database of existing and available natural antiviral compounds. The mechanisms of antimicrobial (antiviral) action of these bioactive flavonoids need to be clearly elucidated, along with the determination of the absence of cytotoxic, mutagenic, or carcinogenic effects. In addition, clinical trials need to be undertaken before any recommendations for use can be made. Future work will focus on determining the antiviral mechanism of myricetin and l-epicatechin. One can only speculate at this point, that these compounds could potentially affect the capsid structure and integrity of the virus. Transmission electron microscopy studies will help us understand if any changes to the structure of feline calicivirus (FCV-F9) do indeed occur. In addition, the viral nucleic acid or viral RNA-dependent RNA polymerase activity could also be adversely affected by these compounds, which requires further in-depth investigation.
Conclusions
The present study demonstrates that the antiviral effects of myricetin, l-epicatechin, tangeretin, and naringenin are virus-dependent, titer-dependent, and dose-dependent. Among the four chemicals, myricetin and l-epicatechin had greater antiviral effects than tangeretin and naringenin. These two compounds were more effective in reducing FCV-F9 than MNV-1. The understanding of the antiviral mechanisms of these flavonoids against food borne viruses needs further in-depth research.
References
Cheesbrough, J. S., Green, J., Gallimore, C. I., Wright, P. A., & Brown, D. W. G. (2000). Widespread environmental contamination with Norwalk-like viruses (NLV) detected in a prolonged hotel outbreak of gastroenteritis. Epidemiology and Infection, 125(1), 93–98.
Cheng, P. C., & Wong, G. (1996). Honey bee propolis: Prospects in medicine. Bee World, 77(1), 8–15.
Chiang, L. C., Chiang, W., Liu, M. C., & Lin, C. C. (2003). In vitro antiviral activities of Caesalpinia pulcherrima and its related flavonoids. Journal of Antimicrobial Chemotherapy, 52(2), 194–198.
Chu, S. C., Hsieh, Y. S., & Lin, J. Y. (1992). Inhibitory effects of flavonoids on moloney murine leukemia virus reverse transcriptase activity. Journal of Natural Products, 55(2), 179–183.
Cody, V., Middleton, E., & Harborne, J. B. (Eds.). (1986). Plant flavonoids in biology and medicine: Biochemical, pharmacological, and structure–activity relationships. New York: Alan R. Liss, Inc.
Cushnie, T. P. T., & Lamb, A. J. (2005). Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents, 26(5), 343–356.
Debiaggi, M., Tateo, F., Pagani, L., Luini, M., & Romero, E. (1990). Effects of propolis flavonoids on virus infectivity and replication. Microbiologica, 13(3), 207–213.
D’Souza, D. H., Sair, A., Williams, K., Papafragkou, E., Jean, J., Moore, C., et al. (2006). Persistence of caliciviruses on environmental surfaces and their transfer to food. International Journal of Food Microbiology, 108(1), 84–91.
D’Souza, D. H., & Su, X. W. (2010). Efficacy of chemical treatments against murine norovirus, Feline calicivirus, and MS2 Bacteriophage. Foodborne Pathogens and Disease, 7(3), 319–326.
El Gharras, H. (2009). Polyphenols: Food sources, properties and applications—a review. International Journal of Food Science & Technology, 44(12), 2512–2518.
Jassim, S. A. A., & Naji, M. A. (2003). Novel antiviral agents: A medicinal plant perspective. Journal of Applied Microbiology, 95(3), 412–427.
Kuusi, M., Nuorti, J. P., Maunula, L., Minh, N. N. T., Ratia, M., Karlsson, J., et al. (2002). A prolonged outbreak of Norwalk-like calicivirus (NLV) gastroenteritis in a rehabilitation centre due to environmental contamination. Epidemiology and Infection, 129(1), 133–138.
Kwon, H. J., Kim, H. H., Ryu, Y. B., Kim, J. H., Jeong, H. J., Lee, S. W., et al. (2010). In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorganic & Medicinal Chemistry, 18(21), 7668–7674.
Liu, A. L., Wang, H. D., Lee, S. M. Y., Wang, Y. T., & Du, G. H. (2008). Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorganic & Medicinal Chemistry, 16(15), 7141–7147.
Lyu, S. Y., Rhim, J. Y., & Park, W. B. (2005). Antiherpetic activities of flavonoids against herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) in vitro. Archives of Pharmacal Research, 28(11), 1293–1301.
Matias, A. A., Serra, A. T., Silva, A. C., Perdigao, R., Ferreira, T. B., Marcelino, I., et al. (2010). Portuguese winemaking residues as a potential source of natural anti-adenoviral agents. International Journal of Food Sciences and Nutrition, 61(4), 357–368.
Mead, P. S., Slutsker, L., Dietz, V., McCaig, L. F., Bresee, J. S., Shapiro, C., et al. (1999). Food-related illness and death in the United States. Emerging Infectious Diseases, 5(5), 607–625.
Mukoyama, A., Ushijima, H., Nishimura, S., Koike, H., Toda, M., Hara, Y., et al. (1991). Inhibition of rotavirus and enterovirus infection by tea extract. Japanese Journal of Medical Science and Biology, 44(4), 181–186.
Ramachandran, K. V. (1956). On the Tukey test for the equality of means and the HARTLEY test for the equality of variances. Annals of Mathematical Statistics, 27(3), 825–831.
Regal, R. R. (1986). PC statistician PC ANOVA. American Statistician, 40(2), 164–167.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V., Widdowson, M. A., Roy, S. L., et al. (2011). Foodborne illness acquired in the United States–major pathogens. Emerging Infectious Disease, 17(1), 7–15.
Siebenga, J. J., Vennema, H., Zheng, D. P., Vinje, J., Lee, B. E., Pang, X. L., et al. (2009). Norovirus illness is a global problem: Emergence and spread of Norovirus GII.4 variants, 2001–2007. Journal of Infectious Diseases, 200, 802–812.
Su, X., & D’Souza, D. H. (2011). Grape seed extract for the control of human enteric viruses. Applied Environmental Microbiology, 77, 3982–3987.
Su, X., Howell, A. B., & D’Souza, D. H. (2010a). The effect of cranberry juice and cranberry proanthocyanidins on the infectivity of human enteric viral surrogates. Food Microbiology, 27(4), 535–540.
Su, X. W., Sangster, M. Y., & D’Souza, D. H. (2010b). In Vitro Effects of Pomegranate Juice and Pomegranate Polyphenols on Foodborne Viral Surrogates. Foodborne Pathogens and Disease, 7(12), 1473–1479.
Tait, S., Salvati, A. L., Desideri, N., & Fiore, L. (2006). Antiviral activity of substituted homoisoflavonoids on enteroviruses. Antiviral Research, 72(3), 252–255.
Wobus, C. E., Karst, S. M., Thackray, L. B., Chang, K. O., Sosnovtsev, S. V., Belliot, G., et al. (2004). Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biology, 2(12), 2076–2084.
Yao, L. H., Jiang, Y. M., Shi, J., Tomas-Barberan, F. A., Datta, N., Singanusong, R., et al. (2004). Flavonoids in food and their health benefits. Plant Foods for Human Nutrition, 59(3), 113–122.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Su, X., D’Souza, D.H. Naturally Occurring Flavonoids Against Human Norovirus Surrogates. Food Environ Virol 5, 97–102 (2013). https://doi.org/10.1007/s12560-013-9106-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-013-9106-4