Abstract
Colonial heterocorals are uncommon faunal component of the Serpukhovian limestones of the Montagne Noire and Pyrenees in southern France. Four species are introduced into the newly established genus Semenomalophyllia. They all share the typical septal arrangement of heterocorals, at least in early stage of development. The fused axial ends of septa commonly withdraw during the ontogeny of larger sized species such as Semenomalophyllia herbigi and S. perretae. In S. weyeri, the septa are withdrawn from the axis and arranged in two series (‘minor’ and ‘major’) that show a striking morphological convergence with rugose corals. The corallites of the smallest species S. webbi are very similar to the solitary heterocorallian Heterophyllia ornata and possibly evolved from it. S. weyeri and S. perretae are commonly colonised by alcyonacean octocorals as indicated by the occurrence of sclerites covering the corallites. These are described as Lafustalcyon vachardi gen. et sp. nov., a new taxon yet only known in the Serpukhovian strata, and only affecting heterocoral colonies. In addition to the alcyonacean octocorals, a diverse assemblage of epibionts colonised the heterocorals: calcifying microbes, bryozoans, foraminifers, microconchids, crinoids, boring organisms, and microproblematica. Syn-vivo relationships can be demonstrated only between Semenomalophyllia and Lafustalcyon as the first commonly bio-immured the second. Other encrusting organisms could have colonised either living, erected colonies or broken or tilted dead colonies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As recently summarised by Weyer (2016), the colonial habitus in Heterocorallia has long been discussed. The occurrence of branching specimens in the Upper Devonian of Poland and Germany (Różkowska 1969; Wrzołek 1981; Weyer 1995) and the lower Carboniferous of England and China (Scrutton 1977; Lin et al. 1992) suggests the possibility for heterocorals to develop colonies. However, in many cases, the distinction between genuine offsets and separated individuals, which developed after larval attachment on the wall of mature corallites, is inconclusive (Cossey 1997; Weyer 2016). Cossey (1997) and Chwieduk (2001) consider that encrustation of juvenile individuals on mature ones is prevailing, since the internal cavities of the ‘offsets’ and ‘parent corallites’ are never connected. Indeed, the figuration of the ‘offsets’ of the colonial Oligophylloides pachythecus Różkowska, 1969 from the upper Famennian (Upper Devonian) of Poland (Wrzołek 1981) does not show any connection between the cavities of the parent corallites and offsets. Hence, the interpretation as separate individuals is most likely, but true coloniality cannot be ruled out. Similarly, Cossey (1997) illustrated branching Heterophyllia ornata McCoy, 1849 from the Viséan of England and argued that the variable – often high – angle of divergence between the juvenile and mature corallites associated with the absence of change in the morphology of the mature corallite near the ‘offsets’ suggest that the coloniality is the result of encrustation rather than budding. An additional argument is the occurrence of ‘offsets’ on the spines covering the external wall of mature corallites. Weyer (2016) described ‘increase’ in Oligophylloides maroccanus Weyer, 2016 from the Upper Devonian of Morocco but he could not conclude with certainty, if his material is true colonies or not. Hence, he introduced the term ‘paracolonies’ to describe an assemblage of protocorallites encrusting older corallites in a gregarious manner, mimicking genuine colonies with asexual growth. In any kind of corals, genuine colonies produced by asexual increase require the (genetically controlled?) ability of a polyp to produce more than one generation of offsets able to reproduce asexually (Fedorowski and Ogar 2013).
Convincing evidences for colonial heterocorals were reported by Sugiyama (1984) from the upper Viséan of Japan. Radiciphyllia Sugiyama, 1984 is described as an elongate, flexuous coral occasionally forming dendroid colonies. However, only one out of the two species attributed to the genus seems to be colonial: Radiciphyllia toriyamai Sugiyama, 1984. This taxon presents a lateral increase with offsets connected to the cavity of its parent corallite (cf. Sugiyama 1984: pl. 7, fig. 6).
In this study, we document new species of colonies that belong to a new genus of Heterocorallia from the Serpukhovian strata of Montagne Noire and Pyrenees in southern France. The Pyrenees specimens were previously reported by Perret and Semenoff-Tian-Chansky (1971) as “Lithostrotion? cf. tareense Pickett, 1966” that were in part revised by Tourneur et al. (1995) but never published until now.
Geological setting and sampled localities
In southern France, late Viséan and Serpukhovian shallow-water carbonates are found as resedimented blocks (olistoliths) in the Southern Variscan foreland basin (e.g., Delvolvé et al. 1993; Aretz 2016). Hence, they are part of the synorogenic sedimentation, which demonstrates the complete change of the basin dynamics in this part of the Variscan Chain (Aretz 2016) from the late Viséan onward. The in situ platform, on which these carbonates originally formed, is not preserved anywhere in Southern France. However, the carbonates clearly formed in various settings and positions on a mixed carbonate-siliciclastic ramp (e.g., Aretz 2002a; Aretz et al. 2019; Cózar et al. 2019).
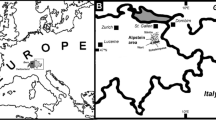
The best outcrops for those shallow-water carbonates are found in the SE Montagne Noire (e.g., Aretz 2002a, 2016; Cózar et al. 2019), the Mouthoumet Massif (Schulze 1982; Bessière and Schulze 1984), and in the northern and central French Pyrenees (e.g., Perret 1971, 1993; Vachard et al. 1991) (Fig. 1a).
a Simplified distribution map of the Palaeozoic rocks in southern France, with the position of the sampled areas in the Pyrenees and in the Montagne Noire; b simplified geological map and distribution of the carbonate olistoliths (Ardengost Limestone) with the approximate position of the sample locality in the Bois de Jezeau (4) east of the Ardengost village (42°91′49.82″N 0.°39′50.39″E); c positions of the sampled localities, (1) Roc de Murviel (43°33′06.66’’N 3°16′34.12’’E), (2) La Serre (43°55′53.21’’N 3°35′64.43’’E) and (3) Serre de Péret (43°57′52.61’’N 3°37′61.32’’E) on a simplified geological map of the Ecailles de Cabrières unit at the SE tip of the Montagne Noire
Facies analysis reveals that these olistoliths majorly represent reefal environments, which are locally highly fossiliferous. The best studied examples are from the Montagne Noire, where a vast spectrum from coral-microbe reefs to microbial mounds and their surrounding peri-reefal facies can be observed (Aretz 2002a, b; Aretz and Herbig 2003; Cózar et al. 2019).
Microbial communities largely contributed to the formation of these reefal carbonates, and they are the major biological constituents in most facies (Aretz 2002a). Other organisms found in relative abundance are foraminifers (Vachard et al. 2016a), calcareous algae (Vachard et al. 2016b), rugose corals (Aretz 2002a, b; Poty and Hecker 2003; Poty et al. 2019), and echinoderms. More localised or less in overall abundance are the occurrences of bryozoans (Ernst et al. 2015), brachiopods (Aretz et al. 2019), tabulate corals, sponges, and gastropods. Note that solitary heterocorals are rare in the Montagne Noire, where only scattered occurrences have been reported in the upper Viséan (Vachard 1977; Aretz 2002a).
New stratigraphical investigations for the Montagne Noire (Poty et al. 2002; Vachard and Aretz 2004; Vachard et al. 2016a, b) show a much more homogeneous age distribution between the different regions in southern France, than previously postulated (e.g., Delvolvé et al. 1993). Based on foraminifers, the youngest olistoliths of the Montagne Noire are dated as Arnsbergian (Poty et al. 2002) (Protvian, i.e., third regional substage of the Serpukhovian in the Russian type-area, sensu Vachard et al. 2016a; Cózar et al. 2019) (Fig. 2). The Ardengost Limestone (e.g., Perret 1971) in the central French Pyrenees is also Arnsbergian in age (Russian substage Zapaltjubian, Vachard pers. com.), so possibly slightly younger than the olistoliths from the Montagne Noire. However, the coral macrofauna yielded by the Roc de Murviel Fm in the Montagne Noire and Ardengost Limestone in the Pyrenees are similar (Poty et al. 2002).

modified from Poty et al. (2002) with additions from Aretz (2016) and Vachard et al. (2016a). The present material comes from the Roc de Murviel Formation. Abbreviations and legend: 1–2: chronostratigraphy, 3: substages, 4: Mississippian foraminiferan zones (Poty et al. 2006), 5: Rugose coral zones (Poty et al. 2006), 6: lithostratigraphy, 7: sea level, Arns.: Arnsbergian substage, Fm.: Formation, R.R.: Roque Redonde Formation, R: regression, T: transgression
Stratigraphical framework of the late Viséan-Serpukhovian of the Montagne Noire,
The vast majority of the collected material described herein is from localities in the Montagne Noire (Fig. 1c). The most productive localities are La Serre vineyard (Fig. 3c–d) and the upper part of the succession at the Roc de Murviel (Fig. 3a), and other specimens were collected in the Serre de Péret locality. Detailed description of the sections can be found in Vachard (1977) and Aretz (2002a). All localities have been attributed to the Roc de Murviel Formation by Poty et al. (2002), and based on rugose corals and carbonate microfossils, a Serpukhovian age has been established for them (Poty et al. 2002; Aretz 2002a; Vachard and Aretz 2004; Vachard et al. 2016a; Cózar et al. 2019) (Fig. 2). The Serre de Péret section exposes the upper part of the Roc de Murviel Formation and yielded the younger Serpukhovian fauna (both corals and foraminifers) of the Montagne Noire (Poty et al. 2002; Vachard et al. 2016a).
A few specimens have been collected by M.-F. Perret from an olistolith in the Bois de Jezeau east of Ardengost (Perret and Semenoff-Tian-Chansky 1971) (Figs. 1b, 3d). This block belongs to the Ardengost Limestone, also Serpukhovian in age (Perret and Vachard 1977).
The heterocoral colonies occur in the Roc de Murviel Fm with large-sized Axophyllum spp., Gangamophyllum sp., Tizraia sp., Siphonodendron pauciradiale, S. irregulare, Lithostrotion decipiens, Palastraea carbonaria, Pareynia sp., Kizilia sp., Serraphyllum vineensis, S. serraensis, and Melanophyllidium sp. However, the occurrences of the species belonging to the genera Semenomanophyllia gen. nov., Serraphyllum and Melanophyllidium are restricted to the Roc de Murviel Formation. This coral assemblage is the local expression of the Serpukhovian RC9 zone of Poty et al. (2006) in the Montagne Noire. Note that the zone RC9 is based on the disappearance of the corals known in the previous zone and no typical guide taxa can be used globally as most Serpukhovian fauna show a high level of endemism.
Systematic palaeontology
The material is deposited in the Animal and Human Palaeontology Collection of the University of Liège (Belgium) under prefix ULg.PA, in the Palaeontology Collection of the Geological Institute of the University of Cologne (Germany) under prefix GIK, and in the Palaeontology Collection of the University Paul Sabatier in Toulouse (France) under prefix UPS-PAL-MA.
Phylum Coelenterata Frey and Leuckart, 1847
Class Anthozoa Ehrenberg, 1834
Subclass Zoantharia de Blainville, 1830
Superorder Heterocorallia Schindewolf, 1941
Family Heterocorallidae Dybowski, 1873 emend. Fernández-Martínez et al., 2003
Emended diagnosis. Solitary or colonial Heterocorallia with slender corallites. Initial growth stages including four or six septa axially joined, new septa formed by peripheral division of the previous septa. Tabulae complete, steeply sloping outwardly, with confluent edges forming a heterotheca.
Microstructure. Heterotheca (thickened tabulotheca made of outwardly fused tabulae, typical for heterocorals) and septa made of long, concave microlamellae. See Lafuste (1981) and Fernández-Martínez et al. (2003) for the discussion of the differences between Devonian and Carboniferous genera of Heterocorallia in term of microstructure.
Remarks. The diagnosis of the family Heterophyllidae is here emended to include colonial forms, whereas the other diagnostic characters are those highlighted by Fedorowski (1991) and Fernández-Martínez et al. (2003) who emended the original diagnosis given by Dybowski (1873).
Genus Semenomalophyllia gen. nov.
Type species. Semenomalophyllia herbigi gen. et sp. nov., Serpukhovian (lower Carboniferous), La Serre, Montagne Noire, southern France.
Other species included. In addition to the type species S. herbigi sp. nov., S. webbi sp. nov. and S. weyeri sp. nov., both from the Serpukhovian of the Montagne Noire, as well as S. perretae sp. nov. from the Serpukhovian of the French Pyrenees are included.
Remarks. This colonial genus was first discovered by the late Pierre Semenoff-Tian-Chansky in Marie-France Perret’s collection from the Pyrenees. Late Jean Lafuste started to study the microstructure, but he died before finishing it and Francis Tourneur tried to complete the work, but his descriptions remained unpublished. Tourneur et al. (1995) presented the material during the 7th International Congress on Fossil Cnidaria and Porifera in Madrid under the name Anomalophyllia. However, according to the ICZN, the name is considered as nomen nudum and should not be retained (Cossey 1997). A new name is here introduced to circumvent this nomenclatural issue.
Etymology. Semenomalophyllia is a contraction of Semenoff and Anomalophyllia, as a reminder of the name proposed in 1995. A strange name for a strange creature.
Diagnosis. Fasciculate heterocoral with lateral increase. Corallites elongated, subcylindrical or prismatic. Septa commonly joined in the centre of the corallite, but frequently detached in larger corallites where they remain joined in bundles or entirely independent from each other. Septa made of a thin middle blade and stereoplasma with their base embedded in the heterotheca. Peripheral edges of the septa thickened and projected outwardly. Tabulae dome- or mesa-shaped with or without concave-upwards axial part. Calyx convex upwards (axial cone). Increase lateral non-parricidal.
Increase and blastogeny. The increase is similar in all four species of Semenomalophyllia and is here exemplified by serial acetate peels through an offset of S. perretae in colony number UPS-PAL-MA-100, prepared by Pierre Semenoff-Tian-Chansky in the 1990s when he planned to publish the present material. The development of the offsets starts with the widening of the parent corallite which acquires an elliptical section, together with the withdrawing of the septa towards the periphery (peels #53–51 in Fig. 4). Sections of heterotheca form through local thickening of the tabulae in the area separating the parent corallite and its offset. However, this thickening is not persistent and internal cavities of both corallites remain connected through interseptal loculi (#49–46). The offset starts then to stretch towards the exterior, while septa inherited from the parent corallite lengthen (#45–42). While the heterotheca invaginates on both sides of the offset, it becomes thicker, as do some of the septa (#41–40). The connection between the offset and its parent ceases when the heterotheca entirely forms and separates the internal cavities of both corallites. The offset is completely individualised, but remains connected to the parent by flange-like expansions of the theca (#39–38). These expansions resorb as the offset becomes cylindrical and grows parallel to its parent. When the offset is completely independent, its septa lengthen and some of them connect in the centre of the corallite (#36). At this stage, the offset has a diameter of c. 2 mm and 20 septa. The blastogeny of Semenomalophyllia perretae shares with that of the Stylostrotionidae Stylostrotion sudeticum Fedorowski, 1991 the connection of the cavities and inheritance of some septa from the parent corallites, as exemplified by Berkowski (1997). However, all other morphological characters of Semenomalophyllia spp. point to their attribution to the Heterocorallidae Dybowski, 1873 and not to the Stylostrotionidae Fedorowski, 1991. Although the early stage of growth of individual offsets differs from early stages of development of heterocorals (protosepta cannot be distinguished), the insertion of septa seems to follow the pattern described in solitary heterocorals (Hexaphyllia spp. and Heterophyllia spp.) by Poty (1977) and Sugiyama (1984).
Camera lucida drawing of serial acetate peels through an offset of Semenomalophyllia perretae (specimen UPS-PAL-MA-100) from the Serpukhovian of Ardengost, exemplifying the blastogeny in this genus; # number identifies the acetate peel section, whereas the second number corresponds to the distance (in millimetres) from the oldest section. Selected pictures of the acetate peels are presented in the right column
Remarks. Four morpho-species of Semenomalophyllia are distinguished based on their size, number of septa (Fig. 5), and morphological complexity. From the colonial species of the genus Radiciphyllia (upper Viséan of Japan), Semenomalophyllia species differ by their fasciculate habitus, whereas Radiciphyllia species are dendroid. The Japanese species is characterised by the disintegration of the trabeculae within the proximal part of the septa embedded in the external wall, but in Semenomalophyllia, the septa enter the wall without being modified. Semenomalophyllia clearly differs from Oligophylloides by the lack of a thick protoheterotheca and its colonial habitus. Isolated corallites of Semenomalophyllia are very similar to those of Heterophyllia and distinguishing one from the other is not straightforward. However, larger corallites of Semenomalophyllia differ by septa commonly not connected in the axial area. The close morphological relationship between Heterophyllia (e.g., H. ornata) and Semenomalophyllia species indicate a probable phylogenetic link between the solitary genus known from Viséan strata and the younger colonial genus known from Serpukhovian strata.
The palaeobiology of heterocorals has been described by Wrzołek (1981) and Chwieduk (2001), mostly based on Oligophylloides spp., and by Fedorowski (1991). Those authors concluded that the heterocorals had no calyx, but only septal cones developed on the axial structure and that the polyps might have occupied the terminal tip of the corallites and been attached to the septal cones. Semenomalophyllia species with large corallites which have the septa detached from the axis and withdrawn towards the periphery of the corallite points to a different palaeobiological interpretation. Hence, it is suggested that the polyps were attached to the septa, as in rugose corals, rather than attached to the axial cones. This alternative interpretation is nevertheless hypothetical as no axial tip or calyx has been yet described in Semenomalophyllia.
Stratigraphic and geographic distribution. To date, the genus Semenomalophyllia has only been reported from the Montagne Noire and French Pyrenees in limestone olistoliths of Serpukhovian age.
Semenomalophyllia herbigi gen. et sp. nov.
Holotype of Semenomalophyllia herbigi gen. et sp. nov. (specimen ULg.PA.1993–33-1/399) form the Serpukhovian of La Serre; a, e, g–h general view of the colony in transverse section (TS); b–c close-up views of offsetting corallites, TS; d, f longitudinal section (LS) showing two offsets; i–k close-up views of mature corallites bearing juvenile ones, most probably developed from individual larva that settled on the heterotheca, TS (compare to b–c)
Semenomalophyllia herbigi gen. et sp. nov.; a–e, j general view of the colony in transversal section (TS) (specimen ULg.PA.1992–1/88); f, i close-up view of an elongated corallite before emission of an offset, same specimen, TS; g-h general view of the colony in longitudinal and transverse sections (ULg.PA.1999–33-1/532); k close-up view of an elongated corallite before emission of an offset, encrusted with two other corallites probably resulting from the settlement of larvae on the heterotheca, TS (ULg.PA.1993–33-1/399)
Semenomalophyllia herbigi gen. et sp. nov. (a–b) and Semenomalophyllia webbi gen. et sp. nov. (c–j); a close-up view of S. herbigi showing three corallites attached to each other in transverse section (TS) (specimen ULg.PA.1999–33-1/532); b close-up view of S. herbigi showing one corallite with two offsets and one additional corallite attached (on the right), TS (ULg.PA.1999–33-1/532); c corallite of S. webbi showing the typical septal arrangement of Heterocorallia, TS (ULg.PA.1999–1/420); d corallite of S. webbi showing the typical septal arrangement of Heterocorallia, TS (UPS-PAL-MA-101); e general view of colony, TS (GIK 1756); f general view of holotype specimen, TS (ULg.PA.1999–1/420) from the Serpukhovian of La Serre; g, j general views of the colony in longitudinal section (ULg.PA.1999–1/439); h–i general views colony, TS (UPS-PAL-MA-101)
Etymology. The species is dedicated to Hans-Georg Herbig, distinguished geologist and palaeontologist who contributed greatly to the knowledge of the Carboniferous Period, its stratigraphy, sedimentology and biota.
Holotype. Specimen ULg.PA.1993–33-1/399 – Roc de Murviel.
Locality and horizon. Roc de Murviel, Roc de Murviel Formation, RC9 rugose coral zone, Serpukhovian (Poty et al. 2002).
Diagnosis. Fasciculate colonies with corallites 2.6–3.4 mm in diameter, subcylindrical-to-prismatic, 16–20 septa arranged symmetrically.
Studied material. Fragments of six colonies (ULg.PA.1992–1/88, ULg.PA.1999–33-1/399, ULg.PA.1999–33-1/415, ULg.PA.1999–33-1/417, ULg.PA.1999–33-1/449 and ULg.PA.1999–33-1/532, 22 thin sections) from Roc de Murviel and La Serre, Montagne Noire, southern France.
Description. Phaceloid-to-dendroid colonies with offsets and probably paracolonial parts with numerous juvenile corallites settled on mature ones. Rejuvenescence suspected. Corallites tortuous, irregular in size and shape, subcylindrical with elliptical or subpolygonal sections. Corallites 2.6–3.4 mm in diameter (tabularium 2–2.5 mm in diameter) having 16–20 septa (Fig. 5). Heterotheca up to 0.4 mm thick with base of septa embedded and occasionally forming longitudinal ridges outwardly. Septa variable in length but not obviously arranged in cycles or in different orders. Septa made of a thin middle dark blade and stereoplasma. Two septa always connect in the centre (counter-cardinal?). Other septa either radially arranged or forming symmetrical bundles on each side of the alar septa. Some corallites show a pseudofossula with a septum withdrawn towards the periphery but not corresponding to a depression in the tabularium. In longitudinal section, tabulae appear incomplete, dome- or mesa-shaped. There are c. 20 tabulae per vertical centimetre.
Remarks. S. herbigi is characterised by its corallites of intermediate diameter compared to other species. It differs from the smaller species S. webbi by its disjoined septa and from the larger species S. weyeri by septa not differentiated in ‘minor’ and ‘major’ series. From S. perretae of similar size, S. herbigi differs by the smooth aspect of its heterotheca.
Stratigraphic and geographic distribution. The species is restricted to the Serpukhovian strata of the Montagne Noire.
Semenomalophyllia webbi gen. et sp. nov.
Figure 8d–i
2002 Colonial ? heterocoral—Aretz: pl. 14, figs. 7–8.
Etymology. The species is dedicated to Gregory E. Webb, American-Australian geologist and palaeontologist, specialist – among others – of fossil and Recent corals and reefs.
Holotype. Specimen ULg.PA-420 – La Serre.
Locality and horizon. La Serre vineyard, Roc de Murviel Formation, RC9 rugose coral zone, Serpukhovian (Poty et al. 2002).
Diagnosis. Fasciculate colonies with small corallites 2 mm in diameter, with 12–16 septa arranged by pairs or in bundles.
Studied material. Fragments of three colonies (GIK1786, UPS-PAL-MA-101 and ULg.PA.1999–1/439, 12 thin sections) from La Serre, Montagne Noire, southern France.
Description. Phaceloid or dendroid colonies with numerous offsets. Increase lateral with offset rapidly separated from parent corallite and growing perpendicularly to it before bending upwards. One to three offsets develop at the same time. Corallites small, commonly in groups of two or four individuals, 5 mm apart. Corallites 1.6–2.2 mm in diameter, with subcircular-to-subpolygonal sections, having 12–16 septa (Fig. 5). Heterotheca up to 0.2–0.3 mm thick with base of septa embedded. Septa straight or tortuous, commonly convergent two by two or in bundles of two to four septa. In largest corallites, septa occasionally not joined and slightly withdrawn from the axis. Occasionally, a pseudofossula marked by a shorter septum develops. In longitudinal section, tabulae appear incomplete, dome-shaped with sigmoidal shoulders. There are c. 25 tabulae per vertical centimetre.
Remarks. With its small corallites, S. webbi is easily distinguished from the other species of Semenomalophyllia. It differs from small corallites of the somewhat larger S. herbigi by its septa almost always joined in the centre, whereas they are commonly detached in the larger species. As explained in the discussion of the genera, isolated corallites of S. webbi are similar to the solitary Heterophyllia ornata with which it shares a comparable diameter and number of septa. The solitary species, however, is commonly compressed dorso-ventraly and generally displays elliptical transverse sections (Poty 1977), whereas S. webbi is subcircular or subpolygonal.
Distribution. The species is restricted to the Serpukhovian strata of the Montagne Noire and only the La Serre section yielded this species so far.
Semenomalophyllia weyeri gen. et sp. nov.
Figure 9a–l
Semenomalophyllia weyeri gen. et sp. nov.; a general view of the holotype in transversal section (TS) (specimen ULg.PA.1993–6/170) from the Serpukhovian of Serre de Péret; b–c general views of the colony, TS (ULg.PA.1993–6/140); d–g close-up views of corallites showing the development of two series of septa and an offset in (e), TS (ULg.PA.1993–6/170); h colony in longitudinal section (LS) (ULg.PA.1993–6/140); i–k views of colony, LS and TS (UPS-PAL-MA-102); l close-up view of a corallite, TS (ULg.PA.1993–6/140)
1971 Lithostrotion? cf. tareense Pickett, 1966—Perret and Semenoff-Tian-Chansky: 570, pl. 1, fig. 1a–c.
Etymology. The species is dedicated to Dieter Weyer, distinguished and inspiring palaeontologist who tremendously contributed to the study of Palaeozoic corals.
Holotype. Specimen ULg.PA.1993–6/170 – Serre de Péret.
Locality and horizon. Serre de Péret, Roc de Murviel Formation, RC9 rugose coral zone, Serpukhovian (Aretz 2002a).
Diagnosis. Phaceloid-to-dendroid colonies with large corallites 6–7 mm in diameter, with subcircular or elliptical sections, 30–36-long septa arranged radially, differentiated into ‘minor’ and ‘major’ series. External side of the heterotheca usually smooth.
Studied material. Fragments of two colonies (specimens ULg.PA.1993–6/140 and ULg.PA.1993–6/170, 12 thin sections) from Serre de Péret, Montagne Noire, and fragments of one colony (UPS-PAL-MA-102, 5 thin sections) from Jezeau, Pyrenees, southern France. Diagenesis has more or less altered the skeleton, which often becomes blurry with recrystallisation.
Description. Phaceloid-to-dendroid colonies with paracolonial parts where juvenile corallites settled on mature ones. Corallites tortuous, irregular in size, subcylindrical with circular or elliptical sections. Corallites 4–5 mm in diameter (tabularium 2.5–4 mm in diameter) having 30–36 septa (Fig. 5). Heterotheca up to 0.4 mm thick with base of septa embedded. Septa variable in length, some are joined in bundles, and other are withdrawn. Septa of different length, differentiated into ‘major’ and ‘minor’ series, disposed in irregular alternation. In the longitudinal section, tabulae appear as incomplete, flat-topped dome with vesicular aspect. There are c. 25 tabulae per vertical centimetre.
Remarks. S. weyeri is the largest species of the genus and differs from large corallites of S. perretae by the smooth aspect of its heterotheca. From S. herbigi it differs mainly by the differentiation of the septa into ‘minor’ and ‘major’ orders.
Stratigraphic and geographic distribution. The species occurs in the Serpukhovian Roc de Murviel Formation in the Montagne Noire and in the Ardengost Limestone in the Pyrenees.
Semenomalophyllia perretae gen. et sp. nov.
Figure 10a–i
Holotype of Semenomalophyllia perretae gen. et sp. nov. (specimen UPS-PAL-MA-100) from the Serpukhovian of Jezeau; a polished slab of the type specimen in longitudinal section (LS); b–e general view of the colony in transverse section (TS); f–h view of different corallites with lateral offsets in LS; i–j close-up views of corallites, TS; k close-up view of the heterotheca showing septal ridges, TS
Etymology. The species is dedicated to Marie-France Perret, who first discovered the colonial heterocorals in the Pyrenees.
Holotype. Specimen UPS-PAL-MA-100 – Bois de Jezeau near Ardengost, French Pyrenees.
Locality and horizon. Forest road in Jezeau near Ardengost, Ardengost Limestone, RC9 rugose coral zone, Serpukhovian (Poty et al. 2002).
Diagnosis. Phaceloid colonies with large corallites 6–7 mm in diameter, subcircular or elliptical in transverse section, 24–28-long septa arranged radially. Outer surface of heterotheca with prominent longitudinal septal ridges.
Studied material. Only the holotype is available (28 thin sections and 124 serial acetate peels).
Description. Phaceloid colonies with corallites c. 5 mm apart. Corallites subcylindrical, circular, or polygonal in section, with prominent lateral extension of the septa on the outer side of the heterotheca. Corallites 3–3.5 mm in diameter (tabularium 2.8–3 mm in diameter) having 24–28 septa (maximum 33). Heterotheca thin with base of septa embedded and forming longitudinal ridges up to 0.2 mm high. Septa variable in length, withdrawn from the axis, with ‘minor’ septa irregularly alternating with ‘major’ septa in largest corallites. Axial end of the septa is usually free, but one or two septa longer than the corallite diameter occasionally connect. Other septa variable in length but usually not connected. Some septa display rhopaloid axial ends. ‘Minor’ septa commonly confluent with the neighbouring ‘major’ ones. In the longitudinal section, tabulae appear relatively complete, dome- or mesa-shaped, regularly spaced (30 tabulae per vertical centimetre) with shoulder sagging towards the heterotheca with an angle of 50–70°.
Remarks. S. perretae differs from S. herbigi and S. weyeri by the prominent longitudinal ridges on the outer side of the heterotheca.
Stratigraphic and geographic distribution. The species is restricted to the Serpukhovian Ardengost Limestone in the French Pyrenees.
Subclass Octocorallia Haeckel, 1866
Order Alcyonacea Lamouroux, 1812
Genus Lafustalcyon gen. nov.
Etymology. The name is a fusion of Lafuste and alcyon, in dedication to the late Jean Lafuste, French coral specialist who was the first to suspect the true nature of Syringoalcyon, association of tabulate coral and epibionts.
Type species. Lafustalcyon vachardi gen. et sp. nov.
Diagnosis. Set of scale-like to irregular spindle-like sclerites, commonly smooth on the convex side and poorly ornamented on the concave side.
Remarks. The morphology of sclerites among alcyonacean octocorals is known to be very variable (Verseveldt and Bayer 1988; Fabricius and Alderslade 2001) and depends on the position within the organism (e.g., van Ofwegen et al. 2007). The peculiar scale- to needle-like morphology observed in the present material has never been described from Carboniferous octocorals. It seems therefore accurate to include them in a new genus – Lafustalcyon – that differs from other previously described genera. Termieraclyon (type species T. maroccanum Fernández-Martínez et al. (2019) from the upper Viséan of Morocco) displays crook- to spindle-shaped sclerites up to 150 µm thick. ‘Syringoalcyon’ sclerites from the Viséan of Tien-Shan (Ogar 1992) are up to 200 µm thick and more commonly polygonal in section. Palaeozoic occurrences of alcyonacean octocorals are scarce (Reich 2002). Atractosella from the Ordovician and Silurian of the Baltic area and Wales clearly differs by the large dimensions of its sclerites (1–2 mm long in average) (Bengtson 1981; Reich 2002). In the present material, there is no evidence for morphological diversity among the sclerites. All sclerites display the same needle- to scale-like morphology (Fig. 12) whatever their position in the armature of the colony. An explanation might be that only the basal sclerites’ assemblage of the alcyonacean skeleton is preserved and more complex sclerites (club-, rod-, spindle-shaped, verrucose, and spinose) usually occurring in distal part of the corolla (surface layer, polyp armature, etc.) are not preserved in the present material. However, isolated sclerites scattered in the sedimentary matrix around the heterocoral colonies display similar simple morphologies. Moreover, it has to be noted that the preservation of the present fossil material is far from perfect and we cannot exclude a slight recrystallisation of the sclerites, with an obliteration of the ornamentation, resulting in apparently smooth(er) morphologies.
Lafustalcyon vachardi gen. et sp. nov.
Morphology of the sclerites of Lafustalcyon vachardi gen. et sp. nov. (specimen ULg.PA.1999–33-1/417); a view of a set of sclerites showing the typical smooth convex side and crenulated concave side; b shadow view of the sclerite in transverse section (in blue) and c in longitudinal section. Same scale for all sclerites
Epibionts on Semenomalophyllia spp.: Lafustalcyon vachardi gen. et. sp. nov.; a–c, e sclerites preserved on the heterotheca of S. weyeri (specimen ULg.PA.1993–6/140); a, e views in transverse section (TS); b–c views in longitudinal section (LS); d cluster of sclerites between two corallites (ULg.PA.1993–6/140); f sclerites immured between two corallites of S. herbigi, TS (ULg.PA.1992–1/88); g sclerites immured between two corallites of S. herbigi, TS (ULg.PA.33–1/399); h sclerites partly immured in a rejuvenating corallites of S. weyeri, TS (ULg.PA.1993–6/170); i sclerites immured between two corallites of S.webbi, note the heterothecal expansion marked by the arrow, TS (ULg.PA.1999–1/420)
Etymology. The species is dedicated to Daniel Vachard, French foraminifer specialist who investigated the Carboniferous limestones of the Montagne Noire.
Holotype. Specimen ULg.PA.1999–33-1/417 – Roc de Murviel.
Locality and horizon. Roc de Murviel, Roc de Murviel Formation, RC9 rugose coral zone, Serpukhovian (Poty et al. 2002).
Studied material. Numerous specimens coating heterocoral colonies, and uncountable isolated sclerites scattered in the carbonate sediment around the colonies. The most interesting specimens occur in colonies UPS-PAL-MA-102 (Ardengost), ULg.PA.1993–6/140 (Serre de Péret), ULg.PA.1993–6/417 and ULg.PA.1999–33/449 (Roc de Murviel), and ULg.PA.1999–1/420 (La Serre).
Diagnosis. Set of scale-like to spindle-like or needle-like sclerites, commonly smooth or poorly ornamented, 100–400 µm-wide, 400–600 µm high and less than 25 µm thick. Most commonly arranged in densely packed layers on corallites of Semenomalophyllia spp.
Description. Set of sclerites made of single crystal of calcite. Their morphologies vary from curved scales to irregular spindles (collaret spindle sensu Ofwegen et al. 2007). The sclerites are 100–400 µm wide and 400–600 µm high but some reaches 800 µm. They are commonly very thin, never thicker than 25–30 µm. Where cut transversally they are typically curved, with the convex side towards the wall of the corallite they cover. Longitudinally, the curvature is less regular (Fig. 12). Their surface is commonly smooth on the convex side and poorly ornamented with short protuberances or crenulations on the concave side (Fig. 12a, c). In most cases, the sclerites are arranged in densely packed layers around the corallites of Semenomalophyllia, where individual sclerites are c. 30° oblique to the vertical surface of the corallites (Fig. 11a–c). Occasionally, a second layer parallel to the corallite surface occurs (Fig. 11c). In transverse section, the coating is commonly discontinuous, covering half or less of the surface of the corallites. Loose sclerites occur in the sedimentary matrix, some being still grouped parallel to each other. In some cases, groups of sclerites are immured in concave sites between two corallites or between the corallites and another epibiont. In rare cases, they are covered by other epibionts (calcifying microbes or bryozoan).
Stratigraphic and geographic distribution. Serpukhovian Roc de Murviel Formation in the Montagne Noire and Ardengost Formation in the French Pyrenees.
Origin and evolution of Semenomalophyllia gen. nov.
As discussed by Weyer (2016), coloniality among heterocorals has several origins. Paracolonies formed by gregarious assemblages of solitary individuals, occasionally settled and cemented on older individual, seems to be rather common in Oligophylloides (Weyer 2016) and potentially in Heterophyllia (Cossey 1997). Genuine colonies formed by asexual increase are not so common and observed only in the genera Radiciphyllia and Semenomalophyllia, as demonstrated by the connection of the cavities of both parent corallite and offsets.
The origin of Semenomalophyllia is probably to be found in budding Heterophyllia (Cossey 1997, and personal observations). However, no Heterophyllia is known in the Serpukhovian strata of the Montagne Noire, which suggest that the evolution of colonial heterocorals might be possibly happened slightly earlier. The three species of Semenomalophyllia are all from the RC9 biozone but obviously from different stratigraphical horizons within the Roc de Murviel Formation with different rugose coral assemblages. Three localities in the Montagne Noire yielded the colonies: Roc de Murviel (S. herbigi), La Serre (S. herbigi and S. webbi, the later in an apparently stratigraphically higher position in the olistoliths), and Serre de Péret (S. weyeri) that yielded the youngest Serpukhovian coral and foraminiferal assemblage in the Montagne Noire. The facies are similar in all localities and might represent bioclastic accumulations associated with microbial buidups (Aretz 2002a, b, 2016).
A hypothetic phylogenetic lineage of the four species of Semonomalophyllia can be established as follows. S. herbigi has a size and number of septa similar to those of Heterophyllia ornata and could possibly be the oldest species of the Semenomalophyllia lineage. Morphologically similar but larger in size, S. herbigi possibly evolved from S. webbi through peramorphosis (increase in size and complexity). In the same way, S. weyeri possibly evolved from S. herbigi with a clear morphological complexification (differentiation of septa into two series ‘major’ and ‘minor’) and through an increase in size. Nevertheless, the relative stratigraphical position of these three species cannot be precisely established and the proposed lineage is only supported by the observation of morphological characters. The origin of S. perretae is not resolved. With its size and number of septa, the Pyrenees species is similar to S. herbigi, but its heterotheca bearing prominent septal ridges is not present in the potential ancestor, making this species very different from the proposed lineage.
Epibionts and ethology
Epibionts are encrusting organisms which colonise another organism, commonly of larger dimensions (Walker and Miller 1992). Depending on the type of organisms involved and the colonised substrate, several types of epibionts have been defined (epizoan, epiphyte, sclerobiont, etc., see Taylor and Wilson 2002). Where host and encrusting organisms are involved in a syn-vivo relationship, it is referred to as symbiosis, even if the recognition of such association in the fossil record is not easy (Zapalski 2007; Tapanila 2008; Vinn 2017a; Zatoń et al. 2017; Zatoń and Wrzołek 2020).
Overall, Carboniferous symbiotic relationships involving corals are particularly uncommon compared to the rest of the Palaeozoic (Sando 1984; Vinn 2017b). Cases involving heterocorals are even rarer, with the notable exception of Oligophylloides maroccanus Weyer, 2016 (Dworczak et al. 2019) from the Upper Devonian of Morocco. No case has been documented so far in the Carboniferous. We here document the numerous epibionts colonising the skeleton of the newly described species of Semenomalophyllia.
All 16 colonies of all four species display at least one epibiont (Table 1, Figs. 12–13). Besides calcifying microbes that are present on all colonies, the most common epibionts of Semenomalophyllia spp. are heterocorals (larvae settled on mature corallites of the same species—interspecific settlement has not been observed so far) (c. 90% of the colonies are affected), bryozoans (c. 70% of the colonies affected), alcyonacean octocorals (c. 40%), foraminifers, and other carbonate epibionts (50%). Moreover, c. 40% of the colonies are affected by bioerosion (borings) that occurs mainly on species with large corallites, i.e., S. weyeri and S. herbigi, both from the La Serre de Péret outcrop. Indeed, the occurrence and proportion of epibionts vary from species to species. Microbial carbonate structures coat up to 20% of the corallites in each of the 16 analysed colonies. The alcyonacean octocoral Lafustalcyon occurs mostly on S. weyeri (c.16% of the corallites in this species, but in less than 1% in the other species). Bryozoans occur mostly on S. weyeri (c. 10% of the corallites are affected), but heterocoral epibionts affect mostly S. webbi (Fig. 13a–e) and, to a lesser extent, S. herbigi. This repartition is tentatively explained by the size of the corallites and distance between individual branches that offer a support for the epibionts. Lafustalcyon seems to have preferentially occupied colonies with large corallites, mostly the lower living-tissue-free part of the branches.
However, in a single colony, only a few corallites are coated by sclerites, either because the alcyonacean octocorals were small, or because the sclerites are rarely preserved in situ. An argument for the second hypothesis is the common preservation of sclerites in cavities between corallites (Fig. 13f–i) or under microbial coating (Fig. 14h) and bryozoan encrusting colonies (Fig. 15i). As commonly observed (e.g., Reich 2002), entire alcyonacean skeletons are rare compared to isolated sclerites dispersed in the sediment.
Epibionts on Semenomalophyllia spp.: microconchids, bryozoans, calcifying microbes, borings, and heterocorals; a spirorbiform microconchid attached to S. herbigi, view in transverse section (TS) (specimen ULg.PA.1992–1/88); b small Gastrochaenolites isp. boring in S. weyeri coated by a fistuliporid bryozoan, TS (ULg.PA.1993–3/170); c small Gastrochaenolites isp. boring in S. herbigi, TS (ULg.PA.1999–33-1/532); d, e calcifying microbes forming a multi-layered crust on S. herbigi, made of Claracrusta-like layers alternating with dark microbial micrite, TS (ULg.PA.1999–33-1/532 and ULg.PA.1999–1/133); f cylindrical borings in S. weyeri, TS (ULg.PA.1993–6/140); g calcifying microbes on S. herbigi, TS (ULg.PA.1999–33-1/532), the arrow indicates a layer of Aphralysia capriorae; h Aphralysia crust on S. webbi, TS (ULg.PA.1999–1/420); i microbial micrite forming a dome on one side of S. herbigi, TS (ULg.PA.1999–33/449); j protocorallite of S. webbi attached to a larger corallite of the same species, note that the larger corallite was coated by calcifying microbes before the larvae settled on it with its talon (ULg.PA.1999–1/420); k protocorallite of S. herbigi attached on a larger corallite, the arrow indicates a small colony of fistuliporid bryozoan under the protocorallite, LS (ULg.PA.1999–33-1/399); l S. webbi corallites attached to another corallite with talon-like structures, both corallites being coated by calcifying microbes (ULg.PA.1999–1/420); m S. herbigi corallites attached to a large corallite, the arrow indicates alcyonacean sclerite immured between the two corallites, TS (ULg.PA.1992–1/88); n S. herbigi corallite half covered by Aphralysia crusts and by another corallite itself covered by a crust of calcifying microbes, TS (ULg.PA.1999–1/133)
Epibionts on Semenomalophyllia spp.: foraminifers, crinoids, algae, and bryozoans; a, b Tetrataxis foraminifer attached to the heterotheca of Semenomalophyllia herbigi (specimens ULg.PA.1999–33-1/532 and ULg.PA.1999–33-1/417), note that on the close-up view (b) the foraminifer is attached to microbial crust (arrow) and not directly in contact with the coral heterotheca; c crinoid roots attached to S. herbigi (ULg.PA.1999–33-1/399); d–g Ungdarella uralica microproblematica attached to S. herbigi, TS (ULg.PA.1999–33-1/532); h–i Cystodactya gallensis bryozoan growing on S. weyeri, TS (ULg.PA.1993–6/140); the arrow indicates a Gastrochaenolites isp. boring through the heterocoral anterior to the bryozoan growth; j Tabulipora howsi bryozoan growing on S. weyeri, TS (ULg.PA.1993–6/170); the arrow indicates a spirorbiform microconchid attached to the bryozoan; k indeterminate fistuliporid bryozoan on S. weyeri, TS (ULg.PA.1993–6/170, close-up view of Fig. 9a), the arrows indicate Gastrochaenolites isp. boring both bryozoan and heterocoral; l Cystodactya gallensis bryozoan growing over sclerites of Lafustalcyon vachardi gen. et sp. nov. encrusting a S. weyeri, TS (ULg.PA.1993–6/170)
The settlement of heterocoral larvae within the colonies is common. Juvenile corallites growing on older ones can be distinguished from genuine offsets by the development of two heterothecae either in contact (Fig. 6i–k) or separated by various epibionts (e.g., Figs. 13i, 14j, n). In such cases, the larvae and host corallite always belong to the same species. No case of interspecific growth was observed. A second case of corallite relationships is characterised by the contact of two corallites of the same colony (or paracolony) that build bridge structures between them, though without connection of their internal cavities. These bridge structures consist of vesicular tissue growing perpendicular to the corallites (Figs. 7b, 15g). Some colonies show extensions of the heterotheca of one corallite covering the neighbouring corallites (similar to those described by Dworczak et al. 2019 in Oligophylloides maroccanus). Occasionally, the extensions of the heterotheca immure other epibionts such as alcyonacean sclerites (Fig. 11h).
Perforations are uncommon, but when colonies are affected, up to 2% of the corallites (all species mixed) show bulb-shaped perforations 2 mm high and 0.8 mm large with aperture varying in diameter from 0.2 to 0.6 mm (Figs. 14b, c, 15h, k). The cavity is commonly filled with fine-grained sediment similar to the matrix, and geopetal structures are not uncommon and indicate that the bored corallite was not vertical when the filling occurred (Fig. 14c). These clavate borings are similar to the ichnogenus Gastrochaenolites known to be a perforating bivalve boring. This ichnogenus is commonly larger – several millimetres to centimetres in diameter – but small specimens have been described in post-Palaeozoic deposits, e.g., Belaústegui et al. (2020). No coral-boring bivalves are known before the Triassic (Stanton and Flügel 1987). Note that Semenomalophyllia weyeri with its large corallites is more affected by borings than the other species, which were probably too small to be bored by the Gastrochaenolites perforating organism. A second type of perforations is represented by unbranched sinuous tunnels 0.2 mm in diameter and up to several millimetres-long filled either with sparite or micrite (Figs. 14f, 15l). The size of the perforations points towards sponges rather than bacteria as trace-makers.
Calcifying microbes and other carbonate micro-organisms are the most common and most abundant epibionts. They coat between 2 and 33% of corallites in all 16 colonies. Besides laminated microbial micrite coating (Fig. 14g, i), Clatracrusta-like sheets of polygonal cells (Fig. 14d–e, l–n), and sheets of small tubes (Aphralysia capriorae, Fig. 14g–h) are rather abundant. Some corallites are coated by microbial crusts up to 2 mm thick. In most cases, however, the crusts are very thin (1–2 layers of cells) and incompletely cover the corallites.
At least three genera of bryozoans grew on Semenomalophyllia colonies. The most common is Fistulipora prolifica forming a sleeve around corallites (Figs. 14b, k, 15k). Cystodactya gallensis forms bifoliate sheets covering only half of a corallite and growing perpendicular to it (Fig. 15 h-i). Tabulipora howsi forming semi-massive colonies attached to corallites are rare in the material. A single case of bryozoan immuring alcyonacean sclerites is reported (Fig. 15l).
As previously reported (Vachard and Aretz 2004), the aoujgaliid carbonate microfossil Ungdarella uralica is characterised by a cylindrical or branched morphology with an initial stage attached to erect objects. In the Roc de Murviel section, such initial stages with their attachment structures are common on corallites of Semenomalophyllia herbigi (Figs. 7j, 15d–g). The cylindrical stage with its hollow axial canal, however, is rarely observed (Fig. 15f).
Spirorbiform microconchids c. 2 mm in diameter are occasional on the corallites of Semenomalophyllia spp. (Figs. 14a, 15j). Tetrataxis conica (Fig. 15a–b) is a common encruster attached to the corallites and to crinoid columnals. The foraminifers are either cemented on the heterotheca or embedded on microbialites covering the corallites. Only one case of crinoid attached to a heterocoral (Fig. 14c) is reported among the c. 1800 corallites of the 16 colonies observed here. This rarity is hypothetically explained by the size of the corallite, too small for a crinoid to settle on it.
Palaeoautecology
The four species of Semenomalophyllia are uncommon encounters in microbial reefs, but they are not restricted to these facies (e.g., in Serre de Péret). As all known heterocorals, they were sessile benthos, as indicated by their attachment structures and re-orientation after tilting (Cossey 1997; Fernández-Martínez et al. 2003). However, they were insignificant reef builders, but they are of peculiar interest for synecology and symbiotic relationships with their numerous epibionts. Besides the epizoan associations described on Oligophylloides maroccanus Weyer, 2016 (Dworczak et al. 2019), the present material represents a unique view on micro-environments created by the heterocoral colonies. Both syn-vivo and post-mortem encrustations are present and a timing of colonisation can be proposed (Table 1).
Coating of the heterotheca with calcifying microbes is very common and potentially occurred both on dead branches of the colonies and on the proximal part of the corallites not covered by the soft tissues of the polyp (Dworczak et al. 2019). As Semenomalophyllia colonies lived in microbial facies, coating by calcifying microbes could have happened several times and during a rather long period of time, before and after the death of the colonies.
The alcyonacean Lafustalcyon vachardi encrusted mainly the colonies of S. weyeri and, to a lesser extent, S. perretae, i.e., heterocorals with a large diameter (> 4 mm). It has to be noted that no alcyonacean occupied colonies other than Semenomalophyllia spp. in the investigated sections despite the occurrence of Siphonodendron colonies with small corallites in the same facies. Several cases of alcyonacean sclerites immured between Semenomalophyllia corallites are observed (Fig. 11f–g), witnessing the syn-vivo colonisation by Lafustalcyon. Overgrowth of the corallites during expansion phases (rejuvenescence) creates small cavities where sclerites are preserved – some might be immured, but this is not obvious (Fig. 11i) – suggesting a contemporaneous growth of the heterocoral and alcyonacean octocoral. A single case of bryozoan immuring sclerites around a heterocoral is also known (Fig. 15l), suggesting that the bryozoan grew when the alcyonacean was still alive or just after it died, but before dispersion of its sclerites.
The exact symbiotic relationships between the alcyonacean octocorals and their host in the fossil record are unknown (see discussion in Fernández-Martínez et al. 2019). Recent alcyonacean octocorals such as Alcyonium spp. are considered as commensal on gorgonians. Some species show an obligate dependence to their host for growing above the sea floor (Buhl-Mortensen and Mortensen 2004). Most Alcyonacea growing on scleractinan corals are nevertheless considered as commensal (Goh et al. 1999).
Borings (both Gastrochaenolites isp. and cylindrical tunnels) occurred in dead parts of the corallites. Geopetal structures within borings indicate that the coral branches were not vertical when they were bored, so it probably happened after the colony died and got broken. There is a clear correlation between the occurrence of borings and bryozoan encrustation. Bryozoan colonies are commonly perforated as well, suggesting a post-mortem boring but with no clue on the stage of encrustation by the bryozoan (during or after the life of the coral). One corallite, however, shows a bryozoan covering an open boring, indicating its implantation after perforation and therefore, most probably post-mortem (Fig. 15h). Conversely, there is a negative correlation between bryozoan encrustation and heterocoral settlement, which, again, suggests that the colonies were dead before the bryozoan colonised their skeletons.
Microconchids, crinoid roots, and foraminifers are cemented either on the heterotheca or on another epibiont (microbes, bryozoan). Tetrataxis foraminifers is commonly found on any kind of corals and is often considered as syn-vivo epibionts loosely attached to the substrate (Cossey and Mundy 1990). Microproblematica such as Ungdarella uralica were implanted directly on the heterotheca and their upward growth suggests that they grew when the corallites were alive or at least when the colony was still in upright position. All these organisms obviously used the heterocoral skeleton to reach a higher tiering. Therefore, it would be logical that their implantation predates the death of the colony or, at least, its tilting. Table 1 summarises the probable relationships of the heterocoral colonies and their epibionts.
Finally, it has to be noted that the abundance of calcifying microbes and microproblematica on the colonies, as well as green algae (dasycladacean, cf. Vachard and Aretz 2004) in the surrounding sediment points to photic conditions in the habitat of Semenomalophyllia spp. and their epibionts. Contrarily, the (para-)colonies of Oligophylloides marroccanus and O. pachythecus developed in deeper water settings (Chwieduk 2001; Weyer 1997, 2016; Dworczak et al. 2019).
Conclusions
Colonial heterocorals are uncommon faunal components of the Serpukhovian reefal facies in the Montagne Noire and the French Pyrenees. Despite scarce occurrences, their presence is highly significant stratigraphically as they are restricted to the RC9 coral zone. Four morpho-species belonging to the newly described genus Semenomalophyllia are established. They all share the typical septal arrangement of the Heterocorallia in early stage of development or throughout the entire growth as in the small-sized species S. webbi. The fused axial ends of septa commonly disappear during the ontogeny in larger sized species such as S. herbigi and S. perretae. In S. weyeri, the septa are withdrawn from the axis and arranged in two cycles (‘minor’ and ‘major’, arranged in an imperfect alternation). This case shows a striking morphological convergence with rugose corals. S. weyeri and S. perretae are commonly colonised by alcyonacean octocorals as indicated by the occurrence of sclerites covering some corallites. These are descried as Lafustalcyon vachardi gen. et sp. nov., so far only known in the Serpukhovian strata of southern France, and only affecting heterocoral colonies. In addition to the alcyonacean octocoral, a diverse assemblage of epibionts colonised the heterocorals: microbes, bryozoans, foraminifers, microconchids, crinoids, several types of borings, and microproblematica.
The abundance and diversity of epibionts on heterocoral (para-)colonies, both in Semenomalophyllia spp. and Oligophylloides spp. is remarkable in comparison with the very abundant but less commonly encrusted rugose corals colonies. The epibionts – at least those which colonised the colonies when they were alive and/or erected – might certainly have taken advantage of this higher tiering. Though no satisfactory reason explains why rugose corals lack a similar microcosm.
References
Aretz, M. 2002a. Habitatanalyse und Riffbildungspotential kolonialer rugoser Korallen im Unterkarbon (Mississippium) von Westeuropa. Kölner Forum für Geologie und Paläontologie 10: 1–155.
Aretz, M. 2002b. Rugose corals and associated carbonate microfossils from the Brigantian (Mississippian) of Castelsec (Montagne Noire, Southern France). Geobios 35: 187–200.
Aretz, M. 2016. The Kulm facies of the Montagne Noire (Mississippian, southern France). Geologica Belgica 19: 69–80.
Aretz, M., M. Legrand-Blain, D. Vachard, and A. Izart. 2019. Gigantoproductid and allied productid brachiopods from the “Calcaires à Productus” (late Viséan-Serpukhovian; Montagne Noire, southern France): Taxonomy and palaeobiogeographical position in the Palaeotethys. Geobios 55: 17–40.
Aretz, M., and H.-G. Herbig. 2003. Contribution of rugose corals to Late Viséan and Serpukhovian bioconstructions in the Montagne Noire (Southern France). SEPM Special Publications 78: 119–132. (=AAPG Memoir 83).
Belaústegui, Z., F. Muñiz, R. Domènech, and R., and J. Martinell. 2020. Ichnogeny and bivalve bioerosion: Examples from shell and wood substrates. Ichnos 27(3): 277–283. https://doi.org/10.1080/10420940.2020.1744584.
Bengtson, S. 1981. Atractosella, a Silurian Alcyonacean Octocoral. Journal of Paleontology 55: 281–294.
Berkowski, B. 1997. Calyxcorallia, their relation to Heterocorallia and Rugosa. A blastogenetic study of Stylostrotion sudeticum Fedorowski, 1991. Bolletín de la Real Sociedad Española de Historia Natural, sección Geológica 91: 153–162.
Bessière, G., and H. Schulze. 1984. Le Massif de Mouthoumet (Aude, France): Nouvelle définition des unités structurales et essai d’une reconstruction paléogéographique. Bulletin Société Géologique de France (Série 7) 26: 885–894.
Blainville, H.M.D. de. 1830. Zoophytes. In Dictionnaire des Sciences Naturelles. Tome 60: Règne organisé, Zoologie, Zoophytes. Strasbourg, Paris: F.G. Levrault.
Buhl-Mortensen, L., and P.A. Mortensen. 2004. Symbiosis in deep-water corals. Symbiosis 37: 33–61.
Chwieduk, E. 2001. Biology of the Famennian heterocoral Oligophylloides pachythecus. Palaeontology 44: 1189–1226.
Cossey, P.J. 1997. Hexaphyllia: A spiny heterocoral from Lower Carboniferous reef limestones in Derbyshire, England. Palaeontology 40: 1031–1059.
Cossey, P.J., and D.J.C. Mundy. 1990. Tetrataxis, a loosely attached limpet-like foraminifer from the Upper Palaeozoic. Lethaia 23: 311–322.
Cózar, P., A. Izart, I.D. Somerville, M. Aretz, I. Coronado, and D. Vachard. 2019. Environmental controls on the development of Mississippian microbial carbonate mounds and platform limestones in southern Montagne Noire (France). Sedimentology 66: 2392–2424.
Delvolvé, J.-J., P. Souquet, D. Vachard, M.-F. Perret, and P. Aguirre. 1993. Caractérisation d’un bassin d’avant-pays dans le Carbonifère des Pyrénées : Faciès, chronologie de la tectonique synsédimentaire. Comptes Rendus de l’Académie des Sciences (série II) 316: 959–966.
Dworczak, P.G., B. Berkowski, and M. Jakubowicz. 2019. Epizoans immured in the heterocoral Oligophylloides maroccanus Weyer, 2017: a unique record from the Famennian (Upper Devonian) of Morocco. Lethaia 53: 452–461.
Dybowski, W. 1873. Monographie der Zoantharia sclerodermata rugosa aus der Silurformation Estlands, Nord-Livlands und der Insel Gotland, nebst einer Synopsis aller palaeozoischen Gattungen dieser Abtheilung und einer Synonymik der dazu gehörigen, bereits bekannten Arten. Archiv für die Naturkunde Liv-, Ehst- und Kurlands (Serie 1) 5: 257–532.
Ehrenberg, C.G. 1834. Beiträge zur physiologischen Kenntniss der Corallenthiere im allgemeinen, und besonders des rothen Meeres, nebst einem Versuche der physiologischen Systematik derselben. Abhandlungen der Königlichen Akademie der Wissenschaften zu Berlin, Physikalische Abhandlungen 1: 225–380.
Ernst, A., P.N. Wyse Jackson, and M. Aretz. 2015. Bryozoan fauna from the Mississippian (Visean) of Roque Redonde (Montagne Noire, southern France). Geodiversitas 37: 151–213.
Fabricius, K.K., and P.P. Alderslade. 2001. Soft corals and sea fans: A comprehensive guide to the tropical shallow water genera of the central‐west Pacific, the Indian Ocean and the Red Sea. Townsville: Australian Institute of Marine Science.
Fedorowski, J. 1991. Dividocorallia, a new subclass of Palaeozoic Anthozoa. Bulletin de l’Institut royal des Sciences naturelles de Belgique, Sciences de la Terre 61: 21–105.
Fedorowski, J., and V.V. Ogar. 2013. Early Bashkirian Rugosa (Anthozoa) from the Donets Basin, Ukraine. Part 4. Cordibia, a new protocolonial genus. Acta Geologica Polonica 63: 297–314.
Fernández-Martínez, E., F. Tourneur, and A. López-Alcántara. 2003. A new Middle Devonian heterocoral from Spain. Acta Palaeontologica Polonica 48: 531–546.
Fernández-Martínez, E., I. Coronado, S. Rodríguez, F. Tourneur, and M. Badpa. 2019. Alcyonacea awakens: Palaeobiology and palaeoecology of Palaeozoic octocorals known from their sclerites. Geological Journal 54: 3593–3618.
Frey, J.F.H.C., and K.G.R. Leuckart. 1847. Beiträge zur Kenntniss wirbelloser Thiere mit besonderer Berücksichtigung der Fauna des norddeutschen Meeres. Braunschweig: Vieweg.
Goh, N.K., P.K. Ng, and L.M. Chou. 1999. Notes on the shallow water gorgonian-associated fauna on coral reefs in Singapore. Bulletin of Marine Science 65: 259–282.
Haeckel, E. 1866. Generelle Morphologie der Organismen, 2, Anthozoa. Berlin: Georg Reimer.
Lafuste, J. 1981. Microstructure des Hétérocoralliaires (Cnidaria; Carbonifère). Annales De Paléontologie, Invertébrés 67: 1–12.
Lamouroux, M. 1812. Extrait d’un mémoire sur la classification des Polypiers coralligènes non entièrement pierreux. Nouveau Bulletin des Sciences. Société Philomatique (III) 63: 181–188.
Lin, Y.D., Z.X. Huang, S.Z. Wu, X.D. Peng, and C.Z. Qiu. 1992. The Classification and Geological Significance of the Heterocorals. In Professional Papers of Carboniferous Corals of China, eds. Y.D. Lin, Z.X. Huang, S.Z. Wu, and X.D. Peng. Changchun: Science and Technology Press. (in Chinese, English summary).
McCoy, F. 1849. On some new genera and species of Palaeozoic corals and foraminifera. Annals and Magazine of Natural History (series 2) 3: 1–20 and 119–136.
Ogar, V.V. 1992. Discovery of the tabulate genus Syringoalcyon in the Lower Carboniferous of Tien Shan. Paleontologicheskii Zhurnal 26: 110–112. (in Russian).
Perret, M.-F., and P. Semenoff-Tian-Chansky. 1971. Coralliaires des calcaires carbonifères d’Ardengost (Hautes-Pyrénées). Bulletin de la Société d’Histoire naturelle de Toulouse 107: 567–594.
Perret, M.-F., and D. Vachard. 1977. Algues et pseudo-algues des calcaires Serpoukhoviens d’Ardengost (Hautes-Pyrénées). Annales de Paléontologie 63: 85–156.
Perret, M.-F. 1971. Les calcaires carbonifères d'Ardengost (Hautes Pyrénées). Thèse 3e cycle, Université de Toulouse.
Perret, M.-F. 1993. Recherches micropaléontologiques et biostratigraphiques (conodontes, foraminifères) dans le Carbonifère pyrénéen. Strata (série 2) 21: 1–597.
Pickett, J.W. 1966. Lower Carboniferous coral faunas from the New England District of New South Wales. Memoirs of the Geological Survey of New South Wales, Palaeontology 15: 1–38.
Poty, E. 1977. Données nouvelles sur les Hétérocoralliaires du Dinantien belge. Annales de la Société Géologique de Belgique 100: 233–243.
Poty, E., and M.R. Hecker. 2003. Parallel evolution in European rugose corals of the genus Lonsdaleia McCoy, 1849 (Lower Carboniferous). Bulletin de l’Institut royal des Sciences naturelles de Belgique, Sciences de la Terre 73: 109–135.
Poty, E., M. Aretz, and L. Barchy. 2002. Stratigraphie et sédimentologie des “Calcaires à Productus” du Carbonifère inférieur de la Montagne Noire (Massif Central, France). Compte Rendu de l’Académie des Sciences, Géosciences 334: 843–848.
Poty, E., F.-X. Devuyst, and L. Hance. 2006. Upper Devonian and Mississippian foraminiferal and rugose coral zonations of Belgium and northern France: A tool for Eurasian correlations. Geological Magazine 143: 829–857.
Poty, E., J. Denayer, and M. Aretz. 2019. The upper Mississippian of the Montagne Noire (south Central Massif, France): a small endemic coral area controlled by reefal facies. In 13th International Symposium on Fossil Cnidaria and Porifera 2019, Modena, Italy – Abstract Book, eds. F. Bosselini, M. Aretz, C.A. Papazzoni, and A. Vescogni: 51.
Reich, M. 2002. Skleren von Alcyonacea (Anthozoa: Octocorallia) aus einem Silur-Geschiebe Nordddeutschlands. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte 2002(9): 551–561.
Różkowska, M. 1969. Famennian Tetracoralloid and Heterocoralloid fauna from the Holy Cross Mountains (Poland). Acta Palaeontologica Polonica 14: 1–187.
Sando, W.J. 1984. Significance of epibionts on horn corals from the Chainman Shale (upper Mississippian) of Utah. Journal of Paleontology 58: 185–196.
Schindewolf, O.H. 1941. Zur Kenntnis der Heterophylliden, einer eigentümlichen paläozoischen Korallengruppe. Paläontologische Zeitschrift 22: 213–306.
Schulze, H. 1982. Deckenbau und Flysch-Sedimentation im Variszikum des Massivs von Mouthoumet (Süd-Frankreich). PhD Thesis. University of Göttingen.
Scrutton, C. 1977. New Reports. United Kingdom. Fossil Cnidaria. Newsletter of the International Association for the Study of Fossil Cnidaria 6: 12–13.
Stanton, R.J., and E. Flügel. 1987. Palaeoecology of Upper Triassic reefs in the Northern Calcareous Alps: Reef communities. Facies 16: 157–186.
Sugiyama, T. 1984. Heterocorallia from the Akiyoshi Limestone, Southwest Japan. Part 1, Systematic Paleontology. Bulletin of the Akiyoshi-Dai Museum of Natural History 19: 27–67.
Tapanila, L. 2008. Direct evidence of ancient symbiosis using trace fossils. In From Evolution to Geobiology: Research Questions Driving Paleontology at the Start of a New Century: Paleontological Society Short Course, October 4, 2008, eds. P.H. Kelley and R.K. Bambach. Paleontological Society Paper 14: 271–287.
Taylor, P.D., and M.A. Wilson. 2002. A new terminology for marine organisms inhabiting hard substrates. Palaios 17: 522–525.
Tourneur, F., P. Semenoff-Tian-Chansky, M.-F. Perret, and J. Lafuste. 1995. A new genus of heterocorals from the Lower Carboniferous of the Pyrénées. In VII International Symposium on Fossil Cnidaria and Porifera, Madrid, Spain, September 12–15, 1995, Abstracts, eds. M.J. Comas-Rengifo, A. Rerejón, S. Rodríguez and W.J. Sando. Madrid: Departamento de Paleontología, Facultad de Ciencias Geológicas, Universidad Complutense.
Vachard, D. 1977. Etude stratigraphique et micropaléontologique (algues et foraminifères) du Viséen de la Montagne Noire (Hérault, France). Mémoires de l’Institut Géologique de l’Université de Louvain 29: 111–195.
Vachard, D., and M. Aretz. 2004. Biostratigraphical precisions on the Early Serpukhovian (Late Mississippian), by means of a carbonate algal microflora (cyanobacteria, algae and pseudo-algae) from La Serre (Montagne Noire, France). Geobios 37: 643–666.
Vachard, D., J.-J. Delvolvé, and M. Hansotte. 1991. Foraminifères, algues et pseudo-algues du Serpoukhovien du Massif de l’Arize (Carbonifère inférieur des Pyrénées). Geobios 24: 251–256.
Vachard, D., P. Cozar, M. Aretz, and A. Izart. 2016a. Late Visean-early Serpukhovian cyanobacteria and algae from the Montagne Noire (France); taxonomy and biostratigraphy. Bulletin of Geosciences 91: 433–466.
Vachard, D., P. Cozar, M. Aretz, and A. Izart. 2016b. Late Visean-Serpukhovian foraminifers in the Montagne Noire (France): Biostratigraphic revision and correlation with the Russian substages. Geobios 49: 469–498.
Verseveldt, J., and F.M. Bayer. 1988. Revision of the genera Bellonella, Eleutherobia, Nidalia and Nidaliopsis (Octocorallia: Alcyoniidae and Nidalliidae), with descriptions of two new genera. Zoologische Verhandelingen 245: 3–131.
Vinn, O. 2017a. Symbiosis between Devonian corals and other invertebrates. Palaios 32: 382–387.
Vinn, O. 2017b. Symbiosis in Late Devonian-Mississippian corals: A review. Palaeobiodiversity and Palaeoenvironments 97: 723–729.
Walker, S.E., and W. III, Miller. 1992. Organism—substrate relations: Toward a logical terminology. Palaios 7: 236–238.
Weyer, D. 1995. Heterocorallia aus Famenne-Cephalopodenkalken im Rheinischen Schiefergebirge und Tafilalt. Abhandlungen und Berichte für Naturkunde (Museum für Naturkunde Magdeburg) 18: 103–135.
Weyer, D. 1997. News about Famennian Heterocorallia in Germany and Morocco. Boletín de la Real Sociedad Espańola de Historia Natural (sección Geológica) 91: 145–151.
Weyer, D. 2016. Solitary and/or colonial growth in the Palaeozoic superorder Heterocorallia Schindewolf, 1941 (Eifelian–Serpukhovian). Freiberger Forschungshefte (C: Paläontologie, Stratigraphie, Fazies) 550: 59–101.
Wrzołek, T. 1981. Coral growth in Oligophylloides pachythecus Różkowska, 1969. Acta Palaeontologica Polonica 25: 513–517.
Zapalski, M.K. 2007. Parasitism versus commensalism: The case of tabulate endobionts. Palaeontology 50: 1375–1380.
Zatoń, M., and T. Wrzołek. 2020. Colonization of rugose corals by diverse epibionts: Dominance and syn-vivo encrustation in a Middle Devonian (Givetian) soft-bottom habitat of the Holy Cross Mountains, Poland. Palaeogeography, Palaeoclimatology, Palaeoecology 556: 1090899. https://doi.org/10.1016/j.palaeo.2020.109899.
Zatoń, M., T. Borszcz, and M. Rakociński. 2017. Temporal dynamics of encrusting communities during the late Devonian: A case study from the Central Devonian Field, Russia. Paleobiology 43: 550–568.
Acknowledgements
Through this paper, the authors want to thank many of their collaborators, namely Jean Lafuste (1930–1990, Paris), Pierre Semenoff-Tian-Chansky (1925–2003, Paris), Marie-France Perret (Toulouse), Daniel Vachard (Lille), Gregory Webb (Brisbane), Dieter Weyer (Berlin), and, of course, Hans-Georg Herbig (Cologne) to whom the present volume is dedicated. The authors of the present paper are extremely grateful to these coral researchers as mentors, collaborators, inspiration, and precious friends. The manuscript benefits from the detailed comments and remarks of the reviewers Tomasz Wrzołek (Katowice) and Esperanza Fernández-Martínez (Leon), as well as from the editors Michael Amler (Cologne) and Mike Reich (Munich).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Michael Amler.
Rights and permissions
About this article
Cite this article
Denayer, J., Poty, E., Tourneur, F. et al. Colonial Heterocorallia (Cnidaria, Anthozoa) and their epibionts from the lower Carboniferous of Montagne Noire and Pyrenees, southern France. PalZ 97, 821–846 (2023). https://doi.org/10.1007/s12542-021-00588-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-021-00588-1