Abstract
Two dissimilar alloys Al 5083 and Mg-AZ31 were diffusion bonded using two different interlayer applying methods, where the interlayer was 30 µm pure silver foil and 4 µm physical vapor deposited silver on Al and Mg substrates. The optimized variables used in diffusion bonding processes were holding time of 60 min and bonding temperature of 470 °C. The X-ray diffraction results confirmed the formation of Mg–Ag, Ag–Zn, and Ag–Al phases namely: MgAg, AgZn, Ag5Zn8, and Ag2Al instead of Al–Mg intermetallics at the interface. This proves the effective role of Ag, as a diffusion barrier and its success in minimizing the formation of harmful intermetallic phases at the interface. The joint with silver PVD coat, as interlayer, gave the higher shear strength of 31.6 MPa.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Recently, there is a strong tendency to make diffusion bonded dissimilar joints between different materials. This is because diffusion bonding is an effective method that can solve many difficulties faced during fusion welding and has appeared as a near net shape forming process [1]. Joining of Al alloy to Mg alloy through welding is an enterprise challenge with potentially principal applications [2]. Mg–Al bimetal is an example of these applications in aircraft engines and components [3]. Also, the Mg–Al layered armor is available in armored vehicles and tanks, and Mg–Al connected pipe is used in bicycle manufacture [4]. The major challenge in the welding of Mg/Al couples is the creation of brittle and hard intermetallic compounds (IMCs) which destroy the strength of the joint [5]. Referring to the Mg–Al dual phase diagram shows that by heating Mg and Al together, Al3Mg2 and Al12Mg17 intermetallic phases will probably form: the former on the Al side and the latter on the Mg side [6]. Inserting an interlayer between the two base materials prohibits direct contact of Al and Mg. As a result, the formation of above intermetallics become controllable [7]. several investigations have been performed to solve such incompatibility in joining Al with Mg. This mission is performed by inserting additional interlayers such as Ni, Zn, Ti, and Ag. These interlayers act as diffusion barriers and minimize the formation of intermetallic phases at the interface [8,9,10,11]. Zhang et al. [9] joined pure aluminium and pure magnesium by diffusion bonding at 440 °C using nickel with a thickness of 6 µm as interlayer and reported a relatively low joint strength of 26 N/mm2 after a bonding time of 60 min. Moreover, Wang et al. [11] achieved the highest bond strength of 14.5 N/mm2 at the bonding temperature of 390 °C and holding time of 30 min with a 25 µm thick silver interlayer.
The other challenge origins from the existence of sticky oxide layer in the both Al and Mg surfaces. The presence of aluminum and magnesium oxides on the surfaces can prevent metal-to-metal contact at the joint interface, which is harmful for the joint quality [12]. This led to the necessity of special surface preparation, to bond aluminum to magnesium.
As a continuation of our previous works [2, 5, 6, 13], in the present study, diffusion bonding of Al/Mg alloys using Ag interlayer was investigated. Pure Ag interlayer was applied as foil and as physical vapor deposition (PVD) coat, between base materials. Applying the Ag interlayer, as PVD coat is used for several reasons such as (1) to reduce or to control the formation of brittle intermetallic phases, (2) to eliminate the harmful effects of Al and Mg oxide films, (3) supplying appropriate surface roughness at the two faying surfaces because surface roughness is one of main parameters that play role in determination of bond strength [3], (4) to provide the possibility of applying low interlayer thicknesses because foils are expensive and they have complicated production process. Due to the nature of the PVD interlayer, it is expected that the diffusion bond behavior of the PVD coated interlayer was different than that of the conventionally foil interlayer welded specimen. The effect of interlayer applying method (PVD and foil interlayers) on microstructure and mechanical properties of diffusion-bonded Al/Ag/Mg joints is represented by describing the microstructural changes of the joints.
2 Materials and methods
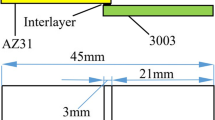
The base materials used in diffusion-bonding experiments were 5083 Al alloy and AZ31C-O Mg alloy. The accurate chemical composition of base metals is listed in Table 1. Specimens with dimension of 15 mm × 15 mm × 3 mm for metallography and 35 mm × 20 mm × 3 mm for shear strength test were prepared by cutting (Figs. 1 and 2). Then two different interlayers including 30 μm pure silver foil (Fig. 1) and Ag physical vapor deposition coat (Fig. 2) was applied to join Al5083 and MgAZ31. In the case of PVD interlayer specimen, before spraying, aluminum and magnesium substrates were prepared by blasting with 36 mesh alumina abrasive blasting grit. The Al and Mg substrates were then PVD coated with pure silver to a thickness of approximately 4 µm (Fig. 3). The PVD parameters were: chamber pressure of 10−5 mbar, working temperature of 80 °C and coating time of 4 h. Image of silver PVD coat for approximately 4 μm thickness is shown in Fig. 3. The microstructure of coating obtained by PVD process does not show the formation of pores. The specimens were ultrasonically cleaned in an acetone bath to remove adhered contaminants and then dried in air. The optimized variables used in our diffusion bonding experiments were bonding temperature of 470 °C, holding time of 60 min, joining pressure of 1 MPa, and heating rate of about 15 °C/min. Vacuum pressure was less than 6 × 10−3 Pa. The assemblies were cooled in the processing chamber under vacuum. For metallurgical examination, the bonded samples were cut transversely. The specimens for optical microscopy were etched in a 5 ml acetic acid + 5 g picric acid + 10 ml water + 100 ml ethanol solution for revealing Mg side of the weld. Scanning electron microscopy (SEM), electron dispersive spectroscopy (EDS) and X-ray diffraction (XRD) were applied to characterize the joints and identify intermetallic compounds. Microhardness measurements of the polished surface of the diffusion bonds were performed according to ASTM E92 standard and with a load of 50 g. The shear strength of the specimen was measured according to ASTM standard D1002-99 [14] at a cross-head speed of 1 mm/min. Finally, the fracture surface of the bonded specimens was studied by SEM.
3 Results and discussion
3.1 Microstructure and compositional changes
Figure 4 shows the microstructure of base metals used in this study. Figure 4a shows a grain size of approximately 31 μm for the Mg alloy base metal. Figure 4b shows the microstructure of 5083 Al alloy with elongated grains along the rolling direction. An optical microscope was employed to check the formation of diffusion layer at the interface. Optical micrographs of the interface for the joints fabricated with foil and PVD coat as interlayer, Fig. 5, describes formation of multilayer joints. The width of the joint decreased from 130 µm for the weld made with foil interlayer (Fig. 5a) to 90 µm for the weld made with PVD coat as interlayer (Fig. 5b). This is due to higher interlayer thickness of the foil (30 µm) in comparison with coated interlayer (≈ 8 µm). figure 5a also shows some coarse grains within the magnesium alloy adjacent to the bond interface. This kind of grain coarsening was also reported by some researchers [15]. But they did not offer a clear explanation for this phenomenon [15]. The presence of inclusions can make a material very resistant to grain growth. However, the resistance to grain growth may breakdown at sufficiently high temperatures. The result is discontinuous grain growth or exaggerated grain growth, Fig. 5a. Dissolution of the second-phase particles or overcoming of their resistance allows a few grains to grow at the expense of others. This is called diffusion induced grain coarsening.
To investigate the microstructure more precisely, a SEM micrograph of the interface and concentration profile of the major elements (Al, Mg, and Ag) across the bonding region of foil interlayer specimen, were taken and are shown in Fig. 6. Diffusion is observable across both Mg–Ag and Ag–Al interfaces. The backscattered electron (BSE) images confirm formation of multilayer joint in this specimen. There are two distinct reaction layers created at the interface between the Mg base and Ag foil in Fig. 6a. The interdiffusion of Al, Mg, and Ag elements is illustrated in Fig. 6b by line scan analysis. The inhomogeneity of the compositions indicates that the joint interface contains various intermetallic phases (Fig. 6b). There are five distinguishable interfacial boundaries in the joint region: I, II, III, IV, and V in Fig. 6b. The amount of Ag decreases from layer III toward both bases (Al and Mg).
The EDS analysis of selected regions (marked as A, B, C, D, and E) in Fig. 6a is represented in Table 2. Point A located adjacent to the Mg base, is probably an Mg-based solid-solution containing few percent Ag. It is illustrated that the major elements in region B are Mg and Ag. Considering the elemental composition and by referring to the Mg–Ag binary alloy phase diagram [16], it is clear that the Mg–Ag intermetallics of MgAg and Mg3Ag are formed in this region. The formation of this region is indicative of active inter-diffusion of Ag and Mg that occurs between the Ag foil and Mg base metal. Point C is the remaining silver foil interlayer. Point D, close to Al-base contains mainly Al, designate Al solid solution. Point E is rich in aluminum (45.2 at%), silver (43.8 at%) and has 11.0 at% of magnesium which indicates that the interdiffusion between Al base and Ag foil is sufficient to form Ag2Al intermetallic. Figure 6a shows cracks in this zone, indicating the brittleness of the weld owing to intermetallics. This is in accordance with previously published works that reported the observation of cracks in the Ag2Al layer near the Al base [11]. The presence of 11.0 at% Mg in this zone, indicate that interdiffusion of Ag and Mg at 470 °C occurs faster in comparison with interdiffusion of Al and Ag.
Figure 7 shows SEM micrograph of the interface and concentration profile of the major elements (Al, Mg, and Ag) across the bonding region of the specimen with PVD coat as interlayer. The joint region appears more homogenied and is free from cracks in comparison with the bond made with Ag foil as interlayer. As can be seen, distribution curve of Al is steeper in comparison with Mg curve. This is an indication of higher diffusion rate of Mg through the interface, at the bonding temperature. Oxygen level was low within the joint suggesting that oxides did not form at the joint region. Regarding the concentration profiles of elements Al, Mg, and Ag across the interface (Fig. 7b), it is concluded that diffusion of Ag is occurred through Mg and Al base metals, so that the marked point in Fig. 7a, located at the interface of silver PVD coat and Mg base metal, contains 70.3 at%Mg, 19.9 at%Al, and 9.8 at%Ag. This atomic ratio of Mg/(Ag + Al) is close to 3/1; therefore, probably the phase ɛ-Mg3(Ag, Al) is formed. The main challenge in the welding of Al and Mg is the easy formation of Mg–Al intermetallic compounds at the interface that are brittle and hard phases. The formation of these intermetallics results in low strength in joint. One approach to solve this problem is to insert interlayers that play the role of diffusion barrier, between Al and Mg alloys. It seems that using Ag interlayer in this study, successfully controlled the diffusion. As mentioned, the thickness of Ag coat, applied on each of base metals was approximately 3 µm (Fig. 3). As can be observed in Fig. 7a, the thickness of this interlayer is decreased from approximately 6 µm to 2 µm, due to diffusion. Besides, there are discontinuities and fractures, observed on the Ag interlayer, indicating fiddling diffusion at the Al side of the joint (Fig. 7a).
It is noteworthy that although excessive diffusion, causes crack formation in the weld (Fig. 6a) due to the widespread formation of IMCs, but interdiffusion and the formation of reaction layer is necessary to achieve a metallurgical bond between the two base metals. For example, Alhaza et al. [17] expressed that formation of Ti-Sn intermetallic phase creates joint at the Ti–6Al–4V interface in dissimilar joining of Al alloy to Ti alloys. Likewise, Jing et al. [4] reported that with increasing welding temperature, high energy and sufficient diffusion can be obtained, appropriate thickness of the intermetallic compounds and good atom connection are formed. Hence, formation of brittle Al–Mg IMCs cannot be completely avoided, but the IMCs could only be reduced. Applying Ag interlayer as PVD coat, was successful in controlling the diffusion between Mg and Al alloys and IMC formation at the interface of the joint. As can be observed from Figs. 6b and 7b the joint width is decreased significantly from 130 to 30 µm by using silver PVD coat instead of Ag foil as interlayer. On the otherhand, applying Ag interlayer as foil (with the thickness of 30 μm), results in formation of layers of IMCs with the thickness of approximately 16 μm (Fig. 6a) in the joint zone that causes crack formation. Applying Ag interlayer as PVD coat, influences the distribution and the thickness of the intermetallics, so that the thickness of these layers reduced to approximately 1.4 μm (Fig. 7a).
3.2 Microhardness
The Vickers microhardness (HMV) profiles of the bonds made with Ag foil and silver PVD coat as interlayer is illustrated in Fig. 8. Fairly high hardness values are observed at the interface. These values are much greater compared with that of recrystallized Al alloy and Mg alloy and greater than the Al and Mg alloy base metals. The sharp increase of microhardness value observed in in the diffusion reaction zone of Mg/Ag foil/Al joint with a maximum value of 266 HV is related to the formation of intermetallic compounds. The hardness profile of Mg/silver PVD coat/Al joint shows a light and soft increase to a maximum value of 166 HV in IMC layer. These results confirm that, applying Ag interlayer as PVD coat, was successful in controlling the diffusion between Mg and Al alloys and IMC formation at the interface of the joint. A fluctuation in hardness values is observed in the joint zone of Fig. 8. This is related to the formation of intermetallics randomly dispersed in the joint. Low hardness values (135 VHN for foil interlayer and 80 VHN for PVD interlayer) in the center of the joints, indicate existence of remained interlayer at the interface. The higher amount of microhardness value inside the remaining foil interlayer of (135 VHN) in comparison with PVD remaining interlayer (80 VHN) can be attributed to higher interdiffusion of Al and Mg bases through the interlayer and enhanced IMC formation in the foil.
3.3 Shear strength
Strength of the diffusion bonded speciens was determined by the single lap shear test. Geometry and dimensions of the joint produced for arranging the shear strength tests are shown in Figs. 1 and 2. The room temperature shear strength of the diffusion bonded joints according to interlayer applying method is shown in Fig. 9. The results showed that the interface microstructure influence the joint’s strength notably. It is clear that the joint with silver PVD coat, as interlayer, gives the higher shear strength of 31.6 MPa. The strength of the joint bonded with Ag foil as interlayer, drops to 21.9 MPa. The decrease in bond strength with using Ag foil as interlayer, can be attributed to aggregation and thickening of the brittle intermetallics near the joint interface. It is believed that initial cracks available in the IMC layers (see Fig. 6a) are subsequently propagate during shear testing. These cracks are considered as the most important cause for the poor operation of these joints [11]. The maximum shear strength value registered in the present study (31.6 Mpa) is higher than the previous study of Zhang et al. [9] that have used nickel interlayer to join Al alloy to Ti alloy and reached to 26 MPa shear strength. The maximum shear strength resulted in the present study is also higher than the value recorded by Wang et al. [11] (14.5 MPa) that have used silver interlayer to join Al alloy to Mg alloy.
3.4 SEM and XRD study of the fracture surfaces
Figure 10 shows the fracture surfaces of the joints made with silver foil and silver PVD coat as interlayer. In both cases, fracture takes place in the diffusion region, because this region contains brittle intermetallic phases. The fractographs show a brittle fracture mode for both the Mg (Fig. 10a) and Al (Fig. 10b) sides of the fracture surfaces of the joint made with Ag foil as interlayer. The fracture surface of the both sides, shown in Fig. 10a, b, exhibit a rough Intergranular fracture, which is indication of brittle failures due to intermetallic compounds. This fracture surface is quiet distinct from the microvoid feature of ductile fracture in the specimen made with silver PVD coat as interlayer (Fig. 10c, d). Therefore, the uncontrollable creation of intermetallic compounds can be the main reason of the low strength in foil interlayer specimen.
Figure 11 shows the XRD diffraction patterns of fractured surfaces of the bond made with Ag foil (Fig. 11a, b) and Ag PVD coat (Fig. 11c, d) as intetlayer. The results of XRD analyses confirm that using silver was successful in controlling the diffusion between Mg and Al alloy bases and no Al–Mg IMCs, namely Mg2Al3, Mg3Al2, and Mg17Al12 are formed at the interface. Formation of these detrimental phases is the main challenge in the solid state welding of Al and Mg owing to their brittleness [2, 5, 6, 11]. The XRD results confirm the formation of Mg–Ag, Ag–Zn, and Ag–Al phases namely: MgAg, AgZn, Ag5Zn8, and Ag2Al instead of Al–Mg intermtallics. This is in accordance with microstructural observations of this study. The absolute hardness values of the IMC compounds is as follow: Mg17A12 > Al3Mg2 > Mg3Ag > MgAg > Ag2Al [11]. The maximum hardness values of Al3Mg2, MgAg, and Ag2Al are 268.8, 223.9, and 176.5 HV, respectively [11, 18]. So the formation of Mg–Ag, Ag–Zn, and Ag–Al phases instead of Al–Mg intermtallics can develop the toughness of the weld and is an evidence of effective role of Ag, as a diffusion barrier and it’s success in minimizing the formation of intermetallic phases at the interface.
Regarding the general disposition of XRD patters, it becomes obvious that by using Ag PVD coat as interlayer (Fig. 11c, d), lower amounts of peaks are observed in XRD patterns in comparison with the case of using Ag foil (Fig. 11a, b). This is in accordance with the microstructural observations. As mentioned, applying Ag interlayer as PVD coat, influences the distribution and the thickness of the Mg–Al intermetallics, so that the thickness of these layers reduced to approximately 1.4 μm (Fig. 7a).
Considering the diffusion coefficients of Ag, Mg, and Al atoms, it can be concluded that Ag atoms diffuse faster in Mg in comparison with Al [11]. The high interdiffusion rate of Mg and Ag results in the creation of MgAg, and Mg3Ag. The formation of Ag2Al shows that bonding temperature of 470 °C and holding time of 60 min were sufficient for the interdiffusion of Al and Ag that occurs with lower rate because of the lower interdiffusion rate of Ag and Al. On the other hand, the XRD patterns show the peaks related to pure silver at both faying surfaces. This fact suggests that, by using these welding variables, the silver interlayer is not consumed due to the displacement of Mg, Ag, and Al and retained its diffusion controlling role. This is a confirmation of employed variables in this study (bonding temperature of 470 °C and holding time of 60 min) and suggests that they were optimistic variables.
4 Conclusion
In this work, the joining of Al5083 alloy to Mg AZ31 alloy was produced by diffusion bonding process using 30-µm-thick Ag foil and silver PVD coat as interlayers. Considering the results achieved, it is concluded that:
-
1.
In the specimen welded with Ag foil as interlayer, active inter-diffusion of Ag and Mg caused the formation of thick layer of Mg–Ag intermetallics of MgAg and Mg3Ag with the thickness of approximately 16 μm at the interface of the Ag foil and Mg base metal. In addition, the interdiffusion between Al base and Ag foil was active and causes Ag2Al intermetallic formation. Crack formation in this zone, indicating a brittle weld owing to intermetallics.
-
2.
In the case of using Ag PVD coat, as interlayer the joint region appeared more homogenied and was free from cracks. In this case, the thickness of intermetallic layers reduced to approximately 1.4 μm.
-
3.
The microhardness value showed sharp high values in the diffusion reaction zone of Mg/Ag foil/Al joint because of the creation of intermetallic phases. The hardness profile of Mg/silver PVD coat/Al joint showed a light and soft increase to a maximum value of 166 HV in IMC layer, suggesting that, applying Ag interlayer as PVD coat, was successful in controlling the diffusion between Mg and Al alloys and IMC formation at the interface of the joint.
-
4.
The joint with silver PVD coat, as interlayer, gave the higher shear strength of 31.6 MPa. The strength of the joint bonded with Ag foil as interlayer, dropped to 21.9 MPa. The decrease in bond strength with using Ag foil as interlayer, can be attributed to aggregation and thickening of the brittle intermetallics near the joint interface.
-
5.
The XRD results confirmed the formation of Mg–Ag, Ag–Zn and Ag–Al phases namely: MgAg, AgZn, Ag5Zn8, and Ag2Al instead of Al–Mg intermtallics at the interface. This is an evidence of effective role of Ag, as a diffusion barrier and it’s success in minimizing the formation of harmful intermetallic phases at the interface.
References
S. Sam, S. Kundu, S. Chatterjee, Diffusion bonding of titanium alloy to micro-duplex stainless steel using a nickel alloy interlayer: interface microstructure and strength properties. Mater. Des. 40, 237–244 (2012)
M.A. Mofid, A. Abdollah-zadeh, C. Hakan Gür, Investigating the formation of intermetallic compounds during friction stir welding of magnesium alloy to aluminum alloy in air and under liquid nitrogen. Int. J. Adv. Manuf. Technol. 71(5), 1493–1499 (2014)
M. Jafarian, M. Saboktakin Rizi, M. Jafarian, Effect of thermal tempering on microstructure and mechanical properties of Mg-AZ31/Al-6061 diffusion bonding. Mater. Sci. Eng., A 666, 372–379 (2016)
S. Jing, W. Ke-hong, Z. Qi, Z. De-ku, H. Jun, G. Jia-qi, Effect of joining temperature on microstructure and properties of diffusion bonded Mg/Al joints. Trans. Nonferrous Met. Soc. China 22, 1961–1966 (2012)
M.A. Mofid, A. Abdollah-zadeh, F. Malek Ghaini, The effect of water cooling during dissimilar friction stir welding of Al alloy to Mg alloy. Mater. Des. 36, 161–167 (2012)
M.A. Mofid, A. Abdollah-zadeh, F. MalekGhaini, C. Hakan Gür, Submerged friction stir welding (SFSW) under water and under liquid nitrogen: an improved method to join Al alloys to Mg alloys. Metall. Mater. Trans. A 43, 5106–5114 (2012)
M. Samavatian, A. Halvaee, A.A. Amadeh, A. Khodabandeh, An investigation on microstructure evolution and mechanical properties during liquid state diffusion bonding of Al2024 to Ti–6Al–4 V. Mater. Charact. 98, 113–118 (2014)
M. Gao, S.W. Mei, X.Y. Li, X.Y. Zeng, Characterization and formation mechanism of laser-welded Mg and Al alloys using Ti interlayer. Scr. Mater. 67, 193–196 (2012)
J. Zhang, G.Q. Luo, Y.Y. Wang, Q. Shen, L.M. Zhang, An investigation on diffusion bonding of aluminum and magnesium using a Ni interlayer. Mater. Lett. 83, 189–191 (2012)
S. Habisch, M. Böhme, S. Peter, T. Grund, P. Mayr, The effect of interlayer materials on the joint properties of diffusion-bonded aluminium and magnesium. Metals 8, 138 (2018)
Y. Wang, G. Luo, L. Li, Q. Shen, L. Zhang, Formation of intermetallic compounds in Mg–Ag–Al joints during diffusion bonding. J. Mater. Sci. 49, 7298–7308 (2014)
L.M. Liu, L.M. Zhao, R.Z. Xu, Effect of interlayer composition on the microstructure and strength of diffusion bonded Mg/Al joint. Mater. Des. 30, 4548–4551 (2009)
M.A. Mofid, E. Loryaei, Investigating microstructural evolution at the interface of friction stir weld and diffusion bond of Al and Mg alloys. J. Mater. Res. Technol. 8(5), 3872–3877 (2019)
ASTM standard D1002. Standard test method for apparent shear strength of single-lap-joint adhesively bonded metal specimens by tension loading (metal-to-metal) (1999)
X.-G. Fan, D.-M. Jiang, Q.-C. Meng, B.-Y. Zhang, T. Wang, Trans. Nonferr. Met. Soc. China 16, 577 (2006)
T.B. Massalski, Okamoto Hiroaki, Binary alloy phase diagrams, 2nd edn. (ASM International, Materials Park, 1990)
A. Alhazaa, T.I. Khan, I. Haq, Transient liquid phase (TLP) bonding of Al7075 to Ti–6Al–4V alloy. Mater. Charact. 61(3), 312–317 (2010)
A. Zolriasatein, R.A. Khosroshahi, M. Emamy, N. Nemati, Mechanical and wear properties of Al–Al3Mg2 nanocomposites prepared by mechanical milling and hot pressing. Int. J. Miner. Metall. Mater. b20(3), 290 (2013)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shakeri, H., Mofid, M.A. Physical Vapor Deposition Assisted Diffusion Bonding of Al Alloy to Mg Alloy Using Silver Interlayer. Met. Mater. Int. 27, 4132–4141 (2021). https://doi.org/10.1007/s12540-020-00731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-020-00731-8