Abstract
Redox/cysteine modification of proteins that regulate calcium cycling can affect contraction in striated muscles. Understanding the nature of these modifications would present the possibility of enhancing cardiac function through reversible cysteine modification of proteins, with potential therapeutic value in heart failure with diastolic dysfunction. Both heart failure and muscular dystrophy are characterized by abnormal redox balance and nitrosative stress. Recent evidence supports the synergistic role of oxidative stress and inflammation in the progression of heart failure with preserved ejection fraction, in concert with endothelial dysfunction and impaired nitric oxide–cyclic guanosine monophosphate–protein kinase G signalling via modification of the giant protein titin. Although antioxidant therapeutics in heart failure with diastolic dysfunction have no marked beneficial effects on the outcome of patients, it, however, remains critical to the understanding of the complex interactions of oxidative/nitrosative stress with pro-inflammatory mechanisms, metabolic dysfunction, and the redox modification of proteins characteristic of heart failure. These may highlight novel approaches to therapeutic strategies for heart failure with diastolic dysfunction. In this review, we provide an overview of oxidative stress and its effects on pathophysiological pathways. We describe the molecular mechanisms driving oxidative modification of proteins and subsequent effects on contractile function, and, finally, we discuss potential therapeutic opportunities for heart failure with diastolic dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High myocardial diastolic stiffness is a common abnormality of diastolic function and a prominent feature of the abnormal diastolic left ventricle (LV) function. Diastolic dysfunction is defined as the inability to fill the ventricle to an adequate preload volume (end-diastolic volume; EDV) at acceptably low pressures. Diastolic abnormalities are mainly observed in heart failure (HF) with preserved ejection fraction (EF) (HFpEF) patients (Paulus 2010; Borlaug and Paulus 2011). HFpEF leads to a complex remodelling of cardiomyocyte structure and function, in addition to remodelling of the non-myocyte compartment. HFpEF is of major clinical importance, accounting for more than 50 % of all cases of heart failure in Western societies, and is accompanied by high levels of morbidity and mortality (Borbély et al. 2009a).
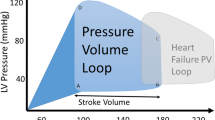
Diastolic function is often conceptualized as the totality of an active process that comprises mid-ventricular ejection, pressure decline to early filling (Leite‐Moreira 2006), which relates to myofilament dissociation and calcium re-uptake, and to the ‘passive’ properties of the myocardial wall, including sarcomere stiffness, extracellular matrix alterations, chamber geometry and the pericardium (Borlaug and Kass 2006). Notably, HFpEF patients are characterized by changes in several of these determinants, including disturbed calcium (Ca2+) handling, abnormal relaxation and ventricular filling, LV hypertrophy with concentric remodelling and increased deposition of extracellular matrix constituents (Paulus 2010; Borlaug and Paulus 2011). Activation of the neurohormonal milieu, including the renin-angiotensin-aldosterone system and the sympathetic nervous system, is common in HF with and without preserved LVEF (Paulus 2010; Borlaug and Paulus 2011). Altogether, these alterations change the time course of ventricular filling and shift the LV diastolic pressure–volume relationship upwards and to the left, indicative of reduced chamber distensibility and compliance, associated with greater than normal filling pressures (high chamber stiffness). By contrast, HF patients with reduced EF (HFrEF) exhibit a diastolic pressure–volume relationship that is displaced to the right (decreased chamber stiffness) (Borlaug and Kass 2011) (Fig. 1).
Pressure–volume loop characteristics in heart failure with reduced ejection fraction (green) (a) and heart failure with preserved ejection fraction (red) (b). Curved arrow depicts the steeper end-systolic pressure–volume relationship in heart failure with preserved ejection fraction compared with heart failure with reduced ejection fraction
Structural elements contributing to myocardial passive stiffness
Over the last decade, studies using isolated skinned cardiomyocytes from HFpEF patients and HFpEF animal models consistently showed a high sarcomere stiffness (Borbély et al. 2005; Hamdani et al. 2009, 2013a, 2014; Hamdani and Paulus 2011; Linke and Hamdani 2014). The technique of cellular membrane removal used in these studies abolished the effect of Ca2+ handling on passive stiffness, allowing the role of the giant myofilament protein titin in passive stiffness to be demonstrated (Borbély et al. 2009b; Bishu et al. 2011; Hamdani et al. 2013a, 2014; Linke and Hamdani 2014). Titin functions as a bidirectional spring, exhibiting a stiffness constant that can be tuned by either isoform switching or post-translational modifications (Krüger and Linke 2009; Linke and Krüger 2010).
HFpEF is associated with serious metabolic co-morbidities. These include obesity, overweight, diabetes mellitus, hypertension, chronic obstructive pulmonary disease, anaemia and chronic kidney disease (Paulus and Tschöpe 2013). In specific, obesity deserves special attention because it induces a systemic proinflammatory state that leads to the production of reactive oxygen species (ROS) by the coronary microvasculature (endothelial cells). This limits nitric oxide (NO) bioavailability (Paulus and Tschöpe 2013) and reduces cGMP concentrations (van Heerebeek et al. 2012; Hamdani et al. 2013a, 2014). Indeed, HFpEF is linked to high myocardial nitrosative/oxidative stress and low intercellular cyclic guanosine monophosphate (cGMP) concentrations (van Heerebeek et al. 2012). The latter lowers protein kinase G (PKG) activity, prominently affecting cardiomyocyte stiffness and inducing titin hypophosphorylation (Fig. 3). Considerable evidence supports the direct modification of titin by oxidative stress, either through phosphorylation by cGMP-PKG, the promotion of disulfide bridge formation within the cardiac titin N2-Bus or by oxidation of Ig-domains (Grützner et al. 2009; Alegre-Cebollada et al. 2014; Linke and Hamdani 2014). These modifications lead to either increased and/or reduced cardiomyocyte stiffness and thereby modulate diastolic LV stiffness.
Despite the fact the underlying mechanisms are still obscure, oxidative stress appears to be an additional important modifier of cardiac passive stiffness. Over the last decade, studies have described a range of proteins that are modified by oxidative stress with a high incidence found on the proteins of the contractile machinery.
The contribution of oxidative stress to heart failure, diastolic stiffness and muscular dystrophies
Cardiac contraction is dynamically regulated on a beat-to-beat basis to accommodate changes in haemodynamic load and to respond to neurohumeral stresses. Control is predominantly signal-regulated via various post-translational modifications, and recently signalling by ROS has emerged as a major physiological control mechanism (Beckendorf and Linke 2015). Striated muscle is subjected to oxidative stress and evidence now suggests that oxidative stress also modulates contractile function in cardiac and skeletal muscle. Redox modifications of proteins are known to result in functionally important changes in protein structure, stability, interactivity, and activity (Haycock et al. 1996; Posterino and Lamb 1996; Gao et al. 1996; Disatnik et al. 1998; Duncan et al. 2005; Dai et al. 2007; Sanchez et al. 2008; Ullrich et al. 2009; Canton et al. 2014). However, due to the limited data available and the fact that most studies have focused mainly on post-translational modifications of single purified contractile proteins, our understanding of the redox-dependent mechanisms that control contraction-relaxation in the intact heart remains poor.
Over the last decade various studies have shown the importance of oxidative/nitrosative stress on the protein modifications (i.e., disulfide bridges, S-glutathionylation, S-nitrosylation and tyrosine nitration and chlorination) involved in excitation–contraction (E–C) coupling and myocyte contraction (Fig. 2). Oxidative stress, defined as the imbalance in excess production but deficient ROS degradation plays an important role in the pathophysiology of cardiac remodelling and development of HF. Oxidative stress can result in direct chemical oxidation of proteins, leading to changes in their structure, function and activity. In addition, it induces subtle changes in intracellular pathways and redox signalling that cause cellular dysfunction and damage (Haycock et al. 1996; Disatnik et al. 1998; Duncan et al. 2005; Dai et al. 2007; Canton et al. 2014) (Fig. 3).
An overview of the main causes of oxidative stress and the induction of oxidative damage in muscle. Abbreviations of causes and modified proteins discussed in the present review: xanthine oxidase (XO), NADPH oxidases (Nox), dihydrobiopterin (BH2), tetrahydrobiopterin (BH4), uncoupled endothelial nitric oxide synthase (eNO synthase), nitric oxide (NO). Free radicals: superoxide (O 2 −), hydroxyl radical (OH•), hydrogen peroxide (H 2 O 2 ), particulate guanylyl cyclase (pGC), soluble guanylyl (sGC), cyclic guanosine monophosphate; (cGMP), cGMP-dependent protein kinase-G (PKG), phosphorylation (P), phospholamban (PLB), sarcoplasmic reticulum (SR), calcium transport ATPase (SERCA), RyR2, calcium (Ca 2+), plasma membrane Ca2+ ATPase (PMCA), L-type calcium channel (LTCC), reactive oxygen species (ROS)
Experimental and clinical studies have reported excess production of ROS in HF (Belch et al. 1991; Hill and Singal 1996; Mallat et al. 1998), myocardial ischemia/reperfusion injury (Canton et al. 2004) and in various muscular dystrophies, such as Duchenne muscular dystrophy (Haycock et al. 1996; Disatnik et al. 1998; Canton et al. 2014) and dysferlinopathy (Terrill et al. 2013). Several antioxidants are known to ameliorate the progress of HF (Takimoto and Kass 2007), and chronic inhibition of xanthine oxidase (XO) has been shown to preserve cardiac function in a transgenic mouse model of cardiomyopathy by reducing myofibrillar protein oxidation (Duncan et al. 2005). Donors of nitroxyl, the reduced congener of NO, were also able to increase force development in intact rat trabeculae (Dai et al. 2007).
In cardiomyocytes, ROS/NOS are typically generated by several intracellular sources including mitochondria, nitric oxide synthases (NO synthase), XO, NADPH oxidase (NOX), NO synthase and cytochrome p450 (Cline and Lehrer 1969; Nishino 1994; Ji 2007; Zangar et al. 2004; Sumimoto et al. 2005; Kim et al. 2008; Nishino et al. 2008) (Fig. 3). Apart from resident cardiac cells, infiltrated leukocytes account for a large portion of ROS and reactive nitrogen species (RNS) in myocardial tissues via production of superoxide (O2 •-) and release of pro-oxidant enzyme systems like leukocyte-derived enzyme myeloperoxidase (MPO). Under physiological conditions, tissue oxidative balance is maintained by utilizing an antioxidant defence system that removes harmful reactive oxygen/nitrogen species (ROS/NOS). The protective mechanisms that oppose ROS-mediated damage in cells include enzymatic pathways, such as the well-characterised catalases and glutathione peroxidase, superoxide dismutases (SODs) and thioredoxin. In addition, a variety of antioxidant molecules, including vitamins A, C, E, glutathione and SH groups of intracellular proteins, constitute an antioxidant buffer (Rahman 2007). The ROS/NOS produced from these sources are oxygen-/nitrogen-based chemical species with high reactivity, and include free radicals such as O2 •-, hydroxyl radical (•OH) and peroxy radicals (ROO•), and nonradicals able to generate free radicals such as hydrogen peroxide (H2O2), nitroxyl and NO (Rahman 2007) (Fig. 2). Diminished capacity of NO synthase to generate NO has been demonstrated (Salvolini et al. 1999; Wong et al. 2010) to be accompanied by increased oxidative stress during diabetic vascular dysfunction (Giugliano et al. 1996). The likely mechanism is via O2 •- which reacts with NO, resulting in formation of peroxynitrite. Peroxynitrite then oxidizes the eNO synthase cofactor, tetrahydrobiopterin (BH4), to dihydrobiopterin (BH2), leading to eNO synthase uncoupling and thus producing O2 •- instead of NO (Fig. 3). This mechanism in turn results in a decrease in eNO synthase expression and activity in endothelial cells (Cosentino et al. 1997; Srinivasan et al. 2004).
In the present review, we focus exclusively on the role of oxidative stress in the modification of the properties and functions of several proteins and consequent effects on diastolic stiffness. Beginning with an overview of the most prominent modulators of diastolic stiffness, we then touch on how oxidative stress can effect these modulators. This is followed by an overview of oxidative modifications of several proteins. Finally, we discuss recent evidence describing oxidative modifications of the giant elastic titin filament in cardiac and skeletal myocytes and how these modifications may interfere with and link to heat shock proteins (HSPs). An overarching theme is the importance of oxidative modification in modulating diastolic stiffness and how modifications can be targeted as therapeutic options in HF, co-morbidities and diastolic stiffness abnormalities.
Reactive oxygen species-responsive protein modification and its intracellular delivery
ROS-induced changes on proteins vary from protein fragmentation to various post-translational modifications. Free radical-induced oxidative modifications of proteins can alter their functional or structural properties (Bolli and Marbán 1999; Kloner and Jennings 2001a, b; Tiago et al. 2006; Solaro 2007; Murphy et al. 2008; Hertelendi et al. 2008; Ding et al. 2011; Gao et al. 2012; Alegre-Cebollada et al. 2014). These modifications modulate muscle force production and regulate passive stiffness in vitro (Bolli and Marbán 1999; Kloner and Jennings 2001a, b; Tiago et al. 2006; Solaro 2007; Murphy et al. 2008; Hertelendi et al. 2008; Ding et al. 2011; Gao et al. 2012; Alegre-Cebollada et al. 2014). Protein oxidation generally occurs at the reactive thio-moieties of cysteine residues (or to a lesser extent methionine), which can be modified through either reversible or irreversible oxidative states (Fig. 2), depending on the nature of ROS and the level of oxidants (Eaton 2006; Thomas and Mallis 2001). The reaction between the cysteine thiolate anion and H2O2 results in the formation of sulfenic acid, which reacts with other thiol groups to form strong disulfide bonds (Sanchez et al. 2008) (Fig. 2). Depending on the available conditions, sulfenics can participate in a variety of reactions. Sulfenics can be further oxidized to sulfinic and sulfonic acids (Sanchez et al. 2008) (Fig. 2). In addition, when levels of oxidized gluthathione (GSSG) are adequate, thiolates can be directly glutathionylated (Fig. 2) through the formation of a mixed disulfide between a protein and glutathione. Although the mechanism by which S-glutathionylation regulates protein function is not fully elucidated, recent evidence shows that S-glutathionylation of proteins prevents irreversible oxidation, but also plays a crucial role in cell signalling processes (Dalle-Donne et al. 2007). Moreover, S-glutathionylation and disulfide bridges can directly impair cardiac function by modifying proteins involved in EC coupling and contractile function, resulting in changes to myocyte contraction, myoplasmic Ca2+ levels and passive stiffness (Linke and Hamdani 2014). Thiolates can also be nitrosylated in the presence of peroxynitrite or NO. (Reid 2001; Dröge 2002; Smith and Reid 2006) (Fig. 2). In summary, the most prevalent reversible cysteine modifications include disulfide bridges, nitrosylation, and sulfenylation (Sanchez et al. 2008). Understanding the influences of oxidative/nitrosative stress on diastolic stiffness is a central objective in attempts to unravel the origin of diastolic dysfunction in HFpEF under 'radical' conditions.
The impact of oxidative stress on collagen and cardiomyocyte-based stiffness
Impact of oxidative stress on collagen stiffness
High diastolic stiffness of the LV plays an important role in minimizing chamber dilatation and the response to increased filling with increased ejection. However, the basic mechanisms underlying increased diastolic stiffness appear to be diverse and dependent on both the causes of disease and specific phenotypes. In HFrEF with diabetes mellitus, increased advanced glycation end (AGE) products and collagen volume fraction appear to play an important role in LV stiffness, whereas increased cardiomyocyte stiffness seems to be more central and significant for LV stiffness in HFpEF patients (van Heerebeek et al. 2008). Evidence indicates the important roles of increased amounts of collagen tissue, the extent of collagen type I and coronary peripheral carboxy-terminal propeptide of procollagen type I in hypertensive patients compared to normotensive patients (Querejeta et al. 2004). Increased collagen synthesis has been observed in HFpEF patients with hypertension without changes in collagen degradation (González et al. 2010a). In concert with increased collagen levels, HFpEF patients with hypertension show an increase in the expression level of a profibrotic growth factor (transforming growth factor β) produced by the accumulated intracardiac inflammatory cells (Westermann et al. 2011). This points to a direct influence of inflammation on myocardial collagen/fibrosis, leading to a triggering of diastolic dysfunction.
The degradation of collagen is regulated by matrix metalloproteinases (MMPs) and enhanced degradation may alter LV remodelling. These cellular events are clearly involved in the development and progression of maladaptive myocardial remodelling and failure (Paulus 2010; Paulus and Tschöpe 2013). The quantity and quality of myocardial collagen is determined by the balance between synthesis and degradation. Synthesis is regulated at both the transcriptional and post-translational levels, while degradation is mediated by MMPs, and its tissue inhibitor (TIPM) (González et al. 2010a). Both MMPs and MPO are released into the extracellular space to propagate inflammatory disorders during neutrophil activation and degranulation. MPO is in addition involved in oxidizing classical peroxidise substrates to radical intermidiates, which converts chloride to hypochlorous acid (MacFarlane and Miller 1994). Hypochlorous acid is a major strong oxidant produced by neutrophils and which might be used as oxidant activity biomarker of neutrophils and MPO-containing cells.
Oxidative stress via ROS-mediation has been implicated in the regulation of MMP activity, (Siwik et al. 2001). ROS are also known to mediate collagen metabolism in a variety of non-cardiac and cardiac cell types including cardiac fibroblasts, both via synthesis and through the activity of degradative enzymes (Siwik et al. 2001). Failing hearts show increases in ROS production that could be the result of mechanical strain, the stimulation of factors such as angiotensin or inflammatory cytokines, and decreases in the activity of mitochondrial electron transport and/or decreases in antioxidant systems (e.g. SOD and glutathione peroxidase).This may suggest a new model in which cellular and molecular processes in HFpEF are influenced by a proinflammatory state induced by multiple comorbidities, such as obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease and anaemia. Aortic stenosis patients with diabetes mellitus, a frequent pathology in older patients, increases LV afterload and exhibits reduced LV distensibility compared to aortic stenosis patients without diabetes mellitus, indicating that disease complicated with co-morbidities may result in more severe diastolic dysfunction (Paulus 2010; Paulus and Tschöpe 2013). Aortic stenosis with diabetes mellitus is associated with increased collagen volume fraction, increased intramyocardial vascular advanced glycation end product deposition, and high cardiomyocyte stiffness, in addition to titin hypophosphorylation (Falcão-Pires et al. 2011; Borbély et al. 2005; van Heerebeek et al. 2006, 2008; Hamdani and Paulus 2011; Hamdani et al. 2013a, b, 2014).
Impact of oxidative stress on cardiomyocyte stiffness
Sarcomeric proteins: thin and thick filaments
The modulation of heart contractility through oxidative stress directly involves the contractile machinery, including thin, thick and elastic filament proteins. The thin filaments are composed of three different types of proteins: actin, tropomyosin, and troponin, together termed the ‘regulatory protein complex’. Cardiac troponin is a three-protein complex with several distinct functions. Troponin I binds tightly to tropomyosin and together they block myosin binding sites on actin molecules. When Ca2+ binds to troponin C, a conformational change takes place in the troponin complex that causes troponin I to shift from the myosin binding site on actin, thereby allowing access to the myosin head. When Ca2+ is removed, the troponin complex resumes its inactivated position, thereby inhibiting myosin-actin binding. Troponin T maintains the interaction with tropomyosin and mediates allosteric effects within the thin filament that influence Ca2+ sensitivity. Many of these proteins undergo oxidation in vivo as a consequence of normal aerobic cellular processes.
During cardiac stunning, ROS depress contractile performance and alter Ca2+-sensitivity of force production (Bolli and Marbán 1999, Kloner and Jennings 2001a, b). Similarly, an increased Ca2+ sensitivity due to S-glutathionylation of troponin I at Cys133 has been demonstrated in rat and human fast-twitch muscle, although this modification did not affect maximal force (Mollica et al. 2012). The S-glutathionylation of troponin I seems to have an influence on the movement of troponin I towards the troponin C bound state, resulting in interaction between troponin I and troponin C at lower Ca2+ levels (Horwitz et al. 1979; Hincke et al. 1979; Chong and Hodges 1982; Aihara et al. 2010; Mollica et al. 2012). The SH groups of troponin subunits, in a reduced state, have been shown to play an important role in producing and maintaining the troponin complex. Oxidized troponin has only a minor effect on the Ca2+-sensitivity of actomyosin ATPase, which is reversible upon dithiothreitol administration. As a consequence oxidized troponin I does not bind to troponin T (Horwitz et al. 1979; Hincke et al. 1979; Chong and Hodges 1982; Aihara et al. 2010). Although troponin I harbours several cysteine residues, some of these cysteines are insensitive to Ca2+-induced conformational changes in native troponin I (Chong and Hodges 1981). Therefore, the impaired contractility could, in part, be unrelated to reduced Ca2+ concentrations but might instead be due to the oxidation of amino acids in myofilament proteins (Gao et al. 1995; Gao et al. 1996; Andrade et al. 1998, 2001; Lamb and Westerblad 2011). In spite of suggestions that SH oxidation of tropomyosin and/or actin in combination with myosin light chain1 (MLC1) (Hertelendi et al. 2008) are potential mediators of oxidative myocardial contractile depression, it is currently difficult to clearly state that oxidation of myofilament proteins causes cardiac dysfunction, since studies have reported equivocal effects in different muscle types and even within a single muscle type. The role of oxidation of myofilament proteins in cardiac dysfunction in the heart is currently confused, as decreased Ca2+ sensitivity has been reported (Posterino and Lamb 1996; Lamb and Posterino 2003; Hertelendi et al. 2008) while others reported no changes or even increases in Ca2+ sensitivity (MacFarlane and Miller 1992; MacFarlane and Miller 1994). Interestingly, slow twitch muscle differed from fast twitch muscle in relation to the addition of cysteine oxidants during muscle contraction (Lamb et al. 1995; Lamb and Posterino 2003), suggesting that oxidative responses may be dependent on positions and differences between cysteines in fast and slow skeletal muscle. The distinct effects of oxidation may also depend on the contractile state in which cysteines are exposed to oxidants (Lamb and Posterino 2003; Murphy et al. 2008; Dutka et al. 2011).
Although the overall biology of oxidative protein modifications remains complex and poorly defined, protein carbonylation is fairly well-characterised (Dukan et al. 2000; Dalle-Donne et al. 2003b; Coirault et al. 2007; Shao et al. 2010; Canton et al. 2011; Ballesteros et al. 2001). Carbonylation is an irreversible, non-enzymatic protein modification, and carbonyl groups are introduced into proteins via a variety of oxidative pathways. ROS can yield highly reactive carbonyl derivatives resulting from the cleavage of peptide bonds by α-amidation, by oxidation of glutamyl residues, or alternatively, through direct oxidation of the Lys, Arg, Pro, and Thr side chains (Dukan et al. 2000; Dalle-Donne et al. 2003b; Coirault et al. 2007; Shao et al. 2010; Canton et al. 2011; Ballesteros et al. 2001). Oxidation can act directly on the carbonyl group, introducing it into proteins by adduction of reactive aldehydes. A final mechanism is the generation of carbonyl groups through the secondary reaction of the primary amino group of Lys residues with reactive carbonyl derivatives (ketoamines, ketoaldehydes, deoxyosones), produced by the reaction of reducing sugars or their oxidation products with lysine residues of proteins (glycation/glycoxidation reactions), eventually leading to the formation of advanced glycation end-products (AGEs).
Several other sarcomeric proteins including myosin, actin and tropomyosin also contain a variety of cysteines that are modified by ROS in specific pathophysiological states (Aksenov et al. 2001; Eaton et al. 2002; Fratelli et al. 2002; Canton et al. 2004, 2006; Fiaschi et al. 2006; Passarelli et al. 2010). However, it has not been fully elucidated which cysteines are involved and how their function is altered. Interestingly, the level of tropomyosin oxidation inversely correlates with contractile function in post-ischaemic porcine hearts (Canton et al. 2006), suggesting that oxidation of myofilament proteins are, at least in part, responsible for myocardial dysfunction. Nevertheless, oxidative modifications are not necessarily involved in impaired cardiac function. Cardiac muscles superfused with nitroxyl (a reactive nitrogen species that displays many unique biochemical and pharmacological features) showed increased force production, perhaps through sensitization of the myofilaments to Ca2+ (Solaro 2007; Ding et al. 2011; Gao et al. 2012). Nitroxyl reacts with the thiol groups on cysteines, forming either a sulfonamide or a disulfide bond when an extra free thiol is present (Dai et al. 2007; Solaro 2007; Ding et al. 2011; Gao et al. 2012). Treatment of isolated rat myofibrils with nitroxyl induced the formation of covalent bonds or a reversible disulfide bond by targeting specific cysteine residues (negatively charged or thiolates) (Dai et al. 2007; Solaro 2007; Ding et al. 2011; Gao et al. 2012). Nitroxyl was also shown to enhance SR Ca2+ uptake and release via cysteine modifications of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), phospholamban (PLB) and ryanodine receptor (RyR), and to increase myofilament Ca2+ responses and contractility in cardiomyocytes (Gao et al. 2012). Although nitroxyl is related to NO, its effects on the heart are independent of cAMP or cGMP. In addition, carbonylation and S-nitrosylation of actin and tropomyosin are correlated with contractile dysfunction and with decrease LV ejection fraction (Canton et al. 2011). Furthermore, Cys190 oxidation of tropomyosin dimers have been found in microembolized dog/pig hearts (Canton et al. 2006), in mice after myocardial infarction (Avner et al. 2012) and in failing rat hearts (Heusch et al. 2010).
Amongst all myofibrillar proteins, actin is a major target of oxidative modifications, and the S-glutathionylation of Cys374 has been found even under non-stress conditions (Dalle-Donne et al. 2003a). The S-glutathionylation of actin is increased after post-ischemic reperfusion and causes decreased actin-tropomyosin interactions (Canton et al. 2004; Chen and Ogut 2006). Furthermore, S-glutathionylation of actin limits polymerization of globular (G)-actin to filament (F)-actin (Wang et al. 2001; Dalle-Donne et al. 2003a). Noteworthy, the deglutathionylation of G-actin leads to a six-fold increase in the rate of polymerization (Wang et al. 2001), indicating the importance of reversible glutathionylation for sarcomere assembly. Additionally, actin glutathionylation was demonstrated to reduce actomyosin ATPase activity via the substitution of glutathionylated F-actin for unmodified F-actin (Pizarro and Ogut 2009).
Cardiac myosin is the key protein in the contractile apparatus of the heart and its interaction with actin is responsible for the ability of the heart muscle to generate force and correspondingly to shorten the beat. In skeletal muscle, the myosin is a hexameric polypeptide consisting of two myosin heavy chain (MHC) and four myosin light chain (MLC) subunits. Each heavy chain extends into one of the paired heads where, together with the light chains, forms a cross-bridge that allows muscle contraction. The myosin ATPase activity is the driving enzyme of the cross-cycle and was shown to be modified upon S-glutathionylation at different MHC sites (Passarelli et al. 2008). Indeed, oxidation of two cysteines near the catalytic centre of the subfragment 1 (S1)-domain in MHC (Cys697 and Cys707) results in a strong inhibition of S1-ATPase activity and reduces maximal force (Tiago et al. 2006). Elevated levels of glucose adducts (glycosylation) have been found on the MHC in post-mortem hearts of diabetic patients, and carbonylation of MHC as a result of reactive carbonyl species (RCS) has been consistently found in rat hearts during diabetes (Shao et al. 2010). These modifications limit contractility by reducing myofilament force generating during diabetes (Shao et al. 2010). In addition, an enhanced level of skeletal MHC carbonylation was reported to be associated with a reduced myosin sliding velocity in in vitro motility studies. Aminoguanidine (Ag) treatment (which inhibits ROS formation (Coirault et al. 2007)) consistently blunted the formation of carbonyl adducts on MHC domains, and decreased myosin-ATPase activity, the extent of myocyte shortening, but did not alter MHC composition (Shao et al. 2010).
Nitroxyl treatment leads to the formation of MHC/MLC heterodimers via disulfide bridges, thus increasing cardiac contractility; likewise, nitroxyl treatment forms actin/tropomyosin heterodimers via actin Cys257 and tropomyosin Cys190 (Gao et al. 2012). In contrast to the many beneficial effects on myocardial contractile function shown by nitroxyl (Gao et al. 2012; Sabbah et al. 2013; Arcaro et al. 2014). Increased peroxynitrite production also induces MLC1 nitration/nitrosylation, leading to its increased degradation by the proteolytic enzyme MMP-2. This in turn suppresses cardiomyocyte contractility during either reoxygenation or reperfusion (Doroszko et al. 2010; Polewicz et al. 2011). Both the MLC1 and MLC2 isoforms are subject to tyrosine nitration and cysteine S-nitrosylation in cardiac models of oxidative stress: MLC1 is S-nitrosylated at Cys138 and nitrated at Tyr73, Tyr141 and Tyr185, and MLC2 at Tyr118, Tyr152 and Tyr182. These changes promoted degradation of both isoforms by MMP-2 (Doroszko et al. 2009, 2010; Polewicz et al. 2011). It has been consistently demonstrated that inhibition of MMP-2 and nitration/nitrosylation of MLC1 attenuates ischemia/reperfusion injury and prevents MLC1 degradation in isolated rat hearts. These findings may suggest a pathological association of MLC tyrosine nitration in ischemia/reperfusion and hypoxia-reoxygenation.
Cardiac myosin binding protein C (cMyBP-C), a known regulator of sarcomeric structure and myocardial contractility, is a thick filament assembly protein that interacts with both myosin and titin to regulate the cross-linkages of myosin in the A-band region of the sarcomere (Sadayappan and de Tombe 2012). cMyBP-C was found to be S-glutathionylated at Cys479, Cys627, and Cys655 of the C3, C4, and C5 domains of MyBP-C in a desoxycorticosterone acetate (DOCA)-salt hypertensive mouse model (Lovelock et al. 2012; Jeong et al. 2013; Patel et al. 2013). These mice exhibited high oxidative stress associated with myocytes and diastolic abnormalities, but no changes in cellular Ca2+-fluxes were seen (Lovelock et al. 2012). Depression of S-glutathionylation in cMyBP-C ameliorates diastolic dysfunction as it reverts in the slow cross-bridge turnover kinetics (Jeong et al. 2013), indicative of a strong correlation between S-glutathionylation and diastolic dysfunction. The S-glutathionylation of cMyBP-C has been implicated in increased myofilament Ca2+-sensitivity, thus suggesting the involvement of S-glutathionylation of cMyBP-C in the maintenance of longitudinal rigidity and cross-bridge kinetics (Patel et al. 2013). In addition, carbonylation of cMyBP-C limited its function associated with its actin-binding properties (Aryal et al. 2014), which may affect the sarcomeric assembly and contractility of cardiac muscle. Therefore, in addition to the well-established phosphorylation of cMyBP-C with its recognized effects on cMyBP-C function (Cazorla et al. 2006; Tong et al. 2008; Sadayappan et al. 2009) the role and therapeutic implications of redox-related post-translational modifications should now be considered, in particular in hypertrophic cardiomyopathy.
Taken together, the above findings, oxidative modifications of thin and thick filament proteins, play crucial roles in sarcomere contraction. The S-glutathionylation of troponin I and cMyBP-C increases Ca2+ sensitivity, while the formation of the heterodimers TnI/actin and MHC/MLC upon nitroxyl treatment greatly increases myofilament responses to Ca2+, without affecting diastolic Ca2+ levels.
Sarcomeric proteins: Elastic filaments
Titin is the largest protein in mammals and spans half a sarcomere from the Z-disk to the M-band. Its human variant is encoded by a single gene consisting of 363 exons, which encodes a protein of 34,350 amino acids with a molecular weight of up to 3800 kDa (Bang et al. 2001). Titin functions as the molecular spring that imparts passive elasticity to muscle. Structurally, titin is composed of 244 individually-folded protein domains that are connected via unstructured peptide sequences (Linke and Hamdani 2014). The folded protein domains unfold when the protein is stretched and refold when tension is removed (Minajeva et al. 2001). Titin alters LV diastolic passive stiffness at physiological sarcomere lengths (SL) and can be modified through isoform switching. Two isoforms of titin are present in adult cardiac muscle: the compliant and longer N2BA isoform, and the stiffer and shorter N2B isoform (Fig. 4) (Linke and Hamdani 2014). The isoforms differ in their I-band segment, which is the only extensible region of titin. Both cardiac N2BA and N2B consist of four distinct elements: (1) proximal tandem Ig-domains (Ig 1–15), (2) distal tandem Ig-domains (Ig 84–105), (3) a spring-like N2B element, and (4) a spring-like PEVK region that is rich in proline, glutamate, valine and lysine. N2BA titin also contains a middle tandem Ig segment with a variable number of Ig-domains, together with an N2A spring element. Another difference is in the PEVK segment, relatively short in the N2B isoform, whereas the PEVK region of N2BA titin is much larger (Labeit and Kolmerer 1995; Bang et al. 2001). Because N2BA titin is more compliant than the stiffer N2B isoform, a higher relative proportion of N2B leads to an increase in passive stiffness.
The effect of oxidative stress on titin-based passive stiffness. The top panel illustrates the different segments of the titin chain (N2BA and N2B isoforms) in a half-sarcomere and illustrates oxidative damage in several regions of titin. a Titin-based passive tension in the heart can be tuned by phosphorylation of the titin spring elements N2-Bus and PEVK by the following kinases: protein kinase-A (PKA); protein kinase-G (PKG); protein kinase-Cα (PKCα); extracellular signal-regulated kinase 2 (ERK2); Ca2+/calmodulin-dependent protein kinase-IIδ (CaMKII). b Schematic illustration of increased titin-based passive stiffness due to reduced PKG-dependent phosphorylation of the titin N2-Bus element in failing human hearts due to oxidative stress. c Under oxidizing conditions, disulfide bonds in the titin N2-Bus are promoted, thus increasing titin-based passive stiffness. d. Under oxidative conditions, S-glutathionylation of cysteines in unfolded titin immunoglobulin (Ig)-domains (due to sarcomere stretch) inhibits domain refolding and thereby reduces titin stiffness. Under oxidative conditions, phosphorylation of Ig-domains and S-glutathionylation of cysteines may or may not inhibit domain refolding. The effect of these modifications together is unknown
Healthy adult human hearts express only ≈30% N2BA, compared to ≈70% N2B (Neagoe et al. 2002), this ratio is altered in several cardiac diseases. Higher expression levels of the complaint N2BA titin isoform have been observed in end-stage heart failure with eccentrically remodeled explanted hearts from ischemic cardiomyopathy (Neagoe et al. 2002) or dilated cardiomyopathy (Makarenko et al. 2004). An increased N2BA/N2B isoform ratio was also accompanied by decreased myofibrillar passive stiffness (these patients had the least diastolic dysfunction). Overall, these studies suggested that the switching of titin isoforms to the more complaint isoform might serve as a compensatory mechanism initiated in a globally stiffened ‘fibrotic’ environment; thus, the contribution of titin to total myocardial passive stiffness decreases as the contribution made by collagen increases. In contrast, a decreased N2BA/N2B ratio was observed in a study of endomyocardial biopsies from HFpEF patients versus HFrEF patients (van Heerebeek et al. 2006). Similarly, a modest decrease in the proportion of N2BA titin was seen in an old dog model of hypertrophy-associated early HFpEF (Bishu et al. 2011, Hamdani et al. 2013a, b, c). This model shows signs of diastolic dysfunction similar to those frequently seen in elderly HFpEF patients: impaired LV relaxation, unaltered coefficient of LV diastolic stiffness and reduced diastolic capacitance, together with elevated natriuretic peptides, normal LV volume but increased LV mass and moderated myocardial fibrosis. However, distinct results have been found in aortic stenosis patients, with either a switch to N2B (Williams et al. 2009) or a switch to N2BA (Borbély et al. 2009b) compared to a donor (non-failing hearts) group. In another variant of heart disease, hypertrophic cardiomyopathy (Chaturvedi et al. 2010), the cardiac titin isoform did not change compared to donor groups. Thus, the co-expression of titin isoforms provides a structural basis for myofibrillar elastic diversity in different vertebrate species and is one of the mechanisms that regulate myocardial passive stiffness. In addition to the long-term changes in isoform ratio that accompany chronic disease and influence passive stiffness, these studies also suggested that increased passive stiffness in HF can arise due to alterations in post-translational modifications of titin, which thus play a prominent role in the modulation of passive stiffness.
Post-translational modifications of titin represent a rapid mechanism to alter tension and are therefore likely to be important in the dynamic modulation of diastolic stiffness. Previous studies have shown that HF and HFpEF are both characterized by a titin phosphorylation deficit (Borbély et al. 2009b; Bishu et al. 2011; Hamdani et al. 2013b, 2014). Titin can be phosphorylated by a variety of kinases, leading to a range of effects on the passive mechanical properties of the sarcomere. Titin is phosphorylated by PKA at the N2B spring element (Yamasaki et al. 2002; Krüger and Linke 2006; Kötter et al. 2013) (Fig. 4), which acutely shifts the diastolic length tension relation downward (enhanced compliance) under the influence of β-adrenergic stimulation and may contribute to enhanced filling. As this effect is more striking in HFpEF patients compared to HFrEF and controls, this suggests that a phosphorylation deficit is an important contributor to diastolic stiffness in HFpEF (Hamdani et al. 2013a, b; Borbély et al. 2009b). PKCα has been shown to induce increased stiffness through phosphorylation of titin at the PEVK region, an effect amplified by pretreatment with protein phosphatase 1 (Hidalgo et al. 2009) (Fig. 4). In contrast, our studies of PKCα showed no effects in mechanical experiments on skinned cardiomyocytes from either an HFpEF large animal model or from their control group (Hamdani et al. 2013a). These findings suggested that increased PEVK phosphorylation due to increased PKCα activity in HF might make only a minor contribution to increased passive diastolic stiffness in HFpEF. Another kinase that was recently shown to phosphorylate titin is the extracellular signal-regulated kinase-2 (ERK2), which phosphorylates titin at the N2B spring element, resulting in reduced stiffness (Raskin et al. 2012) (Fig. 4). We recently identified an additional kinase as a modulator of titin stiffness, calcium calmodulin-dependent kinase II (CaMKII) (Hamdani et al. 2013c) (Fig. 4). Using mass spectrometry, various CaMKII-dependent phosphosites were detected by in vivo quantitative phosphoproteomics. Sites are located within the extensible region of titin at the N2-Bus and PEVK spring elements, while others are found within the A-band, M-line and Z-disk of titin. CaMKII phosphorylation also leads to reduced cardiomyocyte stiffness (Fig. 4). In general, the beneficial effects of the phosphorylation of titin by several kinases (with the exception of PKCα) may include a reduction in myocardial diastolic stiffness and an improvement in ventricular filling.
In addition, PKG, when activated by NO or natriuretic peptides, also phosphorylates titin at the N2-Bus (Krüger et al. 2009; Kötter et al. 2013) (Figs. 3, 4). NO/cGMP/PKG signalling is one of the signalling pathways that is most impaired by oxidative stress and induces hypophosphorylation of titin. PKG-mediated phosphorylation of titin results in reduced titin-based myocardial stiffness in vitro (Borbély et al. 2009b; Hamdani et al. 2013a, b, 2014), and leads to acutely increased cardiac distensibility following short-term cGMP-enhancing treatment with sildenafil and B-natriuretic peptide (BNP) in an HFpEF large animal model in vivo (an old, kidney-wrapped hypertensive dog model) (Bishu et al. 2011). Earlier work demonstrated that phosphorylation of titin by PKA and PKG reduces passive stiffness, suggesting that the chronic phosphorylation deficit observed in HFpEF is most likely due to impaired PKA and PKG signalling. This conclusion is supported by the observation that both PKA and PKG restores the high cardiomyocyte stiffness in HFpEF patients and in large and small animal models of HFpEF (Borbély et al. 2009b; Hamdani et al. 2013a, b, 2014). PKG activity and cGMP concentrations are reduced in the LV myocardium of HFpEF patients (van Heerebeek et al. 2012) and in small (Hamdani et al. 2013b, 2014) and large animal (Hamdani et al. 2013a) models of HFpEF. This in turn resulted from increased myocardial nitrotyrosine levels, indicative of raised obesity-related nitrosative/oxidative stress and low natriuretic peptide levels due to a low LV wall stress caused by concentric LV remodelling (van Heerebeek et al. 2012). In addition, insulin treatment of developing rat cardiomyocytes has been shown to increase the phosphorylation of total titin, this effect probably due to insulin-induced activation of a signalling pathway that includes phosphoinositide 3-kinase (PI3K), NO synthase and thereby PKG (Krüger et al. 2010). Together, these findings suggested that modulation of titin-based stiffness via cGMP-enhancing therapy could be a useful approach to correcting pathologically elevated LV diastolic stiffness, one of the primary characteristics of HFpEF patients.
Oxidative stress and the consequences for titin-based myocardial stiffness
As a direct effect of oxidative stress-related redox modification of titin, disulfide bonding in the cardiac specific N2-Bus decreases the extensibility of N2B, stiffens the whole titin molecule and increases cardiomyocyte passive tension (Fig. 4) (Grützner et al. 2009). The N2-Bus region contains six cysteines, which can form three disulfide bridges under oxidative stress. It has been consistently shown that thioredoxin, an enzyme that catalyses disulfide bond formation and isomerization, reduces the stiffness of isolated human cardiomyofibrils (Grützner et al. 2009). Moreover, internal S–S bridges in the N2-Bus may interfere with the (de)phosphorylation of PKG-dependent stiffness adjustment and may influence ligand binding (Linke 2008), mechanosensing and protein quality control (Sheikh et al. 2008; Linke and Hamdani 2014).
Another direct oxidative stress-related effect on titin-based stiffness is the S-glutathionylation of cryptic cysteines in the Ig-domains of the elastic I-band region, leading to inhibition of folding and weakening of refolded domains (Alegre-Cebollada et al. 2014). It is well established that Ig unfolding controls the elasticity of titin at physiological sarcomere lengths (Linke and Fernandez 2002). Although cryptic cysteines remain inert while parent domains remain folded, they become available for redox modification when mechanical forces accelerate the process of unfolding. Interestingly, most of the Ig-domains within the I-band of titin contain cysteines which appear to be buried. Many of these cysteines are clustered, well conserved and have a potential for disulfide bridge formation under oxidative conditions. This is particularly the case for the proximal and middle Ig-domains (Mayans et al. 2001), while the distal Ig-domains never unfold under physiological conditions (Li et al. 2002). Predictions suggest that 89 of the 93 Ig-domains within the I-band contain cryptic cysteines (Alegre-Cebollada et al. 2014). When I-band Ig-domains unfold, the reaction of cryptic cysteines with oxidized glutathione (GSSG) leads to the formation of S-glutathionylation, which greatly weakens the mechanical properties of the Ig-domain as well as its ability to refold (Fig. 4). This result was obtained for I91 (nomenclature Bang et al. 2001; also known as I27), an Ig-domain at the distal I-band that harbours two buried cysteines. In addition, incubation of single-skinned human cardiomyocytes with GSSG under supraphysiological stretch conditions decreased passive stiffness, and this was consistently reversible upon treatment with reduced glutathione (GSH) (Alegre-Cebollada et al. 2014). In summary, these results revealed a novel mechanism which suggests that the regulation of titin elasticity is controlled by the mechanical unfolding of its Ig-domains during oxidative stress.
Another deleterious effect of chronic oxidative stress is its potential to disturb protein structure and induce protein misfolding. Correct protein folding is an essential aspect of protein function in all organisms, and mechanisms exist to ensure correct protein folding and prevent misfolding. During synthesis proteins translocate into the endoplasmic reticulum (ER), which contains HSPs that function as molecular chaperones. The binding of HSPs enables correctly folded proteins to be distinguished from those that are misfolded. Misfolded proteins are then ubiquitinated and degraded in the cytoplasm by proteasomes. Via this mechanism, molecular chaperones prevent the aggregation of thermally-damaged proteins, mediate unfolding of aggregated proteins, and refold damaged proteins, or, alternatively, target them for efficient degradation (Linke and Hamdani 2014; Bullard et al. 2004; Kötter et al. 2014). Any abnormalities of the chaperones or of the other machinery that regulate polypeptide conformation can contribute to misfolding and aggregation diseases (Saibil 2008). Age and oxidative stress have been firmly linked to a decreased efficiency of molecular chaperones and responses to unfolded proteins (Morimoto 1998; Ahn and Thiele 2003). Misfolded proteins lose their normal functional structure, which can accelerate formation of toxic proteins including oligomers or larger aggregates. Protein aggregates are thought to contribute to pathologies commonly observed in neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease, Huntington's disease, amyotrophic lateral sclerosis and many others. These aggregates may sequester components of the chaperone and degradation systems and thereby reduce their activity (Jiang and Chang 2007). As HSP70 and other heat-inducible chaperones reduce oxidative stress (Kalmar and Greensmith 2009), impairments in heat shock responses may enhance oxidative stress and thereby contribute to the augmented cell death (Burdon et al. 1987; Skibba et al. 1991; McAnulty et al. 2005). Misfolded, dysfunctional proteins have also been shown to be more susceptible to carbonylation compared to their native forms. Rather than dysfunction due to carbonylation and consequent misfolding (Dalle-Donne et al. 2003b), it has been suggested that carbonylation, as an irreversible protein modification, may act as a tagging system for the degradation of an irreparable protein by the proteasomal system (Dukan et al. 2000; Ballesteros et al. 2001). In the heart, α-B-crystallin and HSP27 are induced during ischemic injury, heat stress, or end-stage failure (Martin et al. 1997; Benjamin and McMillan 1998; Knowlton et al. 1998; Yoshida et al. 1999; Dohke et al. 2006). Small HSPs are induced in both myopathic skeletal (Kley et al. 2012) and normal muscles after intense exercise (Paulsen et al. 2009) and during aging (Doran et al. 2007). HSP27 has been shown to preserve cytoskeletal architecture (Mounier and Arrigo 2002) and to play roles in the resistance to oxidative (Huot et al. 1996; Dalle-Donne et al. 2001), thermal and ischemic stress (Vander Heide 2002; Hollander et al. 2004), and to participate in the regulation of cellular redox states (Arrigo 2001) which in turn prevent apoptosis (Mehlen et al. 1996) and correlate with hypoxia (Martin et al. 1997). Since sHSPs are induced during ischemia, acidic stress is a potential trigger. Acidosis has been shown to promote the aggregation of many sHSP, but also to protect against aggregations at low pH (Bennardini et al. 1992; Barbato et al. 1996). It was recently reported that HSP27 or αB-crystallin preferentially translocate under stress conditions to I-band titin springs in both cardiac and skeletal muscles (Bullard et al. 2004 Kötter et al. 2014). The unfolded Ig-domains appear to be better protected against aggregation by HSP27 or αB-crystallin (Kötter et al. 2014), thus reducing myocyte stiffness. Collectively, these data show that multiple stress signals, and in particular oxidative and heat stress, develop in cells under redox challenge and lead to alterations in cellular behaviour and responses.
Titin as a potential biomarker in Chagas’ disease
Increased oxidative/nitrosative/inflammatory stress and inefficient antioxidant capacity has been shown to be associated with Chagas’ disease (Rassi et al. 2010). Chagas’ disease is a chronic parasitic disease of the heart caused by Trypanosoma cruzi. This disease is characterized by cardiomegaly, ventricular dilation and arrhythmia, leading to HF (Rassi et al. 2010). The antioxidant/oxidant imbalance was evident from increased plasma levels of glutathione disulfide and malonyldialdehyde (Wen et al. 2006a), and decreased levels of glutathione (Wen et al. 2006a; de Oliveira et al. 2007) and glutathione peroxidise (Pérez-Fuentes et al. 2003; de Oliveira et al. 2007). In addition, increased levels of phospholipid oxidation products, protein carbonylation, and a heightened glutathione antioxidant defence (Wen et al. 2004) have been shown to associate with oxidative overload in Chagas’ disease (Wen et al. 2006b). Nitrated peptides derived from titin and actin are found in the plasma of patients with Chagas’ disease, and may be useful as biomarkers for the disease (Dhiman et al. 2008). In an experimental setting, oxidation of recombinant titin and actin enhanced their immunogenicity and recognition by sera antibodies from chagasic rats and patients (Dhiman et al. 2012). Treatment with antioxidants (phenylbutylnitrone, vitamin C, and vitamin E) also consistently attenuated oxidative effects in mice (Wen et al. 2006b) and patients with Chagas’ disease (Maçao et al. 2007).
The impact of oxidative stress on the active processes of myocardial relaxation
The diastolic properties of the LV are determined not only by passive distensibility but also by the active processes of myocardial relaxation. This includes mechanisms involved in diastolic Ca2+ sequestration and; abnormalities of this mechanism associates with impaired LV relaxation (Linke and Hamdani 2014). Under normal conditions, the sarcoplasmic reticulum (SR) is the main Ca2+ storage organelle in humans (Ai et al. 2005; Guo et al. 2006). The Ca2+ concentration within the SR is determined by the delicate balance between Ca2+ release through the RyRs and Ca2+ uptake via SERCA (Periasamy and Janssen 2008). Ca2+ is central to a number of cellular functions and impairment of membrane Ca2+ transport has serious deleterious effects, including muscle injury (Zalk et al. 2007; Murphy et al. 2008; Durham et al. 2008; Bellinger et al. 2009; González et al. 2010b; Andersson et al. 2011). Muscle force generation initiates with the rise in cytoplasmic free Ca2+ concentration, whereas the removal of Ca2+ imparts a state of relaxation. Force generation is produced via the cyclic interaction between actin and myosin filaments. This interaction is controlled by the tropomyosin-troponin complex bound to the actin filament (Canton et al. 2006; Chen and Ogut 2006; Horwitz et al. 1979). All mechanisms that regulate Ca2+ in skeletal and cardiac muscles are known to be susceptible to oxidative stress produced by organic free radicals and ROS (Comporti 1989). Increased intracellular Ca2+ is associated with oxidative damage to muscle (Zalk et al. 2007; Durham et al. 2008; Bellinger et al. 2009; Andersson et al. 2011) while the sodium–calcium exchanger is known to contribute to increased intracellular Ca2+ loading during Na+ accumulation (Louch et al. 2010). In addition, oxidative post-translational modifications and Ca2+ mishandling have been shown to be associated with diastolic dysfunction (Linke and Hamdani 2014).
Alterations of the myoplasmic Ca2+ levels during oxidative stress
Calcium and redox signalling cascades are important modulators of cellular function, and concomitant increases in Ca2+ influx and oxidative stress are associated with vascular diseases including hypertension, stroke, and atherosclerosis (Nieves-Cintron et al. 2008; Touyz 2000).
Functional changes in Ca2+ handling have often been linked to post-translational modifications of Ca2+ signalling proteins. While recent evidence indicates a role for oxidative and nitrosative regulation of the Ca2+-sensitive proteome in excitation–contraction (EC) coupling, only phosphorylation is the best-established therapeutic target (Haycock et al. 1996; Posterino and Lamb 1996; Gao et al. 1996; Disatnik et al. 1998; Duncan et al. 2005; Dai et al. 2007; Ullrich et al. 2009; Canton et al. 2014).
Changes in intracellular Ca2+ cycling that are either causal or adaptive have been observed in different models of cardiac disease, including several forms of HF (Zalk et al. 2007; Murphy et al. 2008; Durham et al. 2008; Bellinger et al. 2009; González et al. 2010b; Andersson et al. 2011). Abnormalities in EC coupling components trigger and/or aggravate contractile dysfunction and growing evidence suggests an important role for redox modifications of key signalling components of EC coupling (Haycock et al. 1996; Posterino and Lamb 1996; Gao et al. 1996; Disatnik et al. 1998; Duncan et al. 2005; Dai et al. 2007; Ullrich et al. 2009; Canton et al. 2014). The redox modification of protein kinase activity and direct effects on channels and ion transporters are well-known effects of ROS on ECC (Wagner et al. 2013). Ca2+ channels associated with EC coupling regulate the myoplasmic Ca2+ level. The Ca2+ channels are organized around a system of deep membrane invaginations known as t-tubules. Depolarization of the sarcolemma leads to Ca2+ influx into the sarcoplasm and triggers the release of Ca2+ from the sarcoplasmic reticulum (SR) via the RyR (Ca2+-induced Ca2+ release). Intracellular Ca2+ diffuses to the contractile apparatus and binds to troponin C, ultimately resulting in tropomyosin movement away from myosin-binding sites on actin. Following contraction, the remaining Ca2+ is pumped back into the SR via SERCA. Exposure to H2O2 or GSSG results in the S-glutathionylation of Ca2+-chanels, such as the L-type Ca2+ channels (LTCC), leading to increased diastolic Ca2+ levels and permanent myofilament activation of cardiomyocytes (Viola et al. 2007; Tang et al. 2011). H2O2 has been reported to be involved in the elevation of intracellular calcium concentration in a variety of cell types (Hu et al. 1998; Krippeit-Drews et al. 1999; Redondo et al. 2004). Studies has been suggested that endogenous ROS could serve as an agonist to reinforce the calcium signaling in neutrophils by a positive feedback (Heiner et al. 2003), based on the correlation between an augmented production of H2O2 in primed neutrophils and an enhanced calcium entry. Studies in isolated arterial smooth muscle cells showed that H2O2-dependent Ca2+ influx through L-type Ca2+ channels (Nathan and Gregory, 2012).
β–adrenergic activation via protein kinase A (PKA)-mediated phosphorylation targets several proteins involved in Ca2+ -handling, including the LTCC, SERCA, RyR, and myofilament proteins, such as troponin I and myosin binding protein C (Hamdani et al. 2008). PKA can also be redox-activated through the formation of an inter-disulfide bond between its catalytic subunits (Brennan et al. 2006). A similar mechanism of redox activation has been reported for PKG (Burgoyne et al. 2007). Whether oxidative PKA and PKG activation has any functional consequences in cardiomyocytes remains to be investigated. Prominent examples are the RyRs, which can be modified upon oxidation, S-nitrosation and S-glutathionylation (Wehrens et al. 2005; Meissner 2010; Niggli et al. 2013).The RyRs are known to be sensitive to redox modifications: tetrameric RyR2 contains 364 cysteines, 84 of which have free thiol groups that can be S-nitrosylated (González et al. 2008) glutathionylated (Sánchez et al. 2005).
Similarly, SERCA also undergoes redox modifications. SERCA2a activity is depressed in several pathological conditions associated with increased oxidative and nitrosative stress, such as atherosclerosis (Cohen and Adachi 2006), metabolic syndrome (Balderas-Villalobos et al. 2013), or diabetes mellitus (Shao et al. 2011). This results from the irreversible oxidative modifications of cysteines and other aminoacids. S-glutathionylation of SERCA at Cys674 increases its activity, accelerates Ca2+ uptake, and associates with NO-induced arterial and vascular smooth muscle relaxation (Cohen and Adachi 2006; Adachi et al. 2007). The spontaneous reversibility of S-glutathionylation of SERCA suggests that this modification represents a physiological switch (Adachi et al. 2004). Interestingly, oxidative stress was found to cause an irreversible oxidation of Cys674 to sulfonic acid, thus impairing NO-induced relaxation through the prevention of reversible S-glutathionylation and activation of SERCA in diseased muscles (Cohen and Adachi 2006; Adachi et al. 2007). Similar findings were observed in a study in which oxidative stress decreased activity of SERCA via oxidative modifications of Cys674 and Tyr294/295 (Qin et al. 2014). Taken together, these findings suggest that reduced activity of SERCA due to oxidative stress and the simultaneously increased activity of RyR2 and LTCC could result in diastolic Ca2+ overload, which might in turn lead to slow myocyte relaxation and diastolic dysfunction.
In HF, RyR2 shows diminished levels of S-nitrosylation and associates with increased channel activity (Xu et al. 1998; Donoso et al. 2000; Eu et al. 2000) and disturbed calcium handling and impaired contractility, in spontaneous hypertensive-HF rats (SHHF) (González et al. 2010b). In a canine model of HF, oxidation of RyR2 causes diastolic Ca2+ leakage, an effect that could be partially reversed upon treatment with reducing agents (Terentyev et al. 2008). Hyperphosphorylation of RyR2 at serines 2809 (Wehrens et al. 2006) and 2815 (Ai et al. 2005; Guo et al. 2006) has been proposed to be involved in increased SR leakage and increased activity of RyR2. However, in the latter studies the degree of serine 2809 phosphorylation in SHHF was unchanged, suggesting that excess S-nitrosylation levels may play an important role in increasing the open probability of RyRs, which in turn increases channel activity (leakage). The S-nitrosative modification of cysteines on the RyRs may occur via conversion of NO to peroxynitrite after a chemical reaction with O2 •-. NO activates guanylyl cyclase to produce cGMP, a second messenger with multiple targets in the heart, including PKG and proteins directly involved in intracellular Ca2+ homeostasis (Hammond and Balligand 2012) (Fig. 3).
Contribution of oxidative stress to pathology in muscular dystrophy
Duchenne muscular dystrophy (DMD) is the most common and devastating type of muscular dystrophy. An important pathomechanism is thought to be oxidative stress, which may have a major impact on dystrophic cardiomyopathy. More broadly, elevated oxidative stress could potentially contribute to the damage and weakness of respiratory and limb skeletal muscles, and possibly to the fibrosis observed in DMD. The mdx mouse, which carries a mutation in the dystrophin gene, is a commonly used model for human DMD (Disatnik et al. 1998; Tkatchenko et al. 2000; Hartel et al. 2001). The diaphragm muscle in the mdx mouse is highly susceptible to oxidative stress and shows a disease progression similar to human DMD (Disatnik et al. 1998; Tkatchenko et al. 2000; Hartel et al. 2001). The damage observed in dystrophic cardiomyopathy is mainly caused by abnormalities of Ca2+ signalling, particularly in the early stages of disease (Jung et al. 2007; Prosser et al. 2011). Antioxidant interventions with N-acetylcysteine in mdx mice consistently reduced ROS levels, normalized intracellular Ca2+ levels, improved myofilament function and reduced cardiac inflammation and fibrosis (Williams and Allen 2007). The major source of ROS in dystrophic cardiac tissue seems to be NOX. NOX is upregulated in the mdx mouse, and thus a likely contributor to oxidative stress and the DMD pathology (Williams and Allen 2007; Spurney et al. 2008; Whitehead et al. 2008). Inhibition of NAD(P)H oxidase protected against delayed muscle development and apoptosis through reduced ROS production (Williams and Allen 2007; Whitehead et al. 2008). In addition, a stretch-induced increase in ROS production was completely abolished in the NOX2 knockout mice. The increased intracellular Ca2+ response to mechanical challenge and hypersensitivity of EC coupling were normalized by both ROS scavengers and NOX inhibitors blockers (Jung et al. 2007; Ullrich et al. 2009; Prosser et al. 2011). Taken together, and based on fact that ROS production occurs in the sarcolemmal and t-tubule membranes in close proximity to NOX2, thus sensitising nearby RyRs in the SR, this may suggest that oxidation of RyRs is an early post-translational modification event in dystrophy.
Reduced NO synthase activity in dystrophy may downregulates cGMP signalling pathways. Dystrophic mdx mice with overexpression of guanylyl cyclase consistently show improved cellular integrity, myocardial contractility and energy metabolism (Khairallah et al. 2008). A similarly beneficial effect on cardiac function resulted from treatment of mdx mice with the phosphodiesterase 5 inhibitor sildenafil (Adamo et al. 2010; Percival et al. 2012). Taken together, these findings point to alterations in NO signalling as key contributors to cardiac dystrophy and suggest that the reversal of this redox imbalance might have important pathophysiologic and therapeutic implications.
Cross-talk oxidation and other post-translational modifications within the cell
Oxidation may also influence other post-translational modifications such as phosphorylation, acetylation, ubiquitination, and others. This interplay between different post-translational modifications may represent a complex regulatory network and a broad system that regulates the cell. Oxidative stress typically increases phosphorylation by stimulating protein kinases or inhibiting protein phosphatases (PP) (Meng et al. 2002). A redox-sensitive cysteine found in protein tyrosine phosphatases is highly susceptible to ROS-dependent inactivation, resulting in increased protein tyrosine phosphorylation. Oxidative stress also inactivates the serine/threonine phosphatase calcineurin (Namgaladze et al. 2002). Redox regulation of calcineurin may have an important role in transcriptional programs that regulate cardiac hypertrophy. However, it may not have a direct effect on myofilament proteins, since myofilament protein phosphorylation was reported to be preserved in transgenic mouse models with altered calcineurin activity (Wilkins and Molkentin 2002). However, hypophosphorylation of several myofilament proteins has been attributed to PP1 and 2A (Ke et al. 2004; Humphries et al. 2002; Wu and Solaro 2007). Oxidative stress activates stress kinases, such as several PKC isoforms, PKD, CaMKII and P38 mitogen-activated protein kinases and inactivate PP1 and/or PP2A activity (Jin Jung et al. 2013).
Another intriguing possibility is that phosphorylation of Ig-domains may interfere with S-glutathionylation. Proteomic technologies identified novel phosphorylation sites within the I-band Ig-domains (Hamdani et al. 2013c), although the functional role of this phosphorylation has yet to be established. Theoretically, the phosphorylation of stretched Ig-domains may prevent further S-glutathionylation by blocking cryptic cysteines, facilitating the refolding of these Ig-domains, or, alternatively, phosphorylation itself could disturb the refolding of stretched Ig-domains. Equally, phosphorylation may actually stabilize the conformation of folded Ig-domains, resulting in a lower rate of unfolding and increased passive stiffness (Fig. 4). In summary, the contribution of titin-based passive stiffness upon oxidative stress is highly regulated via a variety of pathways. However, much still remains unknown regarding underlying mechanisms and their diverse interactions.
Conclusion and therapeutic approaches
In conclusion, recent evidence suggests that the contribution of intrinsic cardiac myocyte stiffness to diastolic dysfunction is a promising therapeutic target for HF with diastolic abnormalities, and that oxidative/nitrosative stress-related modifications of several proteins have a major impact on these abnormalities. These findings highlight important mechanisms that may occur in vivo during preload and increased oxidative stress in HFpEF patients. Targeting underlying inflammation, oxidative stress, and age-related stiffness dysfunction may prove to be particularly effective in HFpEF. Nitroxyl donors, such as the newly formulated compound 1-nitrosocyclohexyl acetate (NCA), may have therapeutic benefits in HF due to a direct action of nitroxyl on myofilaments, SERCA2a, and the RyR2, which have overall positive inotropic and lusitropic effects on the heart. Whether nitroxyl donors are beneficial in treating HFpEF still needs to be assessed. On other hand, NO and inhibitors of cGMP-degrading enzymes, some of which are currently approved for treatment of pulmonary arterial hypertension, increase PKG and consequently increase cardiac muscle relaxation through troponin I and titin mediated-PKG phosphorylation (Linke and Hamdani 2014). In addition to these effects, NO signalling also has anti-fibrotic, anti-hypertrophic, and anti-adrenergic actions that oppose adverse cardiac remodelling and thus serve as potential therapeutic strategies for HFpEF. Furthermore, BH4 and eNO synthase activators could be useful in reducing diastolic dysfunction, since the DOCA-salt mouse model is associated with BH4 depletion and uncoupled NO synthase activity, and BH4 administration consistently reduced DOCA-salt-associated MyBP-C glutathionylation and diastolic dysfunction (Lovelock et al. 2012; Jeong et al. 2013; Patel et al. 2013). A successful therapeutic strategy for HFpEF must focus on selecting more consistent patient populations to control for the impact of co-morbidities. Body mass index, smoking, and atrial fibrillation have been identified as traditional risk factors that predict new onset HFpEF; these factors can be important in the prevention and treatment of HFpEF. Finally, aging is associated with increased MMPs levels and increases in MMP-9 in particular, which subsequently leads to diastolic dysfunction. MMP-9 deletion has been shown to attenuate this effect through regulation of the ECM. Selective inhibition of MMP-9 could therefore be a possible avenue for HFpEF treatment.
References
Adachi T, Pimentel DR, Heibeck T, Hou X, Lee YJ, Jiang B, Ido Y, Cohen RA (2004) S-glutathiolation of Ras mediates redox-sensitive signaling by angiotensin II in vascular smooth muscle cells. J Biol Chem 279:29857–29862
Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, Cohen RA (2007) S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10:1200–1207
Adamo CM, Dai DF, Percival JM, Minami E, Willis MS, Patrucco E, Froehner SC, Beavo JA (2010) Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 107:19079–19083
Ahn SG, Thiele DJ (2003) Redox regulation of mammalian heat shock factor 1 is essential for Hsp gene activation and protection from stress. Genes Dev 17:516–528
Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM (2005) Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97:1314–1322
Aihara T, Nakamura M, Ueki S, Hara H, Miki M, Arata T (2010) Switch action of troponin on muscle thin filament as revealed by spin labeling and pulsed EPR. J Biol Chem 285:10671–10677
Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR (2001) Protein oxidation in the brain in Alzheimer's disease. Neuroscience 103:373–383
Alegre-Cebollada J, Kosuri P, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernández JM (2014) S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding. Cell 156:1235–1246
Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, Xie W, Shiomi T, Zalk R, Lacampagne A, Marks AR (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14:196–207
Andrade FH, Reid MB, Allen DG, Westerblad H (1998) Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509:565–575
Andrade FH, Reid MB, Westerblad H (2001) Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J 15:309–311
Arcaro A, Lembo G, Tocchetti CG (2014) Nitroxyl (HNO) for treatment of acute heart failure. Curr Heart Fail Rep 11:227–235
Arrigo AP (2001) Hsp27: novel regulator of intracellular redox state. IUBMB Life 52:303–307
Aryal B, Jeong J, Rao VA (2014) Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity. Proc Natl Acad Sci U S A 111:2011–2016
Avner BS, Shioura KM, Scruggs SB, Grachoff M, Geenen DL, Helseth DL Jr, Farjah M, Goldspink PH, Solaro RJ (2012) Myocardial infarction in mice alters sarcomeric function via post-translational protein modification. Mol Cell Biochem 363:203–215
Balderas-Villalobos J, Molina-Muñoz T, Mailloux-Salinas P, Bravo G, Carvajal K, Gómez-Viquez NL (2013) Oxidative stress in cardiomyocytes contributes to decreased SERCA2a activity in rats with metabolic syndrome. Am J Physiol Heart Circ Physiol 305:H1344–H1353
Ballesteros M, Fredriksson A, Henriksson J, Nyström T (2001) Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J 20:5280–5289
Bang ML, Centner T, Fornoff F, Geach AJ, Gotthardt M, McNabb M, Witt CC, Labeit D, Gregorio CC, Granzier H, Labeit S (2001) The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system. Circ Res 89:1065–1072
Barbato R, Menabò R, Dainese P, Carafoli E, Schiaffino S, Di Lisa F (1996) Binding of cytosolic proteins to myofibrils in ischemic rat hearts. Circ Res 78:821–828
Beckendorf J, Linke WA (2015) Emerging importance of oxidative stress in regulating striated muscle elasticity. Muscle Res Cell Motil 36:25–36
Belch JJ, Bridges AB, Scott N, Chopra M (1991) Oxygen free radicals and congestive heart failure. Br Heart J 65:245–248
Bellinger AM, Reiken S, Carlson C, Mongillo M, Liu X, Rothman L, Matecki S, Lacampagne A, Marks AR (2009) Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15:325–330
Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins: molecular chaperones in cardiovascular biology and disease. Circ Res 83:117–132
Bennardini F, Wrzosek A, Chiesi M (1992) Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res 71:288–294
Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC Jr, Linke WA, Redfield MM (2011) Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 124:2882–2891
Bolli R, Marbán E (1999) Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79:609–634
Borbély A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, Paulus WJ (2005) Cardiomyocyte stiffness in diastolic heart failure. Circulation 111:774–781
Borbély A, Papp Z, Edes I, Paulus WJ (2009a) Molecular determinants of heart failure with normal left ventricular ejection fraction. Pharmacol Rep 61:139–145
Borbély A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, Leite-Moreira AF, Bronzwaer JG, Papp Z, van der Velden J, Stienen GJ, Paulus WJ (2009b) Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res 104:780–786
Borlaug BA, Kass DA (2006) Mechanisms of diastolic dysfunction in heart failure. Trends Cardiovasc Med 16:273–279
Borlaug BA, Paulus WJ (2011) Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 32:670–679
Borlaug BA, Kass DA (2011) Ventricular-vascular interaction in heart failure. Cardiol Clin 29:447–459
Brennan JP, Bardswell SC, Burgoyne JR, Fuller W, Schröder E, Wait R, Begum S, Kentish JC, Eaton P (2006) Oxidant-induced activation of type I protein kinase A is mediated by RI subunit interprotein disulfide bond formation. J Biol Chem 281:21827–21836
Bullard B, Ferguson C, Minajeva A, Leake MC, Gautel M, Labeit D, Ding L, Labeit S, Horwitz J, Leonard KR, Linke WA (2004) Association of the chaperone alphaB-crystallin with titin in heart muscle. J Biol Chem 279:7917–7924
Burdon RH, Gill VM, Rice-Evans C (1987) Oxidative stress and heat shock protein induction in human cells. Free Radic Res Commun 3:129–139
Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P (2007) Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317:1393–1397
Canton M, Neverova I, Menabò R, Van Eyk J, Di Lisa F (2004) Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am J Physiol Heart Circ Physiol 286:H870–H877
Canton M, Skyschally A, Menabò R, Boengler K, Gres P, Schulz R, Haude M, Erbel R, Di Lisa F, Heusch G (2006) Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur Heart J 27:875–881
Canton M, Menazza S, Sheeran FL, Polverino de Laureto P, Di Lisa F, Pepe S (2011) Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol 57:300–309
Canton M, Menazza S, Di Lisa F (2014) Oxidative stress in muscular dystrophy: from generic evidence to specific sources and targets. J Muscle Res Cell Motil 35:23–36
Cazorla O, Szilagyi S, Vignier N, Salazar G, Krämer E, Vassort G, Carrier L, Lacampagne A (2006) Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res 69:370–380
Chaturvedi RR, Herron T, Simmons R, Shore D, Kumar P, Sethia B, Chua F, Vassiliadis E, Kentish JC (2010) Passive stiffness of myocardium from congenital heart disease and implications for diastole. Circulation 121:979–988
Chen FC, Ogut O (2006) Decline of contractility during ischemia-reperfusion injury: actin glutathionylation and its effect on allosteric interaction with tropomyosin. Am J Physiol Cell Physiol 290:C719–C727
Chong PC, Hodges RS (1981) A new heterobifunctional cross-linking reagent for the study of biological interactions between proteins. I. Design, synthesis, and characterization. J Biol Chem 256:5064–5070
Chong PC, Hodges RS (1982) Proximity of sulfhydryl groups to the sites of interaction between components of the troponin complex from rabbit skeletal muscle. J Biol Chem 257:2549–2555
Cline MJ, Lehrer RI (1969) d-amino acid oxidase in leukocytes: A possible d-amino-acid-linked antimicrobial system. Proc Natl Acad Sci U S A 62:756–763
Cohen RA, Adachi T (2006) Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc Med 16:109–114
Coirault C, Guellich A, Barbry T, Samuel JL, Riou B, Lecarpentier Y (2007) Oxidative stress of myosin contributes to skeletal muscle dysfunction in rats with chronic heart failure. Am J Physiol Heart Circ Physiol 292:H1009–H1017
Comporti M (1989) Three models of free radical-induced cell injury. Chem Biol Interact 72:1–56
Cosentino F, Hishikawa K, Katusic ZS, Lüscher TF (1997) High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96:25–28
Dai T, Tian Y, Tocchetti CG, Katori T, Murphy AM, Kass DA, Paolocci N, Gao WD (2007) Nitroxyl increases force development in rat cardiac muscle. J Physiol 580:951–960
Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R (2001) The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med 31:1624–1632
Dalle-Donne I, Giustarini D, Rossi R, Colombo R, Milzani A (2003a) Reversible S-glutathionylation of Cys 374 regulates actin filament formation by inducing structural changes in the actin molecule. Free Radic Biol Med 34:23–32
Dalle-Donne I, Giustarini D, Colombo R, Rossi R, Milzani A (2003b) Protein carbonylation in human diseases. Trends Mol Med 9:169–176
Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A (2007) S-glutathionylation in protein redox regulation. Free Radic Biol Med 43:883–898
de Oliveira TB, Pedrosa RC, Filho DW (2007) Oxidative stress in chronic cardiopathy associated with Chagas disease. Int J Cardiol 116:357–363
Dhiman M, Nakayasu ES, Madaiah YH, Reynolds BK, Wen JJ, Almeida IC, Garg NJ (2008) Enhanced nitrosative stress during Trypanosoma cruzi infection causes nitrotyrosine modification of host proteins: implications in Chagas' disease. Am J Pathol 173:728–740
Dhiman M, Zago MP, Nunez S, Amoroso A, Rementeria H, Dousset P, Nunez Burgos F, Garg NJ (2012) Cardiac-oxidized antigens are targets of immune recognition by antibodies and potential molecular determinants in chagas disease pathogenesis. PLoS ONE 7:e284449
Ding W, Li Z, Shen X, Martin J, King SB, Sivakumaran V, Paolocci N, Gao WD (2011) Reversal of isoflurane-induced depression of myocardial contraction by nitroxyl via myofilament sensitization to Ca2+. J Pharmacol Exp Ther 339:825–831
Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, Rando TA (1998) Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci 161:77–84
Dohke T, Wada A, Isono T, Fujii M, Yamamoto T, Tsutamoto T, Horie M (2006) Proteomic analysis reveals significant alternations of cardiac small heat shock protein expression in congestive heart failure. J Card Fail 12:77–84
Donoso P, Aracena P, Hidalgo C (2000) Sulfhydryl oxidation overrides Mg(2+) inhibition of calcium-induced calcium release in skeletal muscle triads. Biophys J 79:279–286
Doran P, Gannon J, O'Connell K, Ohlendieck K (2007) Aging skeletal muscle shows a drastic increase in the small heat shock proteins alphaB-crystallin/HspB5 and cvHsp/HspB7. Eur J Cell Biol 86:629–640
Doroszko A, Polewicz D, Sawicka J, Richardson JS, Cheung PY, Sawicki G (2009) Cardiac dysfunction in an animal model of neonatal asphyxia is associated with increased degradation of MLC1 by MMP-2. Basic Res Cardiol 104:669–679
Doroszko A, Polewicz D, Cadete VJ, Sawicka J, Jones M, Szczesna-Cordary D, Cheung PY, Sawicki G (2010) Neonatal asphyxia induces the nitration of cardiac myosin light chain 2 that is associated with cardiac systolic dysfunction. Shock 34:592–600
Dröge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Dukan S, Farewell A, Ballesteros M, Taddei F, Radman M, Nyström T (2000) Protein oxidation in response to increased transcriptional or translational errors. Proc Natl Acad Sci U S A 97:5746–5749
Duncan JG, Ravi R, Stull LB, Murphy AM (2005) Chronic xanthine oxidase inhibition prevents myofibrillar protein oxidation and preserves cardiac function in a transgenic mouse model of cardiomyopathy. Am J Physiol Heart Circ Physiol 289:H1512–H1516
Dutka TL, Mollica JP, Lamb GD (2011) Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol 110:705–716. 1985
Durham WJ, Aracena-Parks P, Long C, Rossi AE, Goonasekera SA, Boncompagni S, Galvan DL, Gilman CP, Baker MR, Shirokova N, Protasi F, Dirksen R, Hamilton SL (2008) RyR1 S-nitrosylation underlies environmental heat stroke and sudden death in Y522S RyR1 knockin mice. Cell 133:53–65
Eaton P, Byers HL, Leeds N, Ward MA, Shattock MJ (2002) Detection, quantitation, purification, and identification of cardiac proteins S-thiolated during ischemia and reperfusion. J Biol Chem 277:9806–9811
Eaton P (2006) Protein thiol oxidation in health and disease: techniques for measuring disulfides and related modifications in complex protein mixtures. Free Radic Biol Med 40:1889–1899
Eu JP, Sun J, Xu L, Stamler JS, Meissner G (2000) The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell 102:499–509
Falcão-Pires I, Hamdani N, Borbély A, Gavina C, Schalkwijk CG, van der Velden J, van Heerebeek L, Stienen GJ, Niessen HW, Leite-Moreira AF, Paulus WJ (2011) Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 124:1151–1159
Fiaschi T, Cozzi G, Raugei G, Formigli L, Ramponi G, Chiarugi P (2006) Redox regulation of beta-actin during integrin-mediated cell adhesion. J Biol Chem 281:22983–22991
Fratelli M, Demol H, Puype M, Casagrande S, Eberini I, Salmona M, Bonetto V, Mengozzi M, Duffieux F, Miclet E, Bachi A, Vandekerckhove J, Gianazza E, Ghezzi P (2002) Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc Natl Acad Sci U S A 99:3505–3510
Gao WD, Atar D, Backx PH, Marban E (1995) Relationship between intracellular calcium and contractile force in stunned myocardium. Direct evidence for decreased myofilament Ca2+ responsiveness and altered diastolic function in intact ventricular muscle. Circ Res 76:1036–1048
Gao WD, Liu Y, Marban E (1996) Selective effects of oxygen free radicals on excitation-contraction coupling in ventricular muscle. Implications for the mechanism of stunned myocardium. Circulation 94:2597–2604
Gao WD, Murray CI, Tian Y, Zhong X, DuMond JF, Shen X, Stanley BA, Foster DB, Wink DA, King SB, Van Eyk JE, Paolocci N (2012) Nitroxyl-mediated disulfide bond formation between cardiac myofilament cysteines enhances contractile function. Circ Res 111:1002–1011
Giugliano D, Ceriello A, Paolisso G (1996) Oxidative stress and diabetic vascular complications. Diabetes Care 19:257–267
González DR, Fernández IC, Ordenes PP, Treuer AV, Eller G, Boric MP (2008) Differential role of S-nitrosylation and the NO-cGMP-PKG pathway in cardiac contractility. Nitric Oxide 18:157–167
González A, López B, Querejeta R, Zubillaga E, Echeverría T, Díez J (2010a) Filling pressures and collagen metabolism in hypertensive patients with heart failure and normal ejection fraction. Hypertension 55:1418–1424
González DR, Treuer AV, Castellanos J, Dulce RA, Hare JM (2010b) Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem 285:28938–28945
Grützner A, Garcia-Manyes S, Kötter S, Badilla CL, Fernandez JM, Linke WA (2009) Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys J 97:825–834
Guo T, Zhang T, Mestril R, Bers DM (2006) Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res 99:398–406
Hamdani N, Kooij V, van Dijk S, Merkus D, Paulus WJ, Remedios CD, Duncker DJ, Stienen GJ, van der Velden J (2008) Sarcomeric dysfunction in heart failure. Cardiovasc Res 77:649–658
Hamdani N, Paulus WJ, van Heerebeek L, Borbély A, Boontje NM, Zuidwijk MJ, Bronzwaer JG, Simonides WS, Niessen HW, Stienen GJ, van der Velden J (2009) Distinct myocardial effects of beta-blocker therapy in heart failure with normal and reduced left ventricular ejection fraction. Eur Heart J 30:1863–1872
Hamdani N, Paulus WJ (2011) Treatment of heart failure with normal ejection fraction. Curr Treat Options Cardiovasc Med 13:26–34
Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA (2013a) Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res 97:464–471
Hamdani N, Franssen C, Lourenço A, Falcão-Pires I, Fontoura D, Leite S, Plettig L, López B, Ottenheijm CA, Becher PM, González A, Tschöpe C, Díez J, Linke WA, Leite-Moreira AF, Paulus WJ (2013b) Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail 6:1239–1249
Hamdani N, Krysiak J, Kreusser MM, Neef S, Dos Remedios CG, Maier LS, Krüger M, Backs J, Linke WA (2013c) Crucial role for Ca2(+)/calmodulin-dependent protein kinase-II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res 112:664–674
Hamdani N, Hervent AS, Vandekerckhove L, Matheeussen V, Demolder M, Baerts L, De Meester I, Linke WA, Paulus WJ, De Keulenaer GW (2014) Left ventricular diastolic dysfunction and myocardial stiffness in diabetic mice is attenuated by inhibition of dipeptidyl peptidase 4. Cardiovasc Res 104:423–431
Hammond J, Balligand JL (2012) Nitric oxide synthase and cyclic GMP signaling in cardiac myocytes: from contractility to remodeling. J Mol Cell Cardiol 52:330–340
Hartel JV, Granchelli JA, Hudecki MS, Pollina CM, Gosselin LE (2001) Impact of prednisone on TGF-beta1 and collagen in diaphragm muscle from mdx mice. Muscle Nerve 24:428–432
Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D (1996) Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport 8:357–361
Heiner I, Eisfeld J, Lückhoff A (2003) Role and regulation of TRP channels in neutrophil granulocytes. Cell Calcium 33:533–540
Hertelendi Z, Tóth A, Borbély A, Galajda Z, van der Velden J, Stienen GJ, Edes I, Papp Z (2008) Oxidation of myofilament protein sulfhydryl groups reduces the contractile force and its Ca2+ sensitivity in human cardiomyocytes. Antioxid Redox Signal 10:1175–1184
Heusch P, Canton M, Aker S, van de Sand A, Konietzka I, Rassaf T, Menazza S, Brodde OE, Di Lisa F, Heusch G, Schulz R (2010) The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br J Pharmacol 160:1408–1416
Hidalgo C, Hudson B, Bogomolovas J, Zhu Y, Anderson B, Greaser M, Labeit S, Granzier H (2009) PKC phosphorylation of titin's PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res 105:631–638
Hill MF, Singal PK (1996) Antioxidant and oxidative stress changes during heart failure subsequent to myocardial infarction in rats. Am J Pathol 148:291–300
Hincke MT, McCubbin WD, Kay CM (1979) The interaction between beef cardiac troponin T and troponin I as demonstrated by ultraviolet absorption difference spectroscopy, circular dichroism, and gel filtration. Can J Biochem 57:768–775
Hollander JM, Martin JL, Belke DD, Scott BT, Swanson E, Krishnamoorthy V, Dillmann WH (2004) Overexpression of wild-type heat shock protein 27 and a nonphosphorylatable heat shock protein 27 mutant protects against ischemia/reperfusion injury in a transgenic mouse model. Circulation 110:3544–3552
Horwitz J, Bullard B, Mercola D (1979) Interaction of troponin subunits. The interaction between the inhibitory and tropomyosin-binding subunits. J Biol Chem 254:350–355
Hu Q, Corda S, Zweier JL, Capogrossi MC, Ziegelstein RC et al (1998) Hydrogen peroxide induces intracellular calcium oscillations in human aortic endothelial cells. Circulation 97:268–275
Humphries KM, Juliano C, Taylor SS (2002) Regulation of cAMP-dependent protein kinase activity by glutathionylation. J Biol Chem 277:43505–43511
Huot J, Houle F, Spitz DR, Landry J (1996) HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res 15:273–279
Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC Jr, Solaro RJ (2013) Tetrahydrobiopterin improves diastolic dysfunction by reversing changes in myofilament properties. J Mol Cell Cardiol 56:44–54
Ji LL (2007) Antioxidant signaling in skeletal muscle: A brief review. Exp Gerontol 42:582–593
Jiang C, Chang JY (2007) Isomers of human alpha-synuclein stabilized by disulfide bonds exhibit distinct structural and aggregative properties. Biochemistry 46:602–609
Jin Jung K, Hyun Kim D, Kyeong Lee E, Woo Song C, Pal Yu B, Young Chung H (2013) Oxidative stress induces inactivation of protein phosphatase 2A, promoting proinflammatory NF-κB in aged rat kidney. Free Radic Biol Med 61:206–217
Jung C, Martins AS, Niggli E, Shirokova N (2007) Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signalling and reactive oxygen species-generating pathways. Cardiovasc Res 77:766–773
Kalmar B, Greensmith L (2009) Induction of heat shock proteins for protection against oxidative stress. Adv Drug Deliv Rev 61:310–318
Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ (2004) Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res 94:194–200
Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, Deschepper CF, Petrof BJ, Des Rosiers C (2008) Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci U S A 105:7028–7033
Kim C, Kim JY, Kim JH (2008) Cytosolic phospholipase A2, lipoxygenase metabolites, and reactive oxygen species. BMB Rep 41:555–559
Kley RA, Serdaroglu-Oflazer P, Leber Y, Odgerel Z, van der Ven PF, Olivé M, Ferrer I, Onipe A, Mihaylov M, Bilbao JM, Lee HS, Höhfeld J, Djinović-Carugo K, Kong K, Tegenthoff M, Peters SA, Stenzel W, Vorgerd M, Goldfarb LG, Fürst DO (2012) Pathophysiology of protein aggregation and extended phenotyping in filaminopathy. Brain 135:2642–2660
Kloner RA, Jennings RB (2001a) Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation 104:2981–2989
Kloner RA, Jennings RB (2001b) Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 2. Circulation 104:3158–3167
Knowlton AA, Kapadia S, Torre-Amione G, Durand JB, Bies R, Young J, Mann DL (1998) Differential expression of heat shock proteins in normal and failing human hearts. J Mol Cell Cardiol 30:811–818
Kötter S, Gout L, Von Frieling-Salewsky M, Müller AE, Helling S, Marcus K, Dos Remedios C, Linke WA, Krüger M (2013) Differential changes in titin domain phosphorylation increase myofilament stiffness in failing human hearts. Cardiovasc Res 99:648–656
Kötter S, Unger A, Hamdani N, Lang P, Vorgerd M, Nagel-Steger L, Linke WA (2014) Human myocytes are protected from titin aggregation-induced stiffening by small heat shock proteins. J Cell Biol 204:187–202
Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G (1999) Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol 514:471–481
Krüger M, Linke WA (2006) Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil 27:435–444
Krüger M, Kötter S, Grützner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA (2009) Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 104:87–94
Krüger M, Linke WA (2009) Titin-based mechanical signalling in normal and failing myocardium. J Mol Cell Cardiol 46:490–498
Krüger M, Babicz K, von Frieling-Salewsky M, Linke WA (2010) Insulin signaling regulates cardiac titin properties in heart development and diabetic cardiomyopathy. J Mol Cell Cardiol 48:910–916
Labeit S, Kolmerer B (1995) Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science 270:293–296
Lamb GD, Junankar PR, Stephenson DG (1995) Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 489:349–362
Lamb GD, Posterino GS (2003) Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol 546:149–163
Lamb GD, Westerblad H (2011) Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol 589:2119–2127
Leite‐Moreira AF (2006) Current perspectives in diastolic dysfunction and diastolic heart failure. Heart 92:712–718
Li H, Linke WA, Oberhauser AF, Carrion-Vazquez M, Kerkvliet JG, Lu H, Marszalek PE, Fernandez JM (2002) Reverse engineering of the giant muscle protein titin. Nature 418:998–1002
Linke WA (2008) Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res 77:637–648
Linke WA, Fernandez JM (2002) Cardiac titin: molecular basis of elasticity and cellular contribution to elastic and viscous stiffness components in myocardium. J Muscle Res Cell Motil 23:483–497
Linke WA, Krüger M (2010) The giant protein titin as an integrator of myocyte signaling pathways. Physiology (Bethesda) 25:186–198
Linke WA, Hamdani N (2014) Gigantic business: titin properties and function through thick and thin. Circ Res 114:1052–1068
Louch WE, Hougen K, Mørk HK, Swift F, Aronsen JM, Sjaastad I, Reims HM, Roald B, Andersson KB, Christensen G, Sejersted OM (2010) Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. J Physiol 588:465–478
Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC Jr (2012) Ranolazine improves cardiac diastolic dysfunction through modulation of myofilament calcium sensitivity. Circ Res 110:841–850
Maçao LB, Wilhelm Filho D, Pedrosa RC, Pereira A, Backes P, Torres MA, Fröde TS (2007) Antioxidant therapy attenuates oxidative stress in chronic cardiopathy associated with Chagas' disease. Int J Cardiol 123:43–49
MacFarlane NG, Miller DJ (1992) Depression of peak force without altering calcium sensitivity by the superoxide anion in chemically skinned cardiac muscle of rat. Circ Res 70:1217–1224
MacFarlane NG, Miller DJ (1994) Effects of the reactive oxygen species hypochlorous acid and hydrogen peroxide on force production and calcium sensitivity of rat cardiac myofilaments. Pflugers Arch 428:561–568
Makarenko I, Opitz CA, Leake MC, Neagoe C, Kulke M, Gwathmey JK, del Monte F, Hajjar RJ, Linke WA (2004) Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res 95:708–716
Mallat Z, Philip I, Lebret M, Chatel D, Maclouf J, Tedgui A (1998) Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 97:1536–1539
Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH (1997) Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation 96:4343–4348
Mayans O, Wuerges J, Canela S, Gautel M, Wilmanns M (2001) Structural evidence for a possible role of reversible disulphide bridge formation in the elasticity of the muscle protein titin. Structure 9:331–340
McAnulty SR, McAnulty L, Pascoe DD, Gropper SS, Keith RE, Morrow JD, Gladden LB (2005) Hyperthermia increases exercise-induced oxidative stress. Int J Sports Med 26:188–192
Mehlen P, Schulze-Osthoff K, Arrigo AP (1996) Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem 271:16510–16514
Meissner G (2010) Regulation of Ryanodine Receptor Ion Channels Through Posttranslational Modifications. Curr Top Membr 66:91–113
Meng TC, Fukada T, Tonks NK (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol Cell 9:387–399
Minajeva A, Kulke M, Fernandez JM, Linke WA (2001) Unfolding of titin domains explains the viscoelastic behavior of skeletal myofibrils. Biophys J 80:1442–1451
Mollica JP, Dutka TL, Merry TL, Lamboley CR, McConell GK, McKenna MJ, Murphy RM, Lamb GD (2012) S-glutathionylation of troponin I (fast) increases contractile apparatus Ca2+ sensitivity in fast-twitch muscle fibres of rats and humans. J Physiol 590:1443–1463
Morimoto RI (1998) Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev 12:3788–3796
Mounier N, Arrigo AP (2002) Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones 7:167–176
Murphy RM, Dutka TL, Lamb GD (2008) Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol 586:2203–2216
Namgaladze D, Hofer HW, Ullrich V (2002) Redox control of calcineurin by targeting the binuclear Fe(2+)-Zn(2+) center at the enzyme active site. J Biol Chem 277:5962–5969
Nathan LC, Gregory CA (2012) Hydrogen peroxide mediates oxidant-dependent stimulation of arterial smooth muscle L-type calcium channels. Am J Physiol Cell Physiol 302:C1382–C1393
Neagoe C, Kulke M, del Monte F, Gwathmey JK, de Tombe PP, Hajjar RJ, Linke WA (2002) Titin isoform switch in ischemic human heart disease. Circulation 106:1333–1341
Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF (2008) The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A 105:15623–15628
Niggli E, Ullrich ND, Gutierrez D, Kyrychenko S, Poláková E, Shirokova N (2013) Posttranslational modifications of cardiac ryanodine receptors: Ca(2+) signaling and EC-coupling. Biochim Biophys Acta 1833:866–875
Nishino T (1994) The conversion of xanthine dehydrogenase to xanthine oxidase and the role of the enzyme in reperfusion injury. J Biochem 116:1–6
Nishino T, Okamoto K, Eger BT, Pai EF (2008) Mammalian xanthine oxidoreductase - Mechanism of transition from xanthine dehydrogenase to xanthine oxidase. FEBS J 275:3278–3289
Passarelli C, Petrini S, Pastore A, Bonetto V, Sale P, Gaeta LM, Tozzi G, Bertini E, Canepari M, Rossi R, Piemonte F (2008) Myosin as a potential redox-sensor: an in vitro study. J Muscle Res Cell Motil 29:119–126
Passarelli C, Di Venere A, Piroddi N, Pastore A, Scellini B, Tesi C, Petrini S, Sale P, Bertini E, Poggesi C, Piemonte F (2010) Susceptibility of isolated myofibrils to in vitro glutathionylation: Potential relevance to muscle functions. Cytoskeleton (Hoboken) 67:81–89
Patel BG, Wilder T, Solaro RJ (2013) Novel control of cardiac myofilament response to calcium by S-glutathionylation at specific sites of myosin binding protein C. Front Physiol 4:336
Paulsen G, Lauritzen F, Bayer ML, Kalhovde JM, Ugelstad I, Owe SG, Hallén J, Bergersen LH, Raastad T (2009) Subcellular movement and expression of HSP27, alphaB-crystallin, and HSP70 after two bouts of eccentric exercise in humans. J Appl Physiol 107:570–582
Paulus WJ (2010) Novel strategies in diastolic heart failure. Heart 96:1147–1153
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271
Percival JM, Whitehead NP, Adams ME, Adamo CM, Beavo JA, Froehner SC (2012) Sildenafil reduces respiratory muscle weakness and fibrosis in the mdx mouse model of Duchenne muscular dystrophy. J Pathol 228:77–87
Pérez-Fuentes R, Guégan JF, Barnabé C, López-Colombo A, Salgado-Rosas H, Torres-Rasgado E, Briones B, Romero-Díaz M, Ramos-Jiménez J, Sánchez-Guillén Mdel C (2003) Severity of chronic Chagas disease is associated with cytokine/antioxidant imbalance in chronically infected individuals. Int J Parasitol 33:293–299
Periasamy M, Janssen PM (2008) Molecular basis of diastolic dysfunction. Heart Fail Clin 4:13–21
Pizarro GO, Ogut O (2009) Impact of actin glutathionylation on the actomyosin-S1 ATPase. Biochemistry 48:7533–7538
Polewicz D, Cadete VJ, Doroszko A, Hunter BE, Sawicka J, Szczesna-Cordary D, Light PE, Sawicki G (2011) Ischemia induced peroxynitrite dependent modifications of cardiomyocyte MLC1 increases its degradation by MMP-2 leading to contractile dysfunction. J Cell Mol Med 15:1136–1147
Posterino GS, Lamb GD (1996) Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad. J Physiol 496:809–825
Prosser BL, Ward CW, Lederer WJ (2011) X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333:1440–1445
Qin F, Siwik DA, Pimentel DR, Morgan RJ, Biolo A, Tu VH, Kang YJ, Cohen RA, Colucci WS (2014) Cytosolic H2O2 mediates hypertrophy, apoptosis, and decreased SERCA activity in mice with chronic hemodynamic overload. Am J Physiol Heart Circ Physiol 306:H1453–H1463
Querejeta R, López B, González A, Sánchez E, Larman M, Martínez Ubago JL, Díez J (2004) Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 110:1263–1268
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2:219–236
Raskin A, Lange S, Banares K, Lyon RC, Zieseniss A, Lee LK, Yamazaki KG, Granzier HL, Gregorio CC, McCulloch AD, Omens JH, Sheikh F (2012) A novel mechanism involving four-and-a-half LIM domain protein-1 and extracellular signal-regulated kinase-2 regulates titin phosphorylation and mechanics. J Biol Chem 287:29273–29284
Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375:1388–1402
Redondo PC, Salido GM, Pariente JA, Rosado JA (2004) Dual effect of hydrogen peroxide on store-mediated calcium entry in human platelets. Biochem Pharmacol 67:1065–1076
Reid MB (2001) Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc 33:371–376
Sabbah HN, Tocchetti CG, Wang M, Daya S, Gupta RC, Tunin RS, Mazhari R, Takimoto E, Paolocci N, Cowart D, Colucci WS, Kass DA (2013) Nitroxyl (HNO): A novel approach for the acute treatment of heart failure. Circ Heart Fail 6:1250–1258
Sadayappan S, Gulick J, Klevitsky R, Lorenz JN, Sargent M, Molkentin JD, Robbins J (2009) Cardiac myosin binding protein-C phosphorylation in a {beta}-myosin heavy chain background. Circulation 119:1253–1262
Sadayappan S, de Tombe PP (2012) Cardiac myosin binding protein-C: redefining its structure and function. Biophys Rev 4:93–106
Saibil HR (2008) Chaperone machines in action. Curr Opin Struct Biol 18:35–42
Salvolini E, Rabini RA, Martarelli D, Moretti N, Cester N, Mazzanti L (1999) A study on human umbilical cord endothelial cells: functional modifications induced by plasma from insulin-dependent diabetes mellitus patients. Metabolism 48:554–557
Sanchez R, Riddle M, Woo J, Momand J (2008) Prediction of reversibly oxidized protein cysteine thiols using protein structure properties. Protein Sci 17:473–481
Sánchez G, Pedrozo Z, Domenech RJ, Hidalgo C, Donoso P (2005) Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J Mol Cell Cardiol 39:982–991
Shao CH, Rozanski GJ, Nagai R, Stockdale FE, Patel KP, Wang M, Singh J, Mayhan WG, Bidasee KR (2010) Carbonylation of myosin heavy chains in rat heart during diabetes. Biochem Pharmacol 80:205–217
Shao CH, Capek HL, Patel KP, Wang M, Tang K, DeSouza C, Nagai R, Mayhan W, Periasamy M, Bidasee KR (2011) Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes 60:947–959
Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang X, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW 2nd, Brown JH, Peterson KL, Omens JH, McCulloch AD, Chen J (2008) An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest 118:3870–3880
Siwik DA, Pagano PJ, Colucci WS (2001) Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280:C53–C60
Skibba JL, Powers RH, Stadnicka A, Cullinane DW, Almagro UA, Kalbfleisch JH (1991) Oxidative stress as a precursor to the irreversible hepatocellular injury caused by hyperthermia. Int J Hyperthermia 7:749–761
Smith MA, Reid MB (2006) Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol 151:229–241
Solaro RJ (2007) Nitroxyl effects on myocardium provide new insights into the significance of altered myofilament response to calcium in the regulation of contractility. J Physiol 580:697
Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP (2008) Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord 18:371–381
Srinivasan S, Hatley ME, Bolick DT, Palmer LA, Edelstein D, Brownlee M, Hedrick CC (2004) Hyperglycaemia-induced superoxide production decreases eNOS expression via AP-1 activation in aortic endothelial cells. Diabetologia 47:1727–1734
Sumimoto H, Miyano K, Takeya R (2005) Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun 338:677–686
Takimoto E, Kass DA (2007) Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49(2):241–248
Tang H, Viola HM, Filipovska A, Hool LC (2011) Cav1.2 calcium channel is glutathionylated during oxidative stress in guinea pig and ischemic human heart. Free Radic Biol Med 51:1501–1511
Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, de Blanco EC, Khanna S, Sen CK, Cardounel AJ, Carnes CA, Györke S (2008) Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res 103:1466–1472
Terrill JR, Radley-Crabb HG, Iwasaki T, Lemckert FA, Arthur PG, Grounds MD (2013) Oxidative stress and pathology in muscular dystrophies: focus on protein thiol oxidation and dysferlinopathies. FEBS J 280:4149–4164
Thomas JA, Mallis RJ (2001) Aging and oxidation of reactive protein sulfhydryls. Exp Gerontol 36:1519–1526
Tiago T, Simão S, Aureliano M, Martín-Romero FJ, Gutiérrez-Merino C (2006) Inhibition of skeletal muscle S1-myosin ATPase by peroxynitrite. Biochemistry 45:3794–3804
Tkatchenko AV, Le Cam G, Léger JJ, Dechesne CA (2000) Large-scale analysis of differential gene expression in the hindlimb muscles and diaphragm of mdx mouse. Biochim Biophys Acta 1500:17–30
Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL (2008) Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res 103:974–982
Touyz RM (2000) Oxidative stress and vascular damage in hypertension. Curr Hypertens Rep 2:98–105
Ullrich ND, Fanchaouy M, Gusev K, Shirokova N, Niggli E (2009) Hypersensitivity of excitation-contraction coupling in dystrophic cardiomyocytes. Am J Physiol Heart Circ Physiol 297:H1992–H2003
van Heerebeek L, Borbély A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, Linke WA, Laarman GJ, Paulus WJ (2006) Myocardial structure and function differ in systolic and diastolic heart failure. Circulation 113:1966–1973
van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ (2008) Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation 117:43–51
van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ (2012) Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126:830–839
Vander Heide RS (2002) Increased expression of HSP27 protects canine myocytes from simulated ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 282:H935–H941
Viola HM, Arthur PG, Hool LC (2007) Transient exposure to hydrogen peroxide causes an increase in mitochondria-derived superoxide as a result of sustained alteration in L-type Ca2+ channel function in the absence of apoptosis in ventricular myocytes. Circ Res 100:1036–1044
Wagner S, Rokita AG, Anderson ME, Maier LS (2013) Redox regulation of sodium and calcium handling. Antioxid Redox Signal 18:1063–1077
Wang J, Boja ES, Tan W, Tekle E, Fales HM, English S, Mieyal JJ, Chock PB (2001) Reversible glutathionylation regulates actin polymerization in A431 cells. J Biol Cell 276:47763–47766
Wehrens XH, Lehnart SE, Marks AR (2005) Intracellular calcium release and cardiac disease. Annu Rev Physiol 67:69–98
Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR (2006) Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A 103:511–518
Wen JJ, Vyatkina G, Garg N (2004) Oxidative damage during chagasic cardiomyopathy development: role of mitochondrial oxidant release and inefficient antioxidant defense. Free Radic Biol Med 37:1821–1833
Wen JJ, Yachelini PC, Sembaj A, Manzur RE, Garg NJ (2006a) Increased oxidative stress is correlated with mitochondrial dysfunction in chagasic patients. Free Radic Biol Med 41:270–276
Wen JJ, Bhatia V, Popov VL, Garg NJ (2006b) Phenyl-alpha-tert-butyl nitrone reverses mitochondrial decay in acute Chagas' disease. Am J Pathol 169:1953–1964
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4:44–52
Whitehead NP, Pham C, Gervasio OL, Allen DG (2008) N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol 586:2003–2014
Wilkins BJ, Molkentin JD (2002) Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 541:1–8
Williams IA, Allen DG (2007) The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol 293:H1969–H1977
Williams L, Howell N, Pagano D, Andreka P, Vertesaljai M, Pecor T, Frenneaux M, Granzier H (2009) Titin isoform expression in aortic stenosis. Clin Sci (Lond) 117:237–242
Wong WT, Tian XY, Xu A, Ng CF, Lee HK, Chen ZY, Au CL, Yao X, Huang Y (2010) Angiotensin II type 1 receptor-dependent oxidative stress mediates endothelial dysfunction in type 2 diabetic mice. Antioxid Redox Signal 13:757–768
Wu SC, Solaro RJ (2007) Protein kinase C zeta. A novel regulator of both phosphorylation and de-phosphorylation of cardiac sarcomeric proteins. J Biol Chem 282:30691–30698
Xu L, Eu JP, Meissner G, Stamler JS (1998) Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279:234–237
Yamasaki R, Wu Y, McNabb M, Greaser M, Labeit S, Granzier H (2002) Protein kinase A phosphorylates titin's cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res 90:1181–1188
Yoshida K, Aki T, Harada K, Shama KM, Kamoda Y, Suzuki A, Ohno S (1999) Translocation of HSP27 and MKBP in ischemic heart. Cell Struct Funct 24:181–185
Zalk R, Lehnart SE, Marks AR (2007) Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76:367–385
Zangar RC, Davydov DR, Verma S (2004) Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199:316–331
Compliance with ethical standards
Conflict of interest
Martin Breitkreuz and Nazha Hamdani declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Breitkreuz, M., Hamdani, N. A change of heart: oxidative stress in governing muscle function?. Biophys Rev 7, 321–341 (2015). https://doi.org/10.1007/s12551-015-0175-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-015-0175-5