Abstract
The Khatyspyt Formation in Arctic Siberia is one of only two carbonate settings with Ediacara-type fossils. As a potential hydrocarbon source rock, it contains abundant molecular fossils that may help to expand our understanding of these ecosystems. Unfortunately, however, the molecular fossil record in geological materials is commonly biased by secondary processes such as thermal maturation, migration of bitumen compounds or surface contamination. In this study, we evaluate the preservation of molecular fossils in a sample from the Khatyspyt Formation and elucidate their paleobiological meaning. Our results reveal that the organic matter is remarkably immature (oil window maturity) and shows little effect of biodegradation. Petrographic observations, exterior/interior experiments, and the similarity between free bitumen, mineral-occluded bitumen, and kerogen pyrolysate point to the syngeneity of the molecular fossils. Abundant hopanes, cyclohexylalkanes, and methyl-branched alkanes indicate a bacterial source of the organic matter, likely including cyanobacteria and anaerobic bacteria. At the same time, a carbonaceous compression fossil on top of the sample and abundant steranes indicate the presence of eukaryotes. The steranes show typical distributions for the Ediacaran (i.e., dominance of stigmastane). Given the exceptional preservation of the body fossils, trace fossils, and molecular fossils, the Khatyspyt Formation can be considered a fossil lagerstätte sensu Seilacher (1970: Begriff und Bedeutung der Fossil-Lagerstätten. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte: 34–39). The combined analysis of sedimentary facies, paleontology (body, trace, and molecular fossils), and biogeochemistry will provide a more complete understanding of ecosystems with Ediacara-type fossils.
Kurzfassung
Die Khatyspyt-Formation im arktischen Sibirien ist eines von lediglich zwei karbonatischen Ablagerungsräumen mit Ediacara-Fossilien. Gleichzeitig ist sie auch ein potentielles Kohlenwasserstoff-Muttergestein. Nicht zuletzt aufgrund der daher reichlich enthaltenen molekularen Fossilien birgt sie das Potential, unser Verständnis dieser Ökosysteme zu verbessern. Das Inventar an in geologischen Materialien erhaltenen molekularen Fossilien ist jedoch häufig durch diverse sekundäre Prozesse, wie z. B. thermische Maturierung, die sekundäre Migration von Kohlenwasserstoffen oder Oberflächen-Kontamination, verfälscht. In dieser Studie evaluieren wir die Erhaltung molekularer Fossilien in einer Probe der Khatyspyt-Formation und diskutieren ihre paläobiologische Bedeutung. Unsere Ergebnisse zeigen, dass das organische Material eine bemerkenswert niedrige Reife aufweist (Ölfenster) und kaum durch Biodegradation beeinflusst wurde. Petrographische Beobachtungen, Exterieur/Interieur-Experimente, und die Gleichheit zwischen freiem Bitumen, mineral-gebundenem Bitumen und Kerogen-Pyrolysat unterstreichen die Syngenität der enthaltenen molekularen Fossilien. Hopane, Cyclohexane, und and methylverzweigte Alkane deuten auf die Anwesenheit von Bakterien, wahrscheinlich inklusive Cyanobakterien und anaeroben Bakterien. Gleichzeitig belegen ein organisch erhaltenes Makrofossil auf der Probenoberseite und häufige Sterane, welche ein für das Ediacarium typisches Verteilungsmuster aufweisen (Dominanz von Stigmastan), die Existenz von Eukaryoten. Aufgrund der außergewöhnlichen Erhaltung von Körper-, Spuren- und molekularen Fossilien kann die Khatyspyt-Formation als Fossillagerstätte sensu Seilacher (1970: Begriff und Bedeutung der Fossil-Lagerstätten. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte: 34–39) interpretiert werden. Die zukünftig gemeinschaftliche Analyse von sedimentärer Fazies, Paläontologie (Körper-, Spuren- und molekulare Fossilien) und Biogeochemie wird ein kompletteres Bild von Ökosystemen mit Ediacara-Fossilien ermöglichen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The Phanerozoic began with a bang–the celebrated “Cambrian explosion” of ecological and morphological complexity (e.g., Erwin and Valentine 2013; Zhang et al. 2014, and references therein). Some million years earlier, the Earth was experiencing several major climatic perturbations, possibly to the extent of global glaciations (i.e., “Snowball Earth”; Kirschvink 1992; Hoffman et al. 1998). The Ediacaran (635–541 Ma) is sandwiched between these events and represents one of the most fundamental transitions in the biosphere (Knoll et al. 2004, 2006; Narbonne et al. 2012). The worldwide occurrence of macroscopic complex organisms, including the first undoubted fossil metazoans, is a particularly striking feature. Although these organisms are important with regard to the diversification of complex life on Earth, their biological affinities, paleobiology, and ecology are still enigmatic (e.g., Seilacher 1989, 1992; Seilacher et al. 2003; Narbonne 2005; Grazhdankin et al. 2008; Xiao and Laflamme 2009).
Efforts to gain a better understanding of Ediacaran ecosystems with macroscopic complex organisms continue to be hampered by taphonomic issues. Ediacara-type fossils (see, e.g., Billings 1872; Gürich 1930a, b, 1933 for early descriptions) are usually preserved as imprints in siliciclastic and volcaniclastic rocks (e.g., Narbonne 2005; Callow and Brasier 2009). Consequently, biological details and biogeochemical signatures are commonly poorly preserved (Grazhdankin et al. 2008; Duda et al. 2014a). In the last few years, research has shown that carbonate settings are ideal for obtaining a more complete understanding, as the fossils retain high anatomical resolution (e.g., Xiao et al. 2005; Grazhdankin et al. 2008; Shen et al. 2009; Chen et al. 2014). Moreover, sedimentary features and biogeochemical proxies are also well preserved (Duda et al. 2014a, 2015). However, only two known carbonate settings with macroscopic complex organisms exist worldwide: the Shibantan Member of the Dengying Formation in South China and the Khatyspyt Formation in Arctic Siberia (Russia).
Ediacara-type macrofossils in the Khatyspyt Formation include arboreomorph frond structures, aspidellamorph and mawsonitomorph holdfast structures, rangeomorphs, palaeopascichnids, and microbial colonies (Sokolov and Fedonkin 1984; Vodanjuk 1989; Fedonkin 1990; Grazhdankin et al. 2008; Nagovitsin et al. 2015). The fossils are preserved by carbonate cementation (Grazhdankin et al. 2008), very similar to the Shibantan Member. Another feature is the ubiquitous presence of meniscate backfilled burrows (Rogov et al. 2012, 2013a, b). Similar structures have been observed in the Dengying Formation of South China, although interpreted as compressed annulated tubular structures (Shen et al. 2007; Dong et al. 2008). In contrast to the Shibantan Member, the Khatyspyt Formation also contains carbonaceous compression fossils (Grazhdankin et al. 2008). The Khatyspyt Formation can therefore be considered a fossil lagerstätte sensu Seilacher (1970), providing a unique, albeit not necessarily representative, window into ecosystems with Ediacara-type organisms.
Molecular fossils are produced by all types of organisms, and could offer additional insight into the structure and functioning of ecosystems with Ediacara-type organisms (cf. e.g., Treibs 1934, 1936; Eglinton and Calvin 1967; Brocks and Summons 2003). Unfortunately, however, the molecular fossil record of geological materials is commonly biased by secondary processes such as maturation, migration, and contamination (e.g., Brocks et al. 2003, 2008; Brocks 2011; Duda et al. 2014b; Mißbach et al. 2016). It has been demonstrated, for instance, that molecular fossil analyses on the Shibantan Member are problematic due to the high thermal maturity of the host rock and surface contamination in the outcrops (Duda et al. 2014a, b). Molecular fossil studies on less mature rocks from a similar setting, therefore, could provide a rare view of the geobiology of ecosystems with Ediacara-type fossils.
Neoproterozoic–Phanerozoic sedimentary rocks of Arctic Siberia are generally characterized by low thermal maturities (Kashirtsev 1988, 2003; Kontorovich et al. 2013). This is mainly due to the geological history of the region: strata from the eastern slope of the Olenek uplift, for instance, have never been buried deeper than 1 km, as evidenced by vitrinite reflectance data and molecular fossils (Kashirtsev 1988, 2003; Kontorovich et al. 2013). The Khatyspyt Formation is considered a potential petroleum source rock, with total organic carbon (TOC) content of up to 4.19 % (Kontorovich et al. 2009; Kashirtsev et al. 2010; Parfenova et al. 2010, 2011), and thus may contain abundant molecular fossils (also “organic biomarkers” or “lipid biomarkers”). On the other hand, the preservation of molecular fossils may have been affected by regional processes such as penetration by diatremes.
In this study, we evaluate the preservation of molecular fossils in an outcrop sample from the Khatyspyt Formation that has been kept in storage cabinets at the Trofimuk Institute of Petroleum Geology and Geophysics in Novosibirsk (i.e., room temperature, no protection against potential secondary contamination) since sampling in 2009. First, we critically assess the thermal maturity, biodegradation, and syngeneity of molecular fossils in the hydrocarbon fractions of the extractable portion (i.e., bitumen) and non-extractable portion (i.e., kerogen) of the organic matter. We then elucidate the paleobiological meaning of the obtained data and discuss implications for future studies. Our results highlight the potential of the Khatyspyt Lagerstätte to provide a more complete understanding of ecosystems with Ediacara-type organisms through comprehensive geobiological studies.
Geological setting
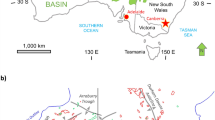
The ca. 190-m-thick Khatyspyt Formation crops out in the Olenek uplift of the northeastern Siberian Platform in Arctic Siberia (Russia) (Fig. 1). It consists of alternating thin layers of limestone and shale, finely laminated thin- to medium-bedded limestone, planar- to wavy-laminated thick-bedded limestone, and intraclastic dolomitized limestone (Knoll et al. 1995; Nagovitsin et al. 2015). It was deposited in distal, low-energy carbonate ramp environments below storm wave base (Knoll et al. 1995).
Sample location (a, b) and stratigraphic overview of the Khatyspyt formation (c) (after Nagovitsin et al. 2015, modified)
The Khatyspyt Formation lies between the Maastakh Formation and the Turkut Formation (Fig. 1). The Khatyspyt Formation, together with the overlying Turkut and Syhargalakh formations, is penetrated by diatremes (Rogov et al. 2015). A U–Pb zircon age of 543.9 ± 0.3 Ma for a tuff breccia from one of these diatremes provides an upper constraint on the deposition of the Khatyspyt and Turkut formations (Bowring et al. 1993; Knoll et al. 1995; Nagovitsin et al. 2015).
The Turkut Formation contains skeletal fossils of the anabaritid Cambrotubulus decurvatus, first appearing 1.4 m above the boundary with the Khatyspyt Formation. The Khatyspyt Formation itself contains the meniscate trace fossil Nenoxites, which is remarkably similar to Helanoichnus helanensis, Shaanxilithes ningqiangensis, Palaeopascichnus minimus, Palaeopascichnus meniscatus, and Palaeopascichnus jiumenensis from China (Shen et al. 2007; Dong et al. 2008). As the latter fossils are a potential biostratigraphic marker for the middle Dengying Formation in South China (Shen et al. 2007), the Khatyspyt Formation could thus be broadly coeval with the Shibantan Member.
Sample and methods
Sample characterization
One sample was investigated for microfacies and molecular fossils (Figs. 1, 2). Petrographic analysis was conducted using a Zeiss SteREO Discovery.V8 stereomicroscope (transmitted and reflected light) linked to an AxioCam MRc5 5-megapixel camera. Bulk C/N/S analysis was performed using a HEKAtech Euro EA CNS analyzer. The total organic carbon (TOC) was determined by combustion infrared detection using a Leco RC612 analyzer.
For the analysis of molecular fossils, all materials were heated to 500 °C for 3 h and/or extensively rinsed with acetone. A laboratory blank prepared and analyzed in parallel was run to monitor laboratory contaminations.
For the analysis of the free bitumen (bitumen 1; see Hallmann et al. 2011), the sample was first separated into exterior and interior parts using a carefully pre-cleaned precision saw (Buehler IsoMet 1000). Both samples were then crushed and powdered using a pebble mill (Retsch MM 301). Ground sample material (ca. 30 g) was extracted with dichloromethane (DCM), DCM/n-hexane (1/1; v/v), and n-hexane using ultrasonication (20 min). For the analysis of mineral-occluded bitumen (bitumen 2; see Hallmann et al. 2011), the extraction residue of the interior part of the sample was decalcified with hydrochloric acid (HCl; 10 %) and then extracted as described for bitumen 1.
For the analysis of the kerogen, the decalcified extraction residue of the interior part of the sample was Soxhlet-extracted using DCM/MeOH (9/1; v/v; 24 h). The kerogen was then isolated using HCl and hydrofluoric acid (HF) (Durand 1980), and again Soxhlet-extracted with DCM/MeOH (9/1; v/v; 24 h). The isolated kerogen was pyrolyzed using a catalytic hydropyrolysis (HyPy) system from Strata Technology Ltd. (Nottingham, UK), following Snape et al. (1989) and Love et al. (1995). Briefly, samples were heated in the presence of a sulfided molybdenum catalyst (the ambient temperature was raised to 250 °C at 50 °C/min, and then from 250 to 500 °C at 8 °C/min) under hydrogen pressure of 150 bar at a flow rate of 5 L/min. The hydrocarbons released from the isolated kerogen were trapped downstream on silica powder cooled with dry ice (see Meredith et al. 2004). The HyPy-pyrolysate was eluted from the silica trap with n-hexane.
All resulting isolates (bitumen 1, bitumen 2, and HyPy-pyrolysate) were desulfurized with activated copper and gently concentrated using a pre-cleaned rotary evaporator and N2 to avoid a major loss of low-boiling compounds (Ahmed and George 2004). The isolates were then dried onto a small amount of silica gel and fractionated by column chromatography (1.5 cm in diameter, 8 cm in height; 7 g of dry silica gel). The saturated fraction (F1) was eluted with 27 mL n-hexane, the aromatic fraction (F2) with 32 mL n-hexane/DCM (1/1; v/v), and a polar residue (F3) with 40 mL DCM/MeOH (1/1; v/v).
Gas chromatography mass spectrometry (GC–MS) analyses were carried out with a Thermo Scientific Trace 1300 Series GC coupled to a Thermo Scientific Quantum XLS Ultra MS. The GC instrument was equipped with a capillary column (Phenomenex Zebron ZB–1MS/Phenomenex Zebron ZB-5, 30 m, 0.1 µm film thickness, inner diameter 0.25 mm). Fractions were injected into a splitless injector and transferred to the GC column at 270 °C. The carrier gas was He, at a flow rate of 1.5 mL/min. The GC oven temperature was ramped from 80 °C (1 min) to 310 °C at 5 °C min−1, and held for 20 min. Electron ionization mass spectra were recorded in full scan mode at an electron energy of 70 eV with a mass range of m/z 50–600 and scan time of 0.42 s.
Pristane/phytane (Pr/Ph), Pr/n-C17, and Ph/n-C18 were calculated based on peak integrals in non-filtered total ion current chromatograms (TICs). The carbon preference indexes (CPI) as well as hopane- [22S/(22S + 22R); Ts/(Ts + Tm)] and sterane-based indexes [20S/(20S + 20R); ββ/(ββ + αα); diasterane/sterane ratios] were calculated by integrating peaks in filtered chromatograms (m/z 85, 191, and 217, respectively) following established formulae (Bray and Evans 1961; Seifert and Moldowan 1978, 1980, 1986; Mello et al. 1987; Killops and Killops 2005; Peters et al. 2005).
Results
Sample characterization
The sample originates from an interval in which Ediacara-type fossils and carbonaceous compression fossils co-occur (8.86–12.29 m in section 0605; see Nagovitsin et al. 2015). The top of the sample contains a carbonaceous compression fossil (Fig. 2a). Thin-section microscopy reveals that the sample consists of layers of dense micritic carbonate (i.e., no pores or voids) without any evidence of biolamination (Fig. 2b). No cracks or fissures are observed.
The TOC content of the analyzed sample was 0.7 wt%. The sulfur and nitrogen content were relatively low (each were 0.02 wt%).
Molecular fossils
Alkanes and acyclic isoprenoids
The chromatograms exhibit no hump of an unresolved complex mixture of organic compounds (UCM) (Fig. 3).
Chromatograms (total ion current; TIC) of the hydrocarbon fractions from bitumen 1, bitumen 2, and the HyPy-pyrolysate. Black circles n-alkanes, blue circles n-alkenes, red diamonds cyclohexylalkanes, Farn farnesane, TMTD 2,6,10 trimethyltridecane, N-Pr norpristane, Pr pristane, Ph phytane, i iso-alkanes, ai anteiso-alkanes
In bitumen 1 and 2, the n-alkanes range from C11 to C36, with a maximum at C17 (Fig. 3). In the HyPy-pyrolysate, the n-alkanes range from C11 to C35, with a maximum at C15 (Fig. 3). A slight odd-over-even carbon number predominance (OEP) is observed for all analyzed fractions. The CPI24–34 index (Bray and Evans 1961) is 1.10 for bitumen 1, 1.06 for bitumen 2, and 1.06 for the HyPy-pyrolysate (Table 1; Fig. 3). Unlike the bitumen fractions, the HyPy-pyrolysate contains alkenes, ranging from C11 to C32 (Fig. 3).
Terminally methyl-branched alkanes (iso- and anteiso-isomers) are present in the range of C13 (i.e., methyl-C12) to C27 (i.e., methyl-C26) in bitumen fractions 1 and 2 and from C12 (i.e., methyl-C11) to C27 (i.e., methyl-C26) in the HyPy-pyrolysate (Fig. 3). In all cases, the terminally branched alkanes are most abundant in the range C15 (i.e., methyl-C14) to C20 (i.e., methyl-C19). Only small amounts of mid-chain methyl-branched alkanes are found in bitumen fractions 1 and 2 (Fig. 3).
Cyclohexylalkanes are prominent compounds in all fractions (Fig. 3), where they range from C11 to C26, with Cmax at C16 and C17. In bitumen fractions 1 and 2, cyclohexylalkanes have an OEP in the range C21+, while no OEP is present in the HyPy-pyrolysate.
Among the acyclic isoprenoids, 2,6,10-trimethyldodecane (farnesane), 2,6,10-trimethyltridecane (TMTD), and 2,6,10-trimethylpentadecane (norpristane) occur in sizeable amounts in all fractions (Fig. 3). Furthermore, 2,6,10,14-tetramethylpentadecane (pristane, Pr) and 2,6,10,14-tetramethylhexadecane (phytane, Ph) are prominent in bitumen fractions 1 and 2 (Pr/n-C17 = 0.48 and 0.52, respectively; Ph/n-C18 = 0.60 and 0.61, respectively), while only very small amounts are present in the HyPy-pyrolysate (Pr/n-C17 = 0.08; Ph/n-C18 = 0.15) (Table 1; Fig. 3). The Pr/Ph ratio is higher in bitumen fractions 1 and 2 (Pr/Ph = 1.14 and 1.13, respectively) than in the HyPy-pyrolysate (Pr/Ph = 0.65) (Table 1).
Tricyclic triterpanes (cheilanthanes)
Cheilanthanes are observed only in trace amounts (bitumen fractions 1 and 2) or close to the detection limit (HyPy-pyrolysate).
Steranes
Both bitumen fractions and the HyPy-pyrolysate include cholestane (C27), ergostane (C28), and stigmastane (C29) isomers (ααα S + R and αββ S + R, respectively) (Fig. 4). Notably, the C27 and C28 steranes are present only in small amounts, whereas the C29 steranes represent the most prominent pseudohomologues (e.g., bitumen 1: C27:C28:C29 = 20:17:64 %; HyPy-pyrolysate: C27:C28:C29 = 18:13:69 %). C27 and C29 diasteranes (S + R) occur in traces in both bitumen fractions but could not be identified in the HyPy-pyrolysate. 4-Methyl stigmastane was found in all fractions (Fig. 4). Additionally, traces of 5α(H)-pregnane and diginane (ααβ) were identified in the bitumen fractions based on published spectra (Philp 1985; Requejo et al. 1997).
Filtered chromatograms of the hydrocarbon fractions from bitumen 1 and the HyPy-pyrolysate (m/z 217 for steranes; m/z 191 for hopanes). Circle 22,29,30-trisnorhop-17(21)-ene, square 17-α(H)-hop-20(21)-ene, rhombus 17-β(H)-hop-20(21)-ene, triangle 17α(H)-homohop-20(21)-ene, Ts and Tm trisnorhopanes (C27), 4Me-C 29 4-methyl stigmastane (tentatively identified). Please note that the hop-17(21)-ene co-elutes with the C30 hopane
Hopanes
Hopanes in bitumen 1 and 2 include C27 (Ts and Tm), C29 (Ts, αβ and βα), C30 (αβ and βα), and the C31–C35 isomers (αβ and βα, S + R, respectively) (Fig. 4). The HyPy-pyrolysate contains the same compounds except the C27 and C29 Ts isomers (Fig. 4). It further shows considerable amounts of hopenes. The most prominent of these compounds were tentatively identified according to published mass spectra (Summons and Jahnke 1992; Meredith et al. 2008) as the C27 22,29,30-trisnorhop-17(21)-ene, the C30 hop-17(21)-ene (co-eluting with C30 hopane), the C30 17α(H)-hop-20(21)-ene, the C30 17β(H)-hop-20(21)-ene, and the C31 17α(H)-homohop-20(21)-ene (Fig. 3).
Discussion
Maturity, biodegradation, and syngeneity of molecular fossils
The CPI values around unity calculated for bitumen fractions 1 and 2 (1.10 and 1.06, respectively; Table 1) indicate that the organic matter is generally thermally mature (Bray and Evans 1961). This is further supported by C31 hopane [22S/(22S + 22R); bitumen 1 = 0.55; bitumen 2 = 0.57; Table 1] and C29 sterane indexes [20S/(20S + 20R); bitumen 1 = 0.48; bitumen 2 = 0.47; ββ/(ββ + αα); bitumen 1 = 0.26; bitumen 2 = 0.28; Table 1], which all suggest early oil window maturity (Seifert and Moldowan 1980, 1986; Killops and Killops 2005; Peters et al. 2005). Low C27 (bitumen 1 = 0.15; bitumen 2 = 0.18; Table 1) and C29 diasterane/sterane ratios (bitumen 1 = 0.08; bitumen 2 = 0.08; Table 1) are also in line with a carbonate-dominated source rock and early oil window maturity (Peters et al. 2005). This estimate agrees with other reports [e.g., 20S/(20S + 20R) sterane stereoisomer indexes of ca. 0.5, Ts/Tm values averaging at 0.58; Kontorovich et al. 2009; see also Parfenova et al. 2010, 2011] and the age of the host rock, although analysis of a larger sample set would be necessary for a more precise maturity evaluation.
The lack of a UCM indicates that the impact of biodegradation is notably low (Fig. 3) (Peters et al. 2005). Biodegradation is controlled by a variety of factors, including temperature, the availability of water, and rock characteristics (e.g., lithology, grain size, porosity, and permeability) (Peters et al. 2005). The generally dense micritic matrix and the virtual absence of pores and fissures in the analyzed sample probably limited the availability of electron acceptors for hydrocarbon-degrading microbes. Furthermore, surface biodegradation during outcrop exposure in recent times was probably hampered by the cold Arctic climate, which prevented the penetration of liquid water into the rock and limited the delivery of nutrients to potential organotrophic microbes.
In addition to biodegradation, the migration of younger hydrocarbons into the rock formation of interest can lead to overprinting of the syngenetic molecular fossil inventory. However, the dense micritic matrix and the absence of pores and fissures in the analyzed rock argue against a major emplacement of younger fluids. The presence of alkenes and hopenes in the HyPy-product generally indicates that these compounds have been covalently bound to the kerogen, although the exact formation pathway of the identified hopenes is still ambiguous (original constituents of the kerogen vs. artificial formation from other hopenes during hydropyrolysis; see Meredith et al. 2008). Further, the organic geochemical indexes for the HyPy-pyrolysate [e.g., CPI = 1.06; C31 hopane 22S/(22S + 22R) = 0.53; C29 sterane 20S/(20S + 20R) = 0.44; Table 1] are slightly lower than those of the bitumen fractions and thus indicate lower maturities of the kerogen-bound moieties. Likewise, the presence of Ts and Tm in both bitumen fractions [Ts/(Ts + Tm); bitumen 1 = 0.39; bitumen 2 = 0.37; Table 1], along with the absence of Ts in the HyPy-pyrolysate, is consistent with a lower maturity of the HyPy product (cf. Bishop et al. 1998; Peters et al. 2005). Such an offset between the bitumen and the corresponding kerogen is typically observed in HyPy studies of source rocks (Love et al. 1995, 2008; Bishop et al. 1998), and supports a syngenetic origin of the bitumen from the Khatyspyt Formation.
A further potential problem that needs to be excluded is contamination during exposure in the outcrop, transport, storage, and saw cutting (Brocks et al. 2003, 2008; Brocks 2011; Schinteie and Brocks 2014; Duda et al. 2014b; French et al. 2015). In addition to the use of comprehensive laboratory blanks, contamination was assessed by comparing bitumen 1 vs. bitumen 2 (see Hallmann et al. 2011) and conducting exterior/interior (E/I) experiments (see Brocks et al. 2008, 2015; Brocks 2011). In the analyzed sample, the bitumen fractions 1 and 2 are virtually identical (Fig. 3), indicating no difference between the free and mineral-occluded compounds. The observed E/I ratios of n-alkanes, hopanes, and steranes range between 0.9 and 1.0 (Table 2), which lends additional support to an in situ origin of the molecular fossils. The observed OEP of cyclohexylalkanes C21+ in the bitumen fractions 1 and 2 could indicate a slight contamination by polyethylene byproducts (Grosjean and Logan 2007; Brocks et al. 2008), especially as there is no such OEP in these compounds in the HyPy-pyrolysate. Apart from that, however, contamination appears to be very minor in all fractions studied.
Paleobiology
Although n-alkanes are ubiquitous in the biosphere and are therefore not specific (e.g., Brocks and Summons 2003), the observed predominance of short-chain n-alkanes with maxima at n-C17 (bitumen fractions 1 and 2) and n-C15 (HyPy-pyrolysate) is generally in line with a bacterial or algal source (e.g., Blumer et al. 1971; Hoffmann et al. 1987; Peters et al. 2005). Pristane and phytane, along with the other short-chain isoprenoids observed, are most commonly considered to derive from the phytol side chain of chlorophyll a (e.g., Tissot and Welte 1984; Philp 1985; Peters et al. 2005, and references therein). This indicates an abundance of photoautotrophs, namely cyanobacteria and algae, in the Khatyspyt environment.
Abundant hopanes indicate the importance of bacterial organic matter sources (e.g., Rohmer et al. 1984; Peters et al. 2005). The presence of mid-chain branched alkanes may point to direct contributions from cyanobacteria (e.g., Gelpi et al. 1970; Shiea et al. 1990; Peters et al. 2005). Terminally methyl-branched alkanes (iso- and anteiso-isomers) in fossil samples are often considered as being derived from iso- and anteiso-fatty acids. These compounds are widespread in anaerobic bacteria, including sulfate-reducing bacteria (see Kaneda 1991, and references therein). However, the lack of distinctive isomer preferences of methyl-branched alkanes could also reflect formation during catagenesis (Kissin 1987). Cyclohexylalkanes may derive from cyclohexyl fatty acids, which occur in various bacteria (De Rosa et al. 1971; Suzuki et al. 1981). It is not clear whether these compounds could also represent diagenetic products of straight-chain fatty acids or polyaldehydes (e.g., Rubinstein and Strausz 1979; Gelin et al. 1994). Taken together, however, the presence of both autotrophic and heterotrophic bacteria in the studied paleoenvironment is likely.
The carbonaceous compression fossil on top of the sample (cf. Grazhdankin et al. 2008) and the abundance of steranes (e.g., Volkman 2003; Peters et al. 2005) indicate the presence of eukaryotes. The observed preference for stigmastane (C29) over cholestane (C27) and ergostane (C28) is typical for the Ediacaran (Knoll et al. 2007; Brocks et al. 2015). Examples include other Siberian platform oils (e.g., Kontorovich et al. 1981; Fowler and Douglas 1987; Summons and Powell 1992; Aref’yev et al. 1993; Kashirtsev et al. 2010; Kelly et al. 2011) and Ediacaran rocks from the South Oman Salt Basin (e.g., Grantham 1986; Grantham et al. 1990; Grosjean et al. 2009). This phenomenon is commonly explained by abundant chlorophyte varieties in Ediacaran ecosystems (Grantham and Wakefield 1988; Summons and Powell 1992; Knoll et al. 2007). Likewise, cheilanthanes may originate from eukaryotic algal lipids, though their exact biological source is still unknown (Brocks and Summons 2003; Brocks and Pearson 2005). Together with the steranes, these compounds indicate that eukaryotic primary producers were abundant in the Khatyspyt ecosystem, which is in good accordance with the most likely chlorophyll-derived molecular fossils pristane and phytane (see above).
Sterane/hopane ratios (Ster/Hop) are commonly used to evaluate the relative importance of bacteria vs. eukaryotes in past environments (e.g., Peters et al. 2005). Ster/Hop ratios in the analyzed sample are well below 1 (bitumen 1 = 0.37; bitumen 2 = 0.32; HyPy-pyrolysate = 0.16; Table 1), suggesting greater contributions from bacteria. However, low Ster/Hop ratios of Precambrian organic matter could also be due to benthic microbial mats that impact the transfer of organic compounds from the water column to the sediment (i.e., the “mat-seal effect”; Pawlowska et al. 2012; Blumenberg et al. 2015). Furthermore, the low Ster/Hop ratios could be due at least in part to the greater thermal stability of hopanes compared to steranes (“thermal taphonomy”) (Requejo 1994; Norgate et al. 1999; Mißbach et al. 2016). Molecular fossil analyses of different biofacies and/or taphofacies of the Khatyspyt Formation could help to determine the influence of these taphonomic processes in greater detail (see “Implications for future studies”).
Implications for future studies
Given the exceptional preservation of body fossils (Grazhdankin et al. 2008), trace fossils (Rogov et al. 2012, 2013a, b), and molecular fossils (this study), the Khatyspyt Formation can certainly be considered a fossil lagerstätte sensu Seilacher (1970). Although fossil lagerstätten provide a unique window into past ecosystems, they are not necessarily representative, as they were formed under rather unusual conditions (Seilacher 1970). The extent to which the Khatyspyt Lagerstätte is similar to other settings with Ediacara-type fossils has yet to be demonstrated. This could be achieved by a critical comparison of different settings with respect to the geological framework, sedimentary facies, paleontological features, and biogeochemical characteristics.
The Shibantan Member in South China is particularly suitable for such a comparison. Both the Khatyspyt Formation and the Shibantan Member consist of bituminous limestones and comprise somewhat similar Ediacara-type fossils (cf. Sun 1986; Xiao et al. 2005; Grazhdankin et al. 2008; Shen et al. 2009; Chen et al. 2014; Duda et al. 2014a, 2015). Unlike the Shibantan Member, however, the Khatyspyt Formation also contains carbonaceous compression fossils (Grazhdankin et al. 2008) and vertical bioturbation (Rogov et al. 2012, 2013a, b). Detailed comparisons of the Khatyspyt Formation and the Shibantan Member will enable scientists to distinguish ecological and taphonomic influences on the respective fossil records.
It has been proposed that the Khatyspyt Formation and the Shibantan Member were at least temporarily stratified, with an anoxic lower and an oxygenated upper water layer (Dzik 2003; Duda et al. 2014a; Kaufman et al. 2014). However, no molecular fossils indicative of stratification (e.g., gammacerane; Sinninghe Damsté et al. 1995), anoxic conditions (e.g., 28,30-bisnorhopane and 25,28,30-trisnorhopane; Peters et al. 2005), or even photic zone anoxia (e.g., isorenieratane or okenane; Koopmans et al. 1996, Brocks and Schaeffer 2008, respectively) were observed in the sample from the Khatyspyt Formation. This may support earlier suggestions of heterogeneous and fluctuating redox conditions in the Khatyspyt and Shibantan environments (Duda et al. 2014a; Kaufman et al. 2014). It remains to be determined, however, whether the analyzed sample is representative of the entire Khatyspyt Lagerstätte. In future studies, more detailed investigations of molecular fossils will improve our understanding of how ecological conditions in these Ediacaran environments fluctuated in time and space.
Conclusions
Organic matter in the Khatyspyt Formation is remarkably immature (oil window maturity) and almost unaffected by biodegradation. Petrographic observations, exterior/interior (E/I) experiments, and the similarity between free bitumen, mineral-occluded bitumen, and HyPy-pyrolysate point to syngeneity of the observed molecular fossils. Abundant hopanes, cyclohexylalkanes, and methyl-branched alkanes indicate a bacterial source of the organic matter, likely including cyanobacteria and anaerobic bacteria. At the same time, the carbonaceous compression fossil on top of the sample and the ample steranes indicate a high abundance of eukaryotes. The steranes show typical distributions for the Ediacaran (i.e., dominance of stigmastane). No molecular fossils indicative of stratification, anoxic conditions, or photic zone anoxia were observed. Given the exceptional preservation of body fossils, trace fossils, and molecular fossils, the Khatyspyt Formation can be considered a fossil lagerstätte sensu Seilacher (1970). The combined analysis of sedimentary facies, paleontology (body, trace, and molecular fossils), and biogeochemistry in future studies will enable a more complete understanding of the Khatyspyt Lagerstätte and will improve our understanding of how ecological conditions fluctuated in time and space.
References
Ahmed, M., and S.C. George. 2004. Changes in the molecular composition of crude oils during their preparation for GC and GC–MS analyses. Organic Geochemistry 35: 137–155.
Aref’yev, O.A., M.N. Zabrodina, G.V. Rusinova, and A.A. Petrov. 1993. Biomarkers of East Siberian crude oils. Petroleum Chemistry 33(6): 474–490.
Billings, E. 1872. On some fossils from the primordial rocks of Newfoundland. Canadian Naturalist 6(4): 465–479.
Bishop, A.N., G.D. Love, A.D. Mcaulay, C.E. Snape, and P. Farrimond. 1998. Release of kerogen-bound hopanoids by hydropyrolysis. Organic Geochemistry 29(4): 989–1001.
Blumenberg, M., V. Thiel, and J. Reitner. 2015. Organic matter preservation in the carbonate matrix of a recent microbial mat—Is there a ‘mat seal effect’? Organic Geochemistry 87: 25–34.
Blumer, M., R. Guillard, and T. Chase. 1971. Hydrocarbons of marine phytoplankton. Marine Biology 8: 183–189.
Bowring, S.A., J.P. Grotzinger, C.E. Isachsen, A.H. Knoll, S.M. Pelechaty, and P. Kolosov. 1993. Calibrating rates of early Cambrian evolution. Science 261: 1293–1298.
Bray, E.E., and E.D. Evans. 1961. Distribution of n-paraffins as a clue to recognition of source beds. Geochimica et Cosmochimica Acta 22: 2–15.
Brocks, J.J. 2011. Millimeter-scale concentration gradients of hydrocarbons in Archean shales: Live-oil escape or fingerprint of contamination? Geochimica et Cosmochimica Acta 75: 3196–3213.
Brocks, J.J., E. Grosjean, and G.A. Logan. 2008. Assessing biomarker syngeneity using branched alkanes with quaternary carbon (BAQCs) and other plastic contaminants. Geochimica et Cosmochimica Acta 72: 871–888.
Brocks, J.J., A.J.M. Jarrett, E. Sirantoine, F. Kenig, M. Moczydłowska, S. Porter, and J. Hope. 2015. Early sponges and toxic protists: possible sources of cryostane, an age diagnostic biomarker antedating Sturtian Snowball Earth. Geobiology 14(2): 129–149.
Brocks, J.J., G.D. Love, C.E. Snape, G.A. Logan, R.E. Summons, and R. Buick. 2003. Release of bound aromatic hydrocarbons from late Archean and Mesoproterozoic kerogens via hydropyrolysis. Geochimica et Cosmochimica Acta 67: 1521–1530.
Brocks, J.J., and A. Pearson. 2005. Building the biomarker tree of life. Reviews in Mineralogy and Geochemistry 59: 233–258.
Brocks, J.J., and P. Schaeffer. 2008. Okenane, a biomarker for purple sulfur bacteria (Chromatiaceae), and other new carotenoid derivatives from the 1640 Ma Barney Creek Formation. Geochimica et Cosmochimica Acta 72: 1396–1414.
Brocks, J.J., and R.E. Summons. 2003. Chapter 8.03 Sedimentary hydrocarbons, biomarkers for early life. In Treatise in geochemistry 8, ed. H.D. Holland, and K. Turekian, 64–103. Amsterdam: Elsevier.
Callow, R.H.T., and M.D. Brasier. 2009. Remarkable preservation of microbial mats in Neoproterozoic siliciclastic settings: Implications for Ediacaran taphonomic models. Earth-Science Reviews 96: 207–219.
Chen, Z., C. Zhou, S. Xiao, W. Wang, C. Guan, H. Hua, and X. Yuan. 2014. New Ediacara fossils preserved in marine limestone and their ecological implications. Scientific Reports 4: 4180.
De Rosa, M., A. Gambacorta, L. Minale, and J.D. Bu’Lock. 1971. Cyclohexane fatty acids from a thermophilic bacterium. Journal of the Chemical Society D: Chemical Communications 21: 1334a.
Dong, L., S. Xiao, B. Shen, and C. Zhou. 2008. Silicified Horodyskia and Palaeopascichnus from upper Ediacaran cherts in South China: tentative phylogenetic interpretation and implications for evolutionary stasis. Journal of the Geological Society 165: 367–378.
Duda, J.-P., M. Blumenberg, V. Thiel, K. Simon, M. Zhu, and J. Reitner. 2014a. Geobiology of a palaeoecosystem with Ediacara-type fossils: the shibantan member (Dengying formation, South China). Precambrian Research 255(1): 48–62. doi:10.1016/j.precamres.2014.09.012.
Duda, J.-P., V. Thiel, J. Reitner, and M. Blumenberg. 2014b. Assessing possibilities and limitations for biomarker analyses on outcrop samples: a case study on carbonates of the shibantan member (Ediacaran Period, Dengying Formation, South China). Acta Geologica Sinica (English Edition) 88: 1696–1704. doi:10.1111/1755-6724.12338.
Duda, J.-P., M. Zhu, and J. Reitner. 2015. Depositional dynamics of a bituminous carbonate facies in a tectonically induced intra-platform basin: the Shibantan Member (Dengying Formation, Ediacaran Period). Carbonates and Evaporites 31(1): 87–99. doi:10.1007/s13146-015-0243-8.
Durand, B. 1980. Kerogen: Insoluble organic matter from sedimentary rocks. Paris: Éditions Technip.
Dzik, J. 2003. Anatomical information content in the Ediacaran fossils and their possible zoological affinities. Integrative and Comparative Biology 43: 114–126.
Eglinton, G., and M. Calvin. 1967. Chemical fossils. Scientific American 216: 32–43.
Erwin, D.H., and J.W. Valentine. 2013. The Cambrian explosion: the construction of animal biodiversity. Greenwood Village: Roberts and Company Publishers Inc.
Fedonkin, M.A. 1990. Systematic description of Vendian Metazoa. In The Vendian System. V2. Paleontology, ed. B.S. Sokolov, and A.B. Iwanowski, 71–120. Berlin: Springer.
Fowler, M.G., and A.G. Douglas. 1987. Saturated hydrocarbon biomarkers in oils of late Precambrian age from Eastern Siberia. Organic Geochemistry 11(3): 201–213.
French, K.L., C. Hallmann, J.M. Hope, P.L. Schoon, J.A. Zumberge, Y. Hoshino, C.A. Peters, S.C. George, G.D. Love, and J.J. Brocks. 2015. Reappraisal of hydrocarbon biomarkers in Archean rocks. Proceedings of the National Academy of Sciences 112: 5915–5920.
Gelin, F., J.W. De Leeuw, J.S. Sinninghe Damsté, S. Derenne, C. Largeau, and P. Metzger. 1994. The similarity of chemical structures of soluble aliphatic polyaldehyde and insoluble algaenan in the green microalga Botryococcus braunii race A as revealed by analytical pyrolysis. Organic Geochemistry 21(5): 423–435.
Gelpi, E., H. Schneider, J. Mann, and J. Oró. 1970. Hydrocarbons of geochemical significance in microscopic algae. Phytochemistry 9: 603–612.
Grantham, P.J. 1986. The occurrence of unusual C27 and C29 sterane predominances in two types of Oman crude oil. Organic Geochemistry 9(1): 1–10.
Grantham, P.J., G.W.M. Lijmbach, and J. Posthuma. 1990. Geochemistry of crude oils in Oman. In Classic petroleum provinces, ed. J. Brooks, 317–328. London: Geological Society Special Publication 50.
Grantham, P.J., and L.L. Wakefield. 1988. Variations in the sterane carbon number distributions of marine source rock derived crude oils through geological time. Organic Geochemistry 12(1): 61–73.
Grazhdankin, D.V., U. Balthasar, K.E. Nagovitsin, and B.B. Kochnev. 2008. Carbonate-hosted Avalon-type fossils in Arctic Siberia. Geology 36: 803–806.
Grosjean, E., and G.A. Logan. 2007. Incorporation of organic contaminants into geochemical samples and an assessment of potential sources: Examples from Geoscience Australia marine survey S282. Organic Geochemistry 38: 853–869.
Grosjean, E., G.D. Love, C. Stalvies, D.A. Fike, and R.E. Summons. 2009. Origin of petroleum in the Neoproterozoic-Cambrian South Oman Salt Basin. Organic Geochemistry 40: 87–110.
Gürich, G. 1930a. Die bislang ältesten Spuren von Organismen in Südafrika. C.R. 15. International Geological Congress, South Africa 1929: 670–680.
Gürich, G. 1930b. Über den Kuibisquarzit in Südwestafrika. Zeitschrift der Deutschen Geologischen Gesellschaft 82: 637.
Gürich, G. 1933. Die Kuibis-Fossilien der Nama-Formation von Südwestafrika. Nachträge und Zusätze. Paläontologische Zeitschrift 15: 137–154.
Hallmann, C., A.E. Kelly, S.N. Gupta, and R.E. Summons. 2011. Reconstructing deep-time biology with molecular fossils. In Quantifying the evolution of early life, ed. M. Laflamme, J.D. Schiffbauer, and S.Q. Dornbos, 355–401. Berlin: Springer.
Hoffman, P.F., A.J. Kaufman, G.P. Halverson, and D.P. Schrag. 1998. A neoproterozoic snowball earth. Science 281: 1342–1346.
Hoffmann, C.F., C.B. Foster, T.G. Powell, and R.E. Summons. 1987. Hydrocarbon biomarkers from Ordovician sediments and the fossil alga Gloeocapsomorpha prisca Zalessky 1917. Geochimica et Cosmochimica Acta 51: 2681–2697.
Kaneda, T. 1991. Iso- and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiological Reviews 55(2): 288–301.
Kashirtsev, V.A. 1988. Natural bitumens of the northeastern Siberian Platform. Yakutsk: Izd. YaF SO AN SSSR. (in Russian).
Kashirtsev, V.A. 2003. Organic geochemistry of naphthides in the east of the Siberian Platform. Yakutsk: Izd. YaF SO AN SSSR. (in Russian).
Kashirtsev, V.A., A.E. Kontorovich, V.L. Ivanov, and A.F. Safronov. 2010. Natural bitumen fields in the northeast of the Siberian Platform. Russian Geology and Geophysics 51: 72–82.
Kaufman, A.J., H. Cui, S. Peek, V. Rogov, D. Grazhdankin, and S. Xiao. 2014. The effect of seawater redox stratification on early metazoans from the terminal Ediacaran Khatyspyt Formation of arctic Siberia. In South China 2014: A symposium and field workshop on Ediacaran and Cryogenian stratigraphy. Abstracts. International Commission on Stratigraphy, Subcommissions on Ediacaran and Cryogenian Stratigraphy: 27.
Kelly, A.E., G.D. Love, J.E. Zumberge, and R.E. Summons. 2011. Hydrocarbon biomarkers of neoproterozoic to lower Cambrian oils from eastern Siberia. Organic Geochemistry 42: 640–654.
Killops, S.D., and V.J. Killops. 2005. Introduction to organic geochemistry, 2nd ed. Malden: Wiley-Blackwell.
Kirschvink, J.L. 1992. Late Proterozoic Low-Latitude Global Glaciation: the Snowball Earth. In The Proterozoic biosphere: A multidisciplinary study, ed. J.W. Schopf, and C. Klein, 51–52. Cambridge: Cambridge University Press.
Kissin, Y.V. 1987. Catagenesis and composition of petroleum: Origin of n-alkanes and isoalkanes in petroleum crudes. Geochimica et Cosmochimica Acta 51: 2445–2457.
Knoll, A.H., M. Walter, G.M. Narbonne, and N. Christie-Blick. 2006. The Ediacaran Period: a new addition to the geologic time scale. Lethaia 39: 13–30.
Knoll, A.H., J.P. Grotzinger, A.J. Kaufman, and P. Kolosov. 1995. Integrated approaches to terminal Proterozoic stratigraphy: an example from the Olenek Uplift, northeastern Siberia. Precambrian Research 73: 251–270.
Knoll, A.H., R.E. Summons, J.R. Waldbauer, and J.E. Zumberge. 2007. The geological succession of primary producers in the oceans. In The evolution of primary producers in the sea, ed. P. Falkowski, and A.H. Knoll, 133–163. Burlington: Elsevier.
Knoll, A.H., M. Walter, G.M. Narbonne, and N. Christie-Blick. 2004. A new period for the geologic time scale. Science 305: 621–622.
Kontorovich, A.E., T. Parfenova, V. Kashirtsev, N. Aksenova, E. Zubova, E. Ivanova, and N. Yudina. 2009. Organic geochemistry of the Vendian Khatyspyt Formation (northeast of the Siberian Platform). Book of abstracts, 24th international meeting on organic geochemistry, 6–11 September 2009, Bremen, Germany: 174.
Kontorovich, A.E., V.S. Surkov, and A.A. Trofimuk. 1981. Geology of petroleum and gas of the Siberian platform. Moscow: Nedra. (in Russian).
Kontorovich, V.A., A.E. Kontorovich, I.A. Gubin, A.M. Zoteev, V.V. Lapkovsky, N.A. Malyshev, M.V. Soloviev, and G.S. Fradkin. 2013. The Neoproterozoic-Phanerozoic section of the Anabar-Lena province: structural framework, geological model, and petroleum potential. Russian Geology and Geophysics 54: 980–996.
Koopmans, M.P., J. Köster, H.M.E. Van Kaam-Peters, F. Kenig, S. Schouten, W.A. Hartgers, J.W. De Leeuw, and S. Damsté. 1996. Diagenetic and catagenetic products of isorenieratene: Molecular indicators for photic zone anoxia. Geochimica et Cosmochimica Acta 60(22): 4467–4496.
Love, G.D., C. Stalvies, E. Grosjean, W. Meredith, and C.E. Snape. 2008. Analysis of molecular biomarkers covalently bound within neoproterozoic sedimentary kerogen. In From evolution to geobiology: Research questions driving paleontology at the start of a new century, ed. P.H. Kelley, and R.K. Bambach. Boulder: The Paleontological Society.
Love, G.D., C.E. Snape, A.D. Carr, and R.C. Houghton. 1995. Release of covalently-bound alkane biomarkers in high yields from kerogen via catalytic hydropyrolysis. Organic Geochemistry 23: 981–986.
Mello, M.R., N. Telnaes, P.C. Gaglianone, M.I. Chicarelli, S.C. Brassel, and J.R. Maxwell. 1987. Organic geochemical characterisation of depositional palaeoenvironments of source rocks and oils in Brazilian marginal basins. Organic Geochemistry 13: 31–45.
Meredith, W., C.A. Russel, M. Cooper, C.E. Snape, G.D. Love, D. Fabbri, and C.H. Vane. 2004. Trapping hydropyrolysates on silica and their subsequent thermal desorption to facilitate rapid fingerprinting by GC–MS. Organic Geochemistry 35: 73–89.
Meredith, M.C.E., A.D. Snape, H.P.Nytoft Carr, and G.D. Love. 2008. The occurrence of unusual hopenes in hydropyrolysates generated from severely biodegraded oil seep asphaltenes. Organic Geochemistry 39(8): 1243–1248.
Mißbach, H., J.-P. Duda, N.K. Lünsdorf, B.C. Schmidt, and V. Thiel. 2016. Testing the preservation of biomarkers during experimental maturation of an immature kerogen. International Journal of Astrobiology 15(3): 165–175. doi:10.1017/S1473550416000069.
Nagovitsin, K.E., V.I. Rogov, V.V. Marusin, G.A. Karlova, A.V. Kolesnikov, N.V. Bykova, and D.V. Grazhdankin. 2015. Revised Neoproterozoic and Terreneuvian stratigraphy of the Lena-Anabar Basin and north-western slope of the Olenek Uplift, Siberian Platform. Precambrian Research 270: 226–245.
Narbonne, G.M. 2005. The ediacara biota: neoproterozoic origin of animals and their ecosystems. Annual Review of Earth and Planetary Sciences 33: 421–442.
Narbonne, G.M., S. Xiao, G.A. Shields, and J.G. Gehling. 2012. Chapter 18—The Ediacaran period. In The geologic time scale 2012, ed. F.M. Gradstein, J.G. Ogg, M.D. Schmitz, and G.M. Ogg, 413–435. Boston: Elsevier.
Norgate, C.M., C.J. Boreham, and A.J. Wilkins. 1999. Changes in hydrocarbon maturity indices with coal rank and type, Buller Coalfield, New Zealand. Organic Geochemistry 30: 985–1010.
Parfenova, T., V. Kashirtsev, L. Borisova, E. Ivanova, B. Kochnev, K. Nagovitsyn, and V. Melenevsky. 2011. Carbonaceous rocks of the Neoproterozoic (Vendian) Khatyspyt Formation as a possible source of oils in the northeastern Siberian Platform. Book of abstracts, 25th international meeting on organic geochemistry, 18–23 September 2011, Interlaken, Switzerland: 434.
Parfenova, T.M., B.B. Kochnev, K.E. Nagovitsin, E.N. Ivanova, V.A. Kashirtsev, and A.E. Kontorovich. 2010. Geochemistry of organic matter of the Khatyspyt Formation (Vend, North-Easten Siberian Platform). Advances in organic geochemistry. Transactions of the Russian scientific conference (Novosibirsk, 11–15 October 2010): 265–268 (in Russian).
Pawlowska, M.M., N.J. Butterfield, and J.J. Brocks. 2012. Lipid taphonomy in the Proterozoic and the effect of microbial mats on biomarker preservation. Geology 41: 103–106.
Peters, K.E., C.C. Walters, and J.M. Moldowan. 2005. The Biomarker Guide Volume 2: Biomarkers and isotopes in petroleum exploration and earth history. Cambridge: The Press Syndicate of the University of Cambridge.
Philp, R.P. 1985. Fossil fuel biomarkers. Applications and spectra. Methods in geochemistry and geophysics 23. Amsterdam: Elsevier.
Requejo, A.G. 1994. Maturation of petroleum source rocks—II. Quantitative changes in extractable hydrocarbon content and composition associated with hydrocarbon generation. Organic Geochemistry 21: 91–105.
Requejo, A.G., G.B. Hieshima, C.S. Hsu, T.C. McDonald, and R. Sassen. 1997. Short-chain (C21 and C22) diasteranes in petroleum and source rocks as indicators of maturity and depositional environment. Geochimica et Cosmochimica Acta 61(13): 2653–2667.
Rogov, V., V. Marusin, N. Bykova, Y. Goy, K. Nagovitsin, B. Kochnev, G. Karlova, and D. Grazhdankin. 2012. The oldest evidence of bioturbation on Earth. Geology 40(5): 395–398.
Rogov, V., V. Marusin, N. Bykova, Y. Goy, K. Nagovitsin, B. Kochnev, G. Karlova, and D. Grazhdankin. 2013a. The oldest evidence of bioturbation on earth: Reply. Geology 41(5): e290.
Rogov, V., V. Marusin, N. Bykova, Y. Goy, K. Nagovitsin, B. Kochnev, G. Karlova, and D. Grazhdankin. 2013b. The oldest evidence of bioturbation on earth: Reply. Geology 41(5): e300.
Rogov, V.I., G.A. Karlova, V.V. Marusin, B.B. Kochnev, K.E. Nagovitsin, and D.V. Grazhdankin. 2015. Duration of the first biozone in the Siberian hypostratotype of the Vendia. Russian Geology and Geophysics 56: 573–583.
Rohmer, M., P. Bouvier-Nave, and G. Ourisson. 1984. Distribution of hopanoid triterpenes in prokaryotes. Journal of General Microbiology 130: 1137–1150.
Rubinstein, I., and O.P. Strausz. 1979. Geochemistry of the thiourea adduct fraction from an Alberta petroleum. Geochimica et Cosmochimica Acta 43: 1387–1392.
Schinteie, R., and J.J. Brocks. 2014. Evidence for ancient halophiles? Testing biomarker syngeneity of evaporites from Neoproterozoic and Cambrian strata. Organic Geochemistry 72: 46–58.
Seifert, W.K., and J.M. Moldowan. 1978. Applications of steranes, terpanes and monoaromatics to the maturation, migration and source of crude oils. Geochimica et Cosmochimica Acta 42: 77–95.
Seifert, W.K., and J.M. Moldowan. 1980. The effect of thermal stress on source-rock quality as measured by hopane stereochemistry. Physics and Chemistry of the Earth 12: 229–237.
Seifert, W.K., and J.M. Moldowan. 1986. Use of biological markers in petroleum exploration. In Methods in Geochemistry and Geophysics 24, ed. R.B. Johns, 261–290. Amsterdam: Elsevier.
Seilacher, A. 1970. Begriff und Bedeutung der Fossil-Lagerstätten. Neues Jahrbuch für Geologie und Paläontologie, Monatshefte: 34–39.
Seilacher, A. 1989. Vendozoa: Organismic construction in the Proterozoic biosphere. Lethaia 22: 229–239.
Seilacher, A. 1992. Vendobionta and Psammocorallia: lost constructions of Precambrian evolution. Journal of the Geological Society 149: 607–613.
Seilacher, A., D. Grazhdankin, and A. Legouta. 2003. Ediacaran biota: The dawn of animal life in the shadow of giant protists. Paleontological Research 7(1): 43–54.
Shen, B., S. Xiao, L. Dong, C. Zhou, and J. Liu. 2007. Problematic macrofossils from Ediacaran successions in the North China and Chaidam Blocks: Implications for their evolutionary roots and biostratigraphic significance. Journal of Paleontology 81(6): 1396–1411.
Shen, B., S. Xiao, C. Zhou, and X. Yuan. 2009. Yangtziramulus zhangi new genus and species, a carbonate-hosted macrofossil from the Ediacaran Dengying Formation in the Yangtze Gorges Area, South China. Journal of Paleontology 83(4): 575–587.
Shiea, J., S.C. Brassel, and D.M. Ward. 1990. Mid-chain branched mono- and dimethyl alkanes in hot spring cyanobacterial mats: A direct biogenic source for branched alkanes in ancient sediments? Organic Geochemistry 15(3): 223–231.
Sinninghe Damsté, J.S., F. Kenig, M.P. Koopmans, J. Köster, S. Schouten, J.M. Hayes, and J.W. de Leeuw. 1995. Evidence for gammacerane as an indicator of water column stratification. Geochimica et Cosmochimica Acta 59: 1895–1900.
Snape, C.E., C. Bolton, R.G. Dosch, and H.P. Stephens. 1989. High liquid yields from bituminous coal via hydropyrolysis with dispersed catalysts. Energy and Fuels 3: 421–425.
Sokolov, B.S., and M.A. Fedonkin. 1984. The Vendian as the terminal system of the Precambrian. Episodes 7(1): 12–19.
Summons, R.E., and L.L. Jahnke. 1992. Hopenes and hopanes methylated in ring-A: Correlation of the hopanoids from extant methylotrophic bacteria with their fossil analogues. In Biological markers in sediments and petroleum, ed. J.M. Moldowan, P. Albrecht, and R.P. Philp, 182–200. New York: Prentice Hall.
Summons, R.E., and T.G. Powell. 1992. Hydrocarbon composition of the late Proterozoic oils of the Siberian platform: implications for the depositional environment of source rocks. In Early organic evolution: implications for mineral and energy resources, ed. M. Schidlowski, S. Golubic, M.M. Kimberley, D.M. McKirdy Sr., and P.A. Trudinger, 296–307. Berlin: Springer.
Sun, W. 1986. Late Precambrian pennatulids (sea pens) from the eastern Yangtze Gorge, China: Paracharnia gen. nov. Precambrian Research 31: 361–375.
Suzuki, K.-I., K. Saito, A. Kawaguchi, S. Okuda, and K. Komagata. 1981. Occurrence of ω-cyclohexyl fatty acids in Curtobacterium pusillum strains. The Journal of General and Applied Microbiology 27: 261–266.
Tissot, B.P., and D.H. Welte. 1984. Petroleum formation and occurrence. Berlin: Springer.
Treibs, A. 1934. Chlorophyll- und Häminderivate in bituminösen Gesteinen, Erdölen, Erdwachsen und Asphalten. Ein Beitrag zur Entstehung des Erdöls. Justus Liebigs Annalen der Chemie 510(1): 42–62.
Treibs, A. 1936. Chlorophyll-und Häminderivate in organischen Mineralstoffen. Angewandte Chemie 49: 682–686.
Vodanjuk, S.A. 1989. Non-skeletal fossil Metazoa from the Khatyspyt Formation of the Olenek Uplift. In Late Precambrian and early Palaeozoic of Siberia. Urgent stratigraphic problems, ed. V.V. Khomentovsky, and J.K. Sovetov, 61–74. Novosibirsk: IGG SO AN SSSR. (in Russian).
Volkman, J.K. 2003. Sterols in microorganisms. Applied Microbiology and Biotechnology 60: 495–506.
Xiao, S., B. Shen, C. Zhou, G. Xie, and X. Yuan. 2005. A uniquely preserved Ediacaran fossil with direct evidence for a quilted bodyplan. Proceedings of the National Academy of Sciences 102: 10227–10232.
Xiao, S., and M. Laflamme. 2009. On the eve of animal radiation: phylogeny, ecology and evolution of the Ediacara biota. Trends in Ecology and Evolution 24: 31–40.
Zhang, X., D. Shu, J. Han, Z. Zhang, J. Liu, and D. Fu. 2014. Triggers for the Cambrian explosion: Hypotheses and problems. Gondwana Research 25: 896–909.
Acknowledgments
This study is dedicated to Adolf Seilacher (1925–2014), a visionary paleontologist and renowned expert on the Ediacara biota. We thank C. Conradt, W. Dröse, A. Hackmann, A. Kral, M. Reinhardt, B. Röring, and A. Reimer for their technical and analytical support. We are indebted to J. Peckmann, M. Rohrssen, and M. Reich, whose comments and suggestions greatly improved the paper. The study was financially supported by the Deutsche Forschungsgemeinschaft (Grant DU 1450/3-1; SPP 1833 “Building a Habitable Earth”), the Research Department of the University of Göttingen, and the Russian Science Foundation (Grant 14-17-00409 “Mechanisms behind Ediacaran and Terreneuvian ecosystem functioning: stability and dynamic processes” to DG). This is publication number 3 of the Early Life Research Group (Department of Geobiology, University of Göttingen; Göttingen Academy of Sciences and Humanities).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Dr. Mike Reich.
Rights and permissions
About this article
Cite this article
Duda, JP., Thiel, V., Reitner, J. et al. Opening up a window into ecosystems with Ediacara-type organisms: preservation of molecular fossils in the Khatyspyt Lagerstätte (Arctic Siberia). PalZ 90, 659–671 (2016). https://doi.org/10.1007/s12542-016-0317-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12542-016-0317-5
Keywords
- Ediacaran
- Fossil lagerstätte
- Ediacara-type organisms
- Carbonaceous compression fossils
- Molecular fossils
- Catalytic hydropyrolysis (HyPy)