Abstract
In this present paper, the electropolishing behavior of Ti–6Al–4V alloy fabricated by additive manufacturing in chloride-containing ethylene glycol electrolyte was surveyed. The impacts of chloride ion on surface quality and oxide film of Ti–6Al–4V were analyzed in dependence on the surface topography, roughness, weight loss ratio and compositions. The visual and microscopic results revealed that the optimally electropolished surface was attained in a 0.4 mol L−1 chloride electrolyte with a decreased surface roughness of 75.04% and a weight loss rate of 4.93%. For lower (C−1Cl ≤ 0.3 mol L−1) or higher concentrations (C−1Cl ≥ 0.5 mol L−1), a smooth and flat surface was not observed due to insufficient reactions or excessive anodic dissolution. During the electropolishing, the titanium oxides nucleated and corresponding surface tension increased, resulting in the formation of a stable TiO2 film on the surface of the Ti–6Al–4V alloy, increasing the corrosion resistance of the specimen.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Additive manufacturing technology (AM), commonly known as 3D printing, has become a research and development hotspot for material preparation because of its simplified production steps, high flexibility, high material use efficiency and near-net shape production [1, 2]. It is widely used to make shaped alloy components that are difficult to produce by conventional methods or other complex components of high cost metals [3]. Titanium and titanium alloys, especially Ti–6Al–4V, have become an important material for the development of additive manufacturing technology in the field of aerospace and human implant applications for their excellent mechanical properties, chemical properties and processing properties [4]. However, the unique production conditions of the additive manufacturing process can also trigger quiet a few of the problems while ensuring efficient production. Because of an unsuitable energy density of the manufacturing process, components of titanium alloys fabricated by AM tend to produce pores and unmelted powder defects, leading to a drop in surface quality [5]. In order to further research and develop this metal material to allow it is widely used in various fields, one of the most important technical means is how to effectively realize the surface modification of the Ti–6Al–4V alloy to obtain a smooth and flat surface. Generally, during post-processing, mechanical polishing, chemical polishing and electrochemical polishing are used to improve the surface quality of titanium alloys to meet their applications [6].
The mechanical polishing operation is simple but it is difficult to achieve uniform leveling of different planes in complex workpieces, which greatly limits the scope of use [7]. The chemical polishing has high production efficiency and it is not limited by the shape of workpieces, but the chemical polishing liquid is mostly composed of a highly corrosive acid solution, which reacts violently in operation and has a certain safety hazards [8]. In contrast, the electropolishing can greatly reduce the stress in the inside and on the surface of the workpieces. It is suitable for metals and alloys with any hardness and is not limited by the shape of the parts. Therefore, in recent years, the electrochemical polishing technology has gradually becoming one of the promising metal surface processing method [9].
For the polishing technology of titanium alloys, a number of researches have been conducted. A previous work compared the effects of different polishing methods on the surface finish of Ti–6Al–4V alloy produced by additive manufacturing technique of direct metal laser sintering from the perspectives of surface roughness and mass measurements [10]. It was observed that the blasting resulted in a surface with uniform roughness, the chemical etching cleaned the surface and reduced its roughness, and the electropolishing exhibited a mirror surface finish. In order to apply additive manufacturing for highly stressed and cyclically loaded Ti–6Al–4V, the as-built surface roughness usually needs to be reduced. Bagehorn and Wehr et al. [11]. developed an enhanced electrolytic polishing process which can decrease the surface roughness of laser beam melted Ti–6Al–4V complex shaped parts by approximately 84% over a 60 min processing time and improve the fatigue performance. Pyka and Burakowski et al. [12] proposed a novel surface modification protocol and applied it to AM titanium alloy-based open porous structures. It was found out that the combination of chemical etching and electrochemical polishing using HF-based solutions can significantly reduce the roughness. Ramver et al. [13] studied the surface integrity of µ-EDMed Ti–6Al–4V Alloy and assessed the quantitative influence of the µ-ECM process on surface roughness. They believed that the µ-ECM process effectively dissolved the thermal damages generated during µ-EDM process and obtained a 72% reduction in average surface roughness of µ-EDMed surface. In terms of specific influencing factors, the quantitative influence of the different process factors of electrochemical polishing had been in studied, indicating that the direct current was the most dominant factor in modifying the surface texture [14]. On this basis, Urlea and Brailovski [15] investigated the electropolishing of powder bed selectively laser-melted Ti–6Al–4V and figured out that by optimizing the current density the effective electropolishing for the Ti–6Al–4V components containing multiple surfaces with respect to the building platform under angles varying from 0° to 135° can be achieved. Similarly, Kuhn [16] compared the electropolishing effects of titanium and its alloys in two solutions consisting of sulfuric acid, hydrofluoric acid, acetic acid and sulfuric acid, phosphoric acid and tartaric acid, and figured out the optimum parameters for their temperature and current density.
In addition, because the Ti–6Al–4V has a tendency to form a protective oxide film, there are also many other references to the electrochemical behavior of Ti–6Al–4V alloys. Li et al. [17] confirmed that the distinctions in the electrochemical anodic dissolution behavior of for laser solid formed Ti–6Al–4V on different planes depended on the microstructural characteristics. The horizontal-planewas more resistant to corrosion than the vertical-plane in 15 wt% NaCl solution. Babilas et al. [18] developed an acid-alcohol electrolyte to analyze the surface modification results of Ti–15Mo alloy. Experimental results showed that an oxide layer composed of TiO2 and MoO3 formed on the alloy surface after electropolishing, which improved the corrosion resistance of the Ti–15Mo alloy.
Although scholars have conducted a lot of researches on electropolishing and electrochemical properties of titanium alloys, a multitude of data focused on the improvement of polishing technology and the adjustment of experimental parameters, such as electrolyte temperature, current density and polishing time etc.,. In the literature, there are practically few reports concerning on the electrolyte components and their specific effects on polishing results are not clear yet. Based on this, this work is an attempt to study the influence of electrolyte components on electropolishing, especially concerning the effects of chloride ion in alcohol-salt systems on the electropolishing of additively manufactured Ti–6Al–4V. To research this problem, the chloride concentration varied from 0.1 to 0.5 mol L−1 within each set of electrolytes. By investigating the polishing behavior of Ti–6Al–4V in different electrolyte concentrations, the influence of chloride ion on the growth of the oxide film and the realization process of the smooth surface in course of the electropolishing were analyzed. Meanwhile, the corrosion resistance of the Ti–6Al–4V alloy in Ringer’s solution before and after electropolishing was evaluated. This is important to understand the polishing mechanism and oxide film formation in electropolishing process and complete the assessment of the surface modification of additively manufactured titanium alloys.
2 Materials and Methods
2.1 Materials and Pretreatment
The Ti–6Al–4V alloy analyzed in this paper was fabricated by using a spherical Ti–6Al–4V powder with an average particle diameter of 0.02 mm as a raw material under the laser additive manufacturing technology. All specimens were fabricated layer-by-layer along the horizontal plane under the guidance of the hierarchical structure of the CAD models. During the fabrication process, argon was used to provide an inert protective atmosphere. The deposition was performed using a printing power of 150 W, a scanning speed of 400 mm s−1, a layer thickness of 50 μm, and a hatch spacing of 75 µm. The compositions of Ti–6Al–4V alloy in weight are: 0.018% N, 0.036% C, 0.2% O, 0.003% H, 1.78% Fe, 6.4% Al, 4.08%V and balance Ti. Analytical-grade ethylene glycol, magnesium chloride, and a small amount of deionized water (a volume fraction of 0.5%) were mixed to obtain various electrolytes with different chloride contents for electrochemical test and electropolishing. The concentration of chloride, C−Cl, in electrolytes was varied from 0.1 to 0.5 mol L−1.
Prior to electropolishing and electrochemical tests, the Ti–6Al–4V alloys were cut into the dimensions of 12 × 12 × 2 mm, and all the specimens were ultrasonically cleaned in acetone and distilled water for 10 min, followed by drying.
2.2 Electropolishing of Ti–6Al–4V alloy
The electropolishing of Ti–6Al–4V was performed using a laboratory power supply. The Ti–6Al–4V specimen was used as the anode and the cathode was made of a stainless steel plate. After a large amount of experiments the electrolytic polishing condition was determined. The process parameters were as follows: the electropolishing process was carried out at a temperature of 25 ± 0.1 °C. The current density was 0.5 A cm−2, the magnetic stirring speed was 500 rpm, and the polishing time was 5–15 min. After electropolishing, the surface roughness Ra and weight loss w were measured by a roughness measuring instrument (MarSurf PS1-M300) and an electronic analytical balance (METTLER). In order to verify the reproducibility of results, 5 measurements of each tested specimen were conducted.
2.3 Surface morphology and electrochemical measurements
Surface morphology of Ti–6Al–4V before or after electropolishing was examined by a scanning electron microscopy (SEM, Quanta FEG 250) and an atomic force microscopy (AFM, ESCALAB250). Surface compositions of alloys were measured by an ESCALAB 250 X-ray photo-electron spectrometer (XPS) with a monochromatic AlKα (1486.6 eV) radiation source operated in 150 W. The detection region by XPS was 3 × 3 μm. To minimize contamination, all specimens were ultrasonically cleaned and dried under a cold air stream before testing. The chemical speciations of elements on the surface oxide film were analyzed using an XPS peak41 software, and charging shifts of the data were corrected using a C 1s peak from the contaminated carbon of 284.6 eV as a reference.
A PARSTAT 2273 electrochemical workstation and a three-electrode cell were utilized for the anodic polarization and other further electrochemistry tests at a temperature of 25 ± 0.1 °C. Electrochemical measurements were performed in Ringer’s solution, which contains 8.6 g L−1 NaCl, 0.3 g L−1 KCl and 0.28 g L−1 CaCl2. The Ti–6Al–4V specimen with an exposed area of 1 cm2 was used as a working electrode, a saturated calomel electrode and a platinum electrode were used as the reference and counter electrodes, respectively. Before measurement, the open circuit potential was recorded to ensure the stability of the measured system. The anodic polarization curve of Ti–6Al–4V alloy was measured from − 1.0 to 0.8 V (vs. SCE) at a scan rate of 1 mV s−1. The frequency range of the impedance spectrum was recorded from 0.01 to 100,000 Hz, and the signal amplitude of the sine wave was 10 mV. The test results were fitted by ZSimpWin. Each electrochemical experiment was conducted at least three times to ensure reproducibility.
3 Results
3.1 Influence of Chloride Ion Content on Electropolishing
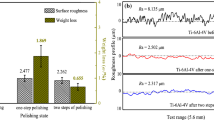
The electropolishing of Ti–6Al–4V alloys using electrolytes with different chloride concentrations were conducted. The corresponding multiple measurement results of the surface roughness Ra and the weight loss w of specimens are summarized in Fig. 1. As shown in Fig. 1, for lower chloride concentrations such as 0.1 mol L−1 and 0.2 mol L−1, the reaction rate was manifestly lower, resulting in a reduced material removal rate. The floating powders and particles on the specimens cannot be completely removed and leveled. Surface roughness Ra of the specimens after electropolishing in these two baths were lower than that of the unpolished alloy, with 5.61 μm and 3.87 μm, respectively. It achieved the purpose of the preliminary polishing, but the roughness and gloss of the specimens were not satisfactory. Increasing the chloride concentration to 0.3 mol L−1 or 0.4 mol L−1, a leveled and brightened surface with lower roughness (Ra = 2.52 μm and 1.13 μm) and weight loss was attained. The surface roughness was significantly reduced comparing to the specimen before electropolishing, reaching 71.79% and 87.36%, and the weight loss rate were only 5.09% and 4.63%, respectively. This implies that a desirable electropolishing effect is achieved after electropolishing in these two electrolytes. However, for higher concentration of 0.5 mol L−1 chloride, due to the continuous metal dissolution process or over-corrosion during the electropolishing [19], a rough and dark surface was observed. Surface roughness of the specimen was 8.24 μm, which was only 7.16% lower compared with the unpolished specimen, and the weight loss rate was as high as 14.77%. This indicates that the electropolishing of Ti–6Al–4V is not feasible in 0.5 mol L−1 chloride electrolyte. Therefore, bringing together the surface roughness and weight loss information, it can be expected that 0.3 mol L−1 and 0.4 mol L−1 chloride electrolytes are better for the electropolishing of Ti–6Al–4V alloy among those investigated solutions and polishing conditions.
3.2 Surface Morphology and Composition
As analyzed above, a pronounced decrease in surface roughness of the electropolished specimen was observed in comparison to the original alloy before electropolishing. Surface morphologies of Ti–6Al–4V alloys before and after electropolishing in different electrolytes are illustrated in Fig. 2.
As shown in Fig. 2a, there are a multitude of pores and unmelted powder defects on the alloy surface after the laser additive manufacturing process, making the surface of the Ti–6Al–4V rough and dull. When the Ti–6Al–4V was electropolished in 0.1 mol L−1 chloride electrolyte, a matt surface with a number of etching pits was observed (Fig. 2b), the specimen did not achieve a pleasurable leveling effect. The appearance of pits was attributed to the milder anode reaction at a lower chloride concentration. Unmelted spherical powder defects were peeled off under the action of a voltage. When the electrolyte concentration was small, a further leveling process was not sufficiently performed. When the Ti–6Al–4V was electropolished in a 0.2 mol L−1 or 0.3 mol L−1 chloride, the surface finishing effect improved significantly, however, a very small amount of shadow stream tracks still appeared (see Fig. 2c, d). It is noticeable that when the specimen was polished in 0.4 mol L−1 chloride, a leveled and brightened surface was achieved (Fig. 2e), the surface gloss and flatness of the specimen were optimal, the surface roughness attained a minimum, which was in agreement with the visual inspection. Specially, in the chloride of 0.5 mol L−1 (Fig. 2f), the specimen turned black brown with the appearance of a large number of deep stream tracks and etch pits on the uneven surface, implying that the anodic reaction paths were adequately activated upon decreasing transport hindrance through increasing electrolyte concentration, the anodic remained in a continuous dissolution state [20]. Under the mass-transfer control, the dissolution of the reaction products was not inhibited, which in turn continued to accelerate the anodic metal dissolution, and it was adverse to the electropolishing [21]. Therefore, basing on the visual inspection of the microscopic morphology, it can be inferred that the electropolishing of Ti–6Al–4V alloy with a selection of 0.4 mol L−1 chloride is most effective and feasible condition in the range of the studied electrolytes, which is consistent with the speculation in Fig. 1.
Figure 3 gives the AFM images of the specimen surface on the original alloy before electropolishing and a well-electropolished specimen in 0.4 mol L−1 chloride ion. The investigated surface area was 3 × 3 µm. Calculated from the AFM data, the maximum peak to valley (Rp-v) of the unelectropolished specimen was 737.39 nm, which was nearly one time bigger than the 285.45 nm for the well-electropolished specimen. This indicates that the real surface area of the original specimen was much greater than its geometrical area compared to the electropolished alloy. After electropolishing the specimen surface was more even and smooth. The surface roughness decreased from 8.33 to 1.09 μm and the surface gloss was distinctly improved.
In the high-energy resolved XPS Ti 2p-spectra, many elements such as Ti, Al, V, Fe, C, N, etc.,. were detected on the surface of the Ti–6Al–4V (Fig. 4a). Since Al on the surface of the Ti–6Al–4V before and after polishing exists in the form of Al2O3, and the content of aluminum in the alloy is relatively small, with 6%. The content of vanadium is much lower, which is difficult to fit the V 2p region with more detail. Therefore, the focus on the XPS spectral composition on the surface of the alloy is mainly the Ti2p spectrum. A large amount of signals of titanium were both detected for the specimens before and after electropolishing. As can be seen from Fig. 4b, in the natural state (before polishing), the XPS Ti 2p spectra consisted of three sets of titanium oxides of Ti 2p1/2, Ti 2p3/2 doublets with binding energies of 456.02, 457.58 and 458.69 eV for Ti2+ (2p3/2), Ti3+ (2p3/2) and Ti4+ (2p3/2). The detected XPS Ti 2p binding energies for the specimen were close to those of TiO, Ti2O3 and TiO2, respectively [22]. Particularly, unoxidized metal titanium (453.66e V, Ti2p3/2) was detected on the surface of the titanium alloy before polishing. However, the XPS Ti 2p spectra of the electropolished specimen showed only one set of doublets with a binding energies of 458.64 eV (Ti4+ 2p3/2), which was close to (Ti4+)–O compounds, suggesting the presence of TiO2. Moreover, there are no signs of (Ti3+)–O bonding or Ti2+ point defects [23]. Since the unoxidized titanium metal has lower binding energies between 453.2 and 454.0 eV [24], which are completely different from the measured binding energies, therefore, it can be inferred that the presence of the unoxidized titanium metal on the analyzed surface can be excluded in the case of the electropolished specimen. The main component in the oxide film formed on the surface of Ti–6Al–4V after electropolishing is TiO2, which has an octahedral structure and exhibits stable properties. The O 1s spectral region on the alloy surface was resolved into three peaks: metal oxides (529.99 eV) assigned to lattice oxygen in the TiOx species, hydroxyl (–OH, peak at 531.01 eV), and adsorbed water (Oads, peak at 532.03 eV).
3.3 Surface Electrochemical Activity
The electrochemical performance of Ti–6Al–4V alloy was investigated in Ringer’s solution, the test results are given in Figs. 5 and 6. As can be seen from the polarization curves for Ti–6Al–4V alloys in Fig. 5, the original alloy before polishing had a higher corrosion potential of − 0.376V. After electropolishing, the self-corrosion potential of the Ti–6Al–4V shifted to − 0.569V. Although the negative shift of the corrosion potential indicates lower thermodynamic stability, the corrosion potential of the specimen does not reflect the corrosion performance [25]. From the polarization curve of Ti–6Al–4V alloys, the corrosion current density of the Ti–6Al–4V after electropolishing decreased to 2.868 × 10−7 A cm−2 comparing to the unpolished Ti–6Al–4V (3.097 × 10−6 A cm−2). In the activated state, the corrosion rate was proportional to the anode current density. The self-corrosion current density of the specimen decreased, the corrosion rate of the anode decreased. In addition, after electropolishing, the specimen showed a broad passivation region in the voltage range of − 0.225 to 0.701 V, indicating that it is easier to form a passivation film with stable protective properties on the surface of the alloy. The anodic dissolution of the metal electrode was suppressed, the corrosion resistance of the alloy increased.
Ti–Al–4V alloys before and after electropolishing were characterized by AC impedance data to estimate the corrosion resistance of specimens, the corresponding Nyquist and Bode plots are shown in Fig. 6a, b. The Nyquist plots for the Ti–6Al–4V electrode in Ringer’s solution consist of two time constants, which correspond to the two depressed semicircles. To fitting EIS data, an equivalent circuit model of two sub-electrochemical branches is used, shown in Fig. 6c. The corresponding fitted parameters are given in Table 1. For the equivalent circuit, Rs stands for the solution resistance, the symbol C1 indicates the capacitance of the electric double layer. R1 represents the resistance of the film layer. C2 is the capacitance of the oxide barrier layer. R2 is the charge transfer resistance. A constant-phase element (CPE) representing a shift from the ideal capacitor behavior was employed to replace the pure capacitance, because of the surface non-uniformity as a result of surface roughness, impurities, dislocations, and formation of a porous layer [26]. The impedance ZCPE of the CPE is defined as:
where Q0 is the constant of CPE expressed in F cm2 s(n−1), j is the imaginary number, ω represents the angular frequency, and n is defined as the diffusion coefficient and − 1 ≤ n ≤ 1. Here n = 1 represents an ideal capacitance. n = 0.5 means the Warburg impedance. n = 0 describes an ideal resistance. n = − 1 represents a pure inductance. When 0.5 < n < 1, CPE indicates the frequency dispersion of the time constant due to local inhomogeneity in the dielectric material. The standard deviation between the measured and fitted values is expressed by a Chi square value (χ2), and the χ2 below 10−3 indicates a reliable description result. It can be seen from Table 1 that the surface film properties of the electropolished Ti–6Al–4V have a direct influence on its electrochemical performance. The changes in passivation film resistance on the Ti–6Al–4V can be attributed to the changes in the ionic or electrical conductivity of the film caused by the changes in the microstructure or chemical compositions of the barrier film. Compared with the unpolished specimen, it was observed that the electropolishing treatment not only decreased the surface roughness but also significantly increased the corrosion resistance of the specimen.
The Fig. 6a shows that the semicircular region in the Ti–6Al–4V after electropolishing process increased compared to that of the unpolished specimen, indicating the increase in corrosion resistance. For the Bodes plots of Ti–6Al–4V before and after electropolishing, the impedance and phase angle varied obviously. As observed previously on the plasma electrolytic treated Ti–6Al–4V [27], the influence of the oxide film causes an abrupt rise for the impedance in the low-frequency region, which is believed to be the typical thin passivating solid film effect of a capacitor. After electropolishing treatment, a similar phenomenon was observed in the Bode plot in Fig. 6b. High |Z| values in the high-frequency region reflect high corrosion resistance of materials. The Fig. 6b shows that the electropolishing process for the additively manufactured Ti–6Al–4V yielded a stable passive film on the surface based on the capacitance. It is speculated that the inferior corrosion resistance of the unpolished specimen is associated with the incompact and unstable nature of the oxides in the passive film [28]. In the high frequency region, the Bode modulus curve shows a constant log |Z| value with a phase angle close to 0°. The appearance of the high frequency platform of modulus |Z| is due to the response of the electrode ohmic resistance [29]. After electropolishing, the maximum phase angle of the Ti–6Al–4V increased from 60° to above 80°, where the maximum phase angle was approximately 10° below 90°. Such a deviation has also been reported by other scholars on researches for films of Ti or titanium alloys [30, 31]. In a addition, it was mentioned that the additively manufactured Ti–6Al–4V alloy also exhibited stronger corrosion resistance than the conventionally produced alloys. Due to the rapid cooling rate, the Ti–6Al–4V prepared by additive manufacturing had less time for the formation of grains and the precipitation of inclusions etc.,. Therefore, the grain boundaries, inclusions, and grain sizes of the AM-produced Ti–6Al–4V were smaller as compared to the conventionally manufactured alloy, leading to a more compact, uniform and developed matrix [32, 33]. This may decrease the formation of corrosive micro (or macro) cell and enhance the corrosion resistance of the alloy.
4 Discussion
In order to investigate the effect of chloride ion on the surface quality of Ti–6Al–4V alloy during electropolishing, the growth processes and evolution regularity of the oxide film on the surface of Ti–6Al–4V alloy were analyzed. During the electropolishing process, after the oxide film formed in the natural state was removed, the Ti4+ ions on the working electrode firstly reacted with Cl− ions in the electrolyte solution to form titanium tetrachloride (TiCl4) [34, 35], as described in Eq. (2).
The TiCl4 was a yellow liquid with high viscosity, and it was difficult to be removed from the surface of the working electrode [36]. Therefore, a layer of TiCl4 gradually formed on the working electrode surface during the electrolpolishing process. Since the electron exchange between the Ti4+ and the Cl− was in an unequal state, the Cl− layer gradually thickened. Because of the polarity of water (polarity index = 1.0) was higher than that of the ethylene glycol (polarity index = 0.79), water molecules run faster toward the working electrode under the action of an electric field [37]. Therefore, the TiCl4 reacted with water molecules to form TiO2 (Eq. 3).
In addition, even if the ethylene glycol can react with TiCl4, the product was still TiO2, following Eqs. (4) and (5) or Eqs. (6) and (7).
TiO2 had strong adhesion to metal Ti and it did not react with other compounds presented in the electrolyte solution [38], so the TiO2 adhered to the surface of the Ti–6Al–4V working electrode to form a stable oxide layer.
Due to the high viscosity, TiCl4 was difficult to dissolve in a polishing solution using ethylene glycol as a solvent. Therefore, in actual electropolishing, to ensure the polishing effect, a certain rate of magnetic stirring must be applied to keep the solution fluidity. Meanwhile, the generation of the high viscosity intermediate product (TiCl4) determined that the solubility and viscosity of the electrolyte were the two main factors restricting the surface quality [39]. Because the solubility of chloride ions in ethylene glycol was small, the viscosity of electrolytes with different chloride concentrations was not much different. Therefore, the concentration of chloride ion was the key to restrict the polishing quality of the additively manufactured Ti–6Al–4V alloy. For the higher concentration of chloride ion (0.5 mol L−1), the TiCl4 layer formed by the preliminary reaction was thick. The thicker TiCl4 layer was not easy to be completely removed from the surface of the titanium alloy during electropolishing, hence it was prone to generate defects such as pits and porous structure, leading to the increase of the surface roughness. Moreover, considering the effect of the pore-related defects, the corrosion resistance of Ti–6Al–4V sample was also substantially lowered. For the lower concentration of chloride ion (0.1 and 0.2 mol L−1), the corresponding current density was small, the reaction proceeded insufficiently, and the generated oxide film was thin and unevenly distributed, resulting in an inapparent surface quality improvement. Therefore, in order to obtain a high-quality surface of Ti–6Al–4V alloy, it is necessary to reasonably adjust the amount of chloride ions to control the main reaction that occurs during the electropolishing to ensure TiCl4 is completely decomposed and only a stable TiO2 oxide layer adhered to the surface of the alloy. The formation process of TiO2 layer on the surface of Ti–6Al–4V alloy is shown in Fig. 7. The formation process of the oxide film in the electropolishing process was divided into three stages: the removal of natural oxide film, the generation of oxide crystal nucleus, and the formation of a TiO2 oxide film [40]. According to the kinetic and surface physicochemical theory, the crystallization nucleation of TiO2 can be summarized as follows: The titanium atoms form Ti4+ aggregation during electrolysis, then the aggregation grows into TiO2 nucleus, and finally the nucleus form an oxide film under the surface tension. The change of Gibbs free energy ΔG of the system in this process is mainly caused by two parts: The change in free energy ΔG1 caused by the oxidation of titanium atoms to form TiO2, and surface energy change ΔG2 triggered by the surface tension of crystal nuclei. As previously analyzed, the chloride ion concentration of 0.4 mol L−1 was the optimal electrolyte condition. The i-t curve obtained during electropolishing of Ti–6Al–4V under this condition is shown in the Fig. 8. The current recorded interval was 30 s.
The i-t curve in the figure corresponds well the above-described formation mechanism of TiO2 oxide film. The current density increases firstly and then decreases with the prolonging of electropolishing time. In the early stage, the current curve was unstable and exhibited a band-like change, which became stable after 750 s of polishing. It indicates that the pitting reaction mainly occurs in the early stage of the electropolishing process, and the current curve of the specimen continuously increases with time increasing [41]. At 630 s, the current density showed a significant drop interval. The oxide film covered on the surface of the titanium alloy was cracked from the metal surface, and the removal of the bulk metal decreased the current density. Afterwards, a new adhesion film layer gradually formed on the metal surface under the action of electrolysis. After the component of the oxide film was stabilized, the corresponding anode current density tended to be stable, the oxide film was continuously leveled and brightened, and the surface quality of the alloy specimen was improved.
5 Conclusions
Surface modification of the Ti–6Al–4V alloy in an alcohol-salt electrolyte was the removal of an original surface-covered oxide film and the formation of a stable TiO2 layer. The chloride ion in the electrolyte controlled the surface polishing effect by regulating the main reactions in the electropolishing. For lower concentration of chloride ion (≤ 0.3 mol L−1), the corresponding anode current density was small, and the reaction was not fully carried out. For higher concentrations of chloride ion (≥0.5 mol L−1), the formed TiCl4 layer was difficult to be removed completely. The concentration of 0.4 mol L−1 chloride ion was the selection of the optimal condition. After electropolishing in this condition, the surface roughness of Ti–6Al–4V was reduced by approximately 75%, and the weight loss rate was only about 5%. During electropolishing, the titanium oxide nucleated and formed a TiO2 layer on the alloy surface, which was more stable in chemical properties than the multi-valent mixture before treatment and had better protection to the substrate.
References
A. Mumith, M. Thomas, Z. Shah, M. Coathup, G. Blunn, Bone Joint J. 100B, 455 (2018)
S.H. Huang, P. Liu, A. Mokasdar, H. Liang, Int. J. Adv. Manuf. Technol. 67, 1191 (2013)
A. Townsend, N. Senin, L. Blunt, R.K. Leach, J.S. Taylor, Precis. Eng. 46, 34 (2016)
A.R. Nassar, E.W. Reutzel, Metall. Mater. Trans. A 46, 2781 (2015)
C. Achillas, D. Aidonis, E. Iakovou, M. Thymianidis, D. Tzetzis, J. Manuf. Syst. 37, 328 (2015)
L.E. Murr, S.M. Gaytan, F. Medina, E. Martinez, J.L. Martinez, Mater. Sci. Eng. A 527, 1861 (2010)
M. Pattabi, K. Ramakrishna, Mater. Sci. Eng. A 486, 14 (2008)
Z.Y. Zhang, Z.F. Shi, Y.F. Du, Appl. Surf. Sci. 427, 409 (2018)
D. Wang, Y. Yang, Z. Yi, X. Su, Int. J. Adv. Manuf. Technol. 65, 1471 (2013)
G.A. Longhitano, M.A. Larosa, A.L.J. Munhoz, C.A.C. Zavaglia, M.C.F. Ierardia, Mater. Res. 18, 838 (2015)
S. Bagehorn, J. Wehr, S. Nixon, A. Balastrier, T. Mertens, H.J. Maier, in Solid Freeform Fabrication (2017), pp. 2516–2529
P. Grzegorz, A. Burakowski, G. Kerckhofs, M. Moesen, S.V. Bael, J. Schrooten, M. Wevers, Adv. Eng. Mater. 14, 363 (2012)
Ramver, A. Dvivedi, P. Kumar, in TMS 2019 148th Annual Meeting and Exhibition Supplemental Proceedings: The Minerals, Metals and Materials Series (Springer, Cham, 2019), pp. 745–753
H. Ramasawmy, L. Blunt, Int. J Mach. Tool Manuf. 42, 1129 (2002)
V. Urlea, V. Brailovski, J. Mater. Process. Technol. 242, 1 (2017)
A. Kuhn, Met. Finish. 102, 80 (2004)
J.Q. Li, X. Lin, M. Zheng, J. Wang, P.F. Guo, T. Qin, M.H. Zhu, W.D. Huang, H.O. Yang, Electrochim. Acta 283, 1482 (2018)
D. Babilasa, E. Urbanczyka, M. Sowa, Electrochim. Acta 205, 256 (2016)
Y.F. Zhang, J.Z. Li, S.H. Che, Int. J. Electrochem. Sci. 13, 4792 (2018)
K. Fushimi, M. Stratmann, A.W. Hassel, Electrochim. Acta 52, 1290 (2006)
Y.L. Cheng, J.H. Cao, M.K. Mao, H.J. Xie, P. Skeldon, Surf. Coat. Technol. 291, 239 (2016)
M.H. Hong, D.H. Lee, K.M. Kim, Y.K. Lee, Thin Solid Films 519, 7065 (2011)
K.S. Lee, I.S. Park, Scr. Mater. 48, 659 (2003)
E.A. Ferreira, N.T.C. Oliveira, S.R. Biaggio, P.A.P. Nascente, R.C. Rocha, N. Bocchi, Surf. Interface Anal. 38, 417 (2006)
N.W. Dai, L.C. Zhang, J.X. Zhang, Q.M. Chen, M.L. Wu, Corros. Sci. 102, 484 (2018)
G.A. Zhang, Y.F. Cheng, Electrochim. Acta 55, 316 (2009)
I.J. Hwang, H.C. Choe, W.A. Brantley, Surf. Coat. Technol. 320, 458 (2017)
Y. Wang, K.Y. Li, F. Scenini, J. Jiao, S.J. Qu, Q. Luo, J. Shen, Surf. Coat. Technol. 302, 27 (2016)
W. Simka, M. Kaczmarek, A.B. Wiechec, G. Nawrat, J. Marciniak, J. Zak, Electrochim. Acta 55, 2437 (2010)
A.K. Shukla, R. Balasubramaniam, S. Bhargava, Intermetallics 13, 631 (2005)
F.T. Cheng, P. Shi, H.C. Man, Surf. Coat. Technol. 187, 26 (2004)
M. Shunmugavel, A. Polishetty, M. Goldberg, R. Singh, G.A. Littlefair, Prototyp. J. 23, 1051 (2017)
N. Hrabe, T. Quinn, Mater. Sci. Eng. A 573, 271 (2013)
E. Godlewska, M. Mitoraj, K. Leszczynska, Corros. Sci. 78, 63 (2014)
S. Kim, S. Park, Y. Jeong, J. Am. Ceram. Soc. 82, 927 (1999)
D. Kim, K. Son, D. Sung, Y. Kim, W. Chung, Corros. Sci. 98, 494 (2015)
I.W. Kim, M.D. Jang, Y.K. Ryu, E.H. Cho, Y.K. Lee, J.H. Park, Anal. Sci. 18, 1357 (2002)
A. Pottier, C. Chanéac, E. Tronc, L. Mazerolles, J. Mater. Chem. 11, 1116 (2001)
M. Pankuch, R. Bell, C.A. Melendrizs, Electrochim. Acta 38, 2777 (1993)
X. Liu, P.K. Chu, C. Ding, Mater. Sci. Eng. R Rep. 47, 49 (2004)
Q.B. Li, W.B. Yang, C.C. Liu, D.A. Wang, J. Liang, Surf. Coat. Technol. 316, 162 (2017)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 51974069); and the Iron and Steel Joint Research Found of National Natural Science Foundation and China Baowu Steel Group Corporation Limited (Grant No. U1760118).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, J., Che, S. et al. Electrochemical Polishing of Additively Manufactured Ti–6Al–4V Alloy. Met. Mater. Int. 26, 783–792 (2020). https://doi.org/10.1007/s12540-019-00556-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-019-00556-0