Abstract
The study of grain coarsening and its kinetics during intercritical annealing is of vital importance for the production of dual phase (DP) steels with appropriate microstructure and properties required for industrial applications. In the present work, the kinetics of grain growth in the two-phase austenite plus ferrite region was studied based on the parabolic grain growth law. It was revealed that the thermally-activated grain growth of ferrite depends on the soaking temperature and the presence of austenite islands, where these two factors compete with each other. As a result, by increasing the temperature, initially the rate of growth increases and the activation energy of grain growth (Q) was determined as 615 kJ/mol, which indicates the high-temperature dependency. However, after the formation of a certain amount of austenite and the formation of chain-network morphology of austenite, the increase of temperature results in a decreased growth rate with the Q value of − 258 kJ/mol. This suggests that the pinning effect counteracts the temperature effect in this stage. The effect of grain size on mechanical properties and work-hardening behavior was also discussed. Higher work-hardening rate was observed for the fine-grained DP microstructures, which was found to be responsible for the better strength–ductility trade off.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Known as mild steel, the low-carbon steels with 0.05 to 0.25 wt% C and up to 0.4 wt% Mn are a group of low-cost materials that can be considered as high-formability flat-rolled steels [1]. The intercritical annealing (IA) of these steels has attracted a recent attention of the researchers [1,2,3]. At the intercritical annealing temperature, a duplex ferrite–austenite microstructure is obtained with the subsequent formation of ferrite–martensite DP microstructure after quenching, which results in a desirable strength-ductility trade off compared with the more common steels for the automotive applications [4,5,6,7,8,9].
The IA conditions significantly affect the microstructure of DP steel as denoted in many studies [10,11,12,13]. It has been revealed that by increasing the IA temperature, the amount of martensite increases with the resulting increased strength and decreased ductility [10,11,12]. Regarding the holding time at the IA temperature, the adjustment of martensite fraction [14], the effect of the presence of alloying elements [15], and the partitioning of manganese [13] have been studied so far. However, one of the important aspects of microstructural development during IA, i.e. the grain coarsening after formation of the ferrite–austenite microstructure, has received less attention [16,17,18].
Intercritical annealing of a 0.16C–0.78Mn–0.27Si steel was studied by Calcagnotto et al. [16], where the presence of austenite and cementite retarded the grain growth. They stated that the longer holding time can result in grain coarsening. In a preliminary study by Nouroozi et al. [3], it was shown that the IA time should be controlled to obtain a fine DP structure with chain-network martensite morphology to govern high work-hardening rate. The kinetics of grain coarsening for DP steels has been studied by Takayama et al. [17] and Park et al. [18] on model low-carbon steels with high-silicon content (3 wt%) to obtain a wide range of intercritical annealing temperature. They showed that the grain boundaries of ferrite migrate under a restriction of pinning by the austenite islands that obeys the parabolic law of \(r^{n} - r_{0}^{n} = Kt\), where r, r0, t, n, and K are the grain radius, initial grain radius, time, grain size exponent, and a constant, respectively. For the single-phase region, n was determined as 2; whereas for the two-phase region, n was considered as 3 or 4 [17, 18].
Despite these excellent works, to the best of authors’ knowledge, there is no reported work on the grain growth kinetics of DP steels with the usual chemistry. It is well-known that the ferrite grain size is an important factor to determine the mechanical properties and work hardening behavior of DP steel [19,20,21,22,23,24,25]. In this regard, it has been revealed that by grain refinement, both strength and ductility of DP steel enhance in contrast to the case of conventional steels [26,27,28]. Since the DP microstructure is obtained by intercritical annealing, the study of grain coarsening and its kinetics is of vital importance for the application of these steels in the industry. The present work aims to deal with this subject using a high-formability low-carbon steel.
2 Experimental Details
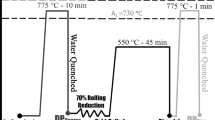
The 0.035C–0.27Mn–0.035Si (wt%) steel sheet was received in the normalized ferritic–pearlitic condition with average ferrite grain size of 17 µm (Fig. 1a). The as-received sheet was austenitized and quenched in water to obtain an essentially martensitic microstructure, which was followed by 50% cold rolling at room temperature. The cold-rolled sheet was considered before intercritical annealing to obtain fine grained DP steels suitable for grain growth experiments. The A1 and A3 temperatures were respectively determined as 736.7 and 890.2 °C based on the equations developed by Hougardy [29]. These critical temperatures were respectively determined as 735.8 and 890.9 °C by the Trzaska equations [30]. However, during intercritical annealing of the same steel at 875 °C from the normalized condition, Maleki et al. [1] obtained a microstructure with ~ 50 vol% martensite due to the low hardenability of this steel with very low C and Mn content. Therefore, up to 875 °C, a dominantly ferritic matrix will be determined for the studied material.
As-received microstructure (a), Cold-rolled microstructure (b), DP microstructures for holding time of 1 min at intercritical annealing temperatures of 775 °C (c), 800 °C (d), 825 °C (e), 850 °C (f), and 875 °C (g), and the dependence of the average grain size on intercritical annealing temperature (h)
Accordingly, the intercritical annealing at temperatures of 775–875 °C for holding durations of 1–240 min followed by water quenching was performed to obtain DP steels with different grain sizes. The LePera and Nital solutions were used for chemical etching for microstructural studies based on an optical microscope and a FEI Nova NanoSEM 450 FE-SEM. The average grain size was obtained based on the concept of standard lineal intercept length. The volume fraction of the martensite was determined via image analysis by consideration of several metallographic and SEM images at different magnifications. The tensile specimens were prepared according to the JIS Z 2201 standard (with the gauge length, the gauge width, and the thickness of 8.76, 4, and 0.65 mm, respectively) for room temperature tests under strain rate of 0.001 s−1. The work-hardening rate was obtained based on the expression of \(\theta = d\sigma /d\varepsilon \left| {_{i} } \right. = \{ \sigma_{i + 1}^{{}} - \sigma_{i - 1}^{{}} \} /\{ \varepsilon_{i + 1} - \varepsilon_{i - 1} \}\) [31,32,33].
3 Results and Discussion
3.1 Grain Growth Behavior
Figure 1 shows the cold-rolled and obtained DP microstructures after holding time of 1 min at different intercritical annealing temperatures followed by water quenching. The cold-rolled sheet shows a deformed lath martensitic microstructure parallel to the rolling direction (RD). In contrast, the DP microstructures are composed of recrystallized ferrite grains and martensite islands at grain boundaries. Image analysis revealed that by increasing the annealing temperature, the volume fraction of martensite increases. Moreover, the ferrite grain size follows the trend of the graph shown in Fig. 1h. It can be seen that the grain size increases by increasing annealing temperature. The kinetics of grain growth become faster by increasing the annealing temperature, which is followed by slower kinetics after a certain temperature. The same trend was observed for other holding durations as discussed below.
The variation of grain size with the holding time for each annealing temperature is shown in Fig. 2. It can be seen that at each temperature, by increasing the holding time, the grain size increases. However, two distinct trends can be identified by increasing the annealing temperature: (1) faster kinetics up to 825 °C and (2) slower kinetics beyond 825 °C. Since grain growth is a thermally-activated process, its kinetics depends strongly on the temperature. So, increasing the annealing temperature results in a faster grain growth rate [34,35,36]. Therefore, the obtained trend up to 825 °C is reasonable. However, by increasing the annealing temperature, the amount of austenite as the second phase increases. Along with the Zener pinning effect of fine particles in pseudo-single phase alloys, the presence of second phase particles in duplex alloys is also known as an effective means for inhibition of grain growth [37, 38]. Therefore, the grain boundary pinning becomes more effective with the resulting decreased grain coarsening rate. These two factors compete with each other, and hence, after formation of a certain amount of martensite, it is possible that the pinning effect counteracts the temperature effect. This is among the first principal reports to show this fact in the case of DP steels (Figs. 1, 2). Based on Table 1, the critical amount of martensite (for the temperature of 825 °C) was obtained as ~ 28 vol%.
Based on Fig. 2, grain growth at 825 °C shows the fastest kinetics, which facilitates the study of grain growth. Figure 3 shows the obtained microstructures after holding at 825 °C for various durations. It can be seen that the microstructures are comprised of equiaxed ferrite grains and martensite islands at grain boundaries. By increasing holding time, the ferrite grain size increases in a normal grain growth manner up to 60 min. Afterward, by continued annealing, very large abnormal grains and much smaller normally grown grains can be seen in the microstructure (e.g. for 240 min). Even by consideration of smaller population of grains in the abnormally grown microstructure, it can be deduced that the kinetics of grain growth is fast and this factor should be taken into account for processing of DP steel.
3.2 Grain Growth Kinetics
The parabolic grain growth kinetics based on Eq. 1 [34, 39] was considered in the present work:
where K is a temperature-dependent constant expressed by Eq. 2 and D, D0, t, and n are respectively the grain radius, initial grain radius, time, and grain size exponent.
where T is the temperature, K0 is a constant, Q is the grain growth activation energy, and R is the gas constant. Since the cold rolled microstructure was considered for intercritical annealing, the value of D0 cannot be determined unambiguously. Therefore, based on the data shown on Fig. 2, Eq. 1 was fitted to the D–t data and the values of n, K, and D0 were calculated from the nonlinear regression analysis. The results are shown in Table 2.
Moreover, it is necessary to obtain a two-phase ferrite–austenite microstructure to study the effect of holding time on grain growth. It was revealed in Fig. 1 that the equilibrium microstructure can be obtained at 1 min annealing. The values of D1min were plotted against D0fitted as shown in Fig. 4. It can be seen that the data fairly follow an equation of D0fitted = D1min. Therefore, the values of D1min were considered as the initial grain size (D0) and the holding time was shifted 1 min backward. Therefore, Fig. 5 was obtained. By fitting Eq. 1 to the D–t data, the values of nfitted and Kfitted were calculated and summarized in Table 3.
While the nonlinear regression method is more accurate, the values of n and K can be also determined by neglecting D0 in Eq. 1, which is the conventional method for investigation of the kinetics of grain growth [34]. Therefore, it is possible to calculate the values of n and lnK for each temperature from the slope and the intercept of the plot of lnt versus lnD based on Eq. 3. The corresponding graphs are shown in Fig. 6 and the resulting values of nlog and Klog are also summarized in Table 3.
The obtained values of n in the present work are superimposed on the grain growth exponent diagram developed by Hu and Rath [40] (Fig. 7). It can be seen that the values of n for growth of ferrite in DP steel are consistent with the expectations from α-iron.
Table 3 reveals that, based on both methods, the obtained values of n and K generally increase by increasing the annealing temperature up to 825 °C and then decrease from temperature above 825 °C. Therefore, it is not possible to interpret the grain growth kinetics based on these values. As a result, the average value of nfitted (Table 3) was considered to obtain the values of Kfitted as shown in Fig. 8 and the results are summarized in Table 4.
Now, based on Table 4, it can be seen that the K values are on the same order of magnitude. Moreover, K increases up to 825 °C and then decreases. Since the value of n is the same for all of the considered temperatures, it suggests that the kinetics of grain growth become faster by increasing the temperature up to 825 °C and then decrease (Eq. 1). Based on Eq. 2, the activation energy of grain growth can be determined from the slope of lnK versus 1/T (Eq. 4). The results are shown in Fig. 9, where two slopes can be identified.
Based on these slopes, the Q values of 615 kJ/mol and − 258 kJ/mol were determined for low temperatures (T ≤ 825 °C) and high temperatures (T ≥ 825 °C), respectively. These observations need more explanations:
-
1.
Low temperature regime (T ≤ 825 °C): In this regime, the effect of increasing temperature on grain growth is more pronounced compared with the pinning effect of austenite islands. Therefore, the grain growth process is highly temperature-dependent in this regime, which is reflected in the obtained high value for activation energy (615 kJ/mol).
-
2.
High temperature regime (T ≥ 825 °C): In this case, the pinning effect becomes dominant, which is related to the formation of a chain-network austenite morphology (the origin of chain-network martensite morphology [3, 19]) at the grain boundaries of ferrite (See the micrograph of the temperature of 825 °C in Fig. 1). In fact, austenite islands have surrounded the ferrite grains in a chain-network manner, where the growth of ferrite grains in every direction can be severely inhibited by the presence of this second phase at grain boundaries. As a result, the temperature-dependency of the grain growth process decreases significantly (the magnitude of Q falls to 258 kJ/mol). The negative value for Q in this regime implies the slower kinetics of grain growth by increasing temperature, which can be easily explained by increasing the volume fraction of austenite and the consequent decreased growth rate of ferrite.
It is noteworthy that the magnitude of the obtained activation energy for T ≥ 825 °C (258 kJ/mol) is near the activation energy for lattice diffusion in α-iron as the main atomic process, which is reported to be 251 kJ/mol [41]. The activation energy for the T ≤ 825 °C regime (615 kJ/mol) cannot be correlated with any atomic mechanism. In this regard, the lattice diffusion activation energy for α-iron and γ-iron is 251 kJ/mol and 270 kJ/mol [41], respectively. During intercritical annealing, the diffusion of C and Mn in austenite and ferrite might be also important [13, 16, 42]. The activation energy for diffusion of C in α-iron and γ-iron is 103 kJ/mol [43] and 125.1 kJ/mol [44], respectively. Moreover, the activation energy for diffusion of Mn in α-iron and γ-iron is in the range of 219.8 and 261.7 kJ/mol [45]. In fact, the so-called “apparent” activation energies cannot be always related to the atom-scale thermally activated process which controls boundary migration (such as solute drag effect, etc.), and hence, great care should be taken in the interpretation of experimentally measured activation energies [37]. Anyway, these results are consistent with the expectation of lower apparent activation energies at higher temperatures [37].
3.3 Mechanical Properties
The tensile stress–strain curves of some samples are shown in Fig. 10a. It can be seen that the yield stress of the 775 °C—1 min and 825 °C—1 min samples are nearly the same but much higher than those of 825 °C—240 min. This can be related to the effect of grain size and presence of martensite. In the case of 775 °C—1 min sample, fine ferrite grains are responsible for the high yield stress. The grain size of 825 °C—1 min sample is coarser but the volume fraction of martensite is higher. The large grain size of 825 °C—240 min sample is responsible for the observed low yield stress.
Similarly, the tensile strength of 775 °C—1 min and 825 °C—1 min samples are nearly the same but much higher than those of 825 °C—240 min. However, the total elongation of 825 °C—240 min is marginally higher than those of other samples when the huge decrease of strength is taken into account (Fig. 10b). These observations should be discussed based on the work-hardening behavior as shown in Fig. 10c. It can be seen that the 775 °C—1 min and 825 °C—1 min samples show similar work-hardening curves but that of 825 °C—240 min sample shows much lower work-hardening rate at each strain. This is responsible for the observed strength-ductility trends.
4 Conclusions
The study of grain coarsening and its kinetics during intercritical annealing is of vital importance for the production of dual phase (DP) steels with appropriate microstructure and properties for industrial applications. In the present work, the kinetics of grain growth in the two-phase austenite plus ferrite region was studied using a high-formability dual phase steel. The following conclusions can be drawn from this study:
-
1.
The grain growth of ferrite was found to depend on the intercritical annealing temperature and the presence of austenite islands, where these two factors compete with each other. By increasing the annealing temperature, initially the rate of growth increased, which indicates the high-temperature dependency. However, after formation of a certain amount of austenite and the formation of chain-network morphology, the increase of temperature resulted in a decreased growth rate. This suggests that the pinning effect counteracts the temperature effect in this stage.
-
2.
It was revealed that the obtained values of n and K generally increase by increasing the annealing temperature up to 825 °C and then decrease. By consideration of the average values of n and also based on the relation of \(K = K_{0} \exp ( - Q/RT)\), the activation energy of grain growth was obtained as 615 kJ/mol and − 258 kJ/mol in the low-temperature and high-temperature regimes, respectively. The negative value for Q implies the slower kinetics of grain growth by increasing temperature, which was explained based on increasing the volume fraction of austenite and the consequent decreased growth rate of ferrite.
-
3.
Fine ferrite grains and higher volume fraction of martensite were found to be responsible for the high yield stress of DP steel. Higher work-hardening rate was observed for the fine-grained DP microstructures, which was found to be responsible for the better strength-ductility trade off.
References
M. Maleki, H. Mirzadeh, M. Zamani, Effect of intercritical annealing on mechanical properties and work-hardening response of high formability dual phase steel. Steel Res. Int. 89, 1700412 (2018)
M. Zamani, H. Mirzadeh, M. Maleki, Enhancement of mechanical properties of low carbon dual phase steel via natural aging. Mater. Sci. Eng. A 734, 178–183 (2018)
M. Nouroozi, H. Mirzadeh, M. Zamani, Effect of microstructural refinement and intercritical annealing time on mechanical properties of high-formability dual phase steel. Mater. Sci. Eng. A 736, 22–26 (2018)
M. Alibeyki, H. Mirzadeh, M. Najafi, Fine-grained dual phase steel via intercritical annealing of cold-rolled martensite. Vacuum 155, 147–152 (2018)
C. Li, X. Yuan, K. Wu, H. Wang, Z. Hu, X. Pan, Nugget formation and its mechanism of resistance spot welded joints in DP600 dual-phase and DC54D ultralow carbon steel. Met. Mater. Int. 23, 543–553 (2017)
W. Bleck, S. Papaefthymiou, A. Frehn, Microstructure and tensile properties in dual phase and trip steels. Steel Res. Int. 75, 704–710 (2004)
J. Kim, W. Lee, D. Kim, J. Kong, C. Kim, M.L. Wenner, K. Chung, Effect of hardening laws and yield function types on spring-back simulations of dual-phase steel automotive sheets. Met. Mater. Int. 12, 293–305 (2006)
U. Prahl, S. Papaefthymiou, V. Uthaisangsuk, W. Bleck, J. Sietsma, S. van der Zwaag, Micromechanics-based modelling of properties and failure of multiphase steels. Comput. Mater. Sci. 39, 17–22 (2007)
S. Papaefthymiou, U. Prahl, W. Bleck, S. van der Zwaag, J. Sietsma, Experimental observations on thecorrelation between microstructure and fracture of multiphase steels. Int. J. Mater. Res. 97, 1723–1731 (2006)
R.G. Davies, Influence of martensite composition and content on the properties of dual phase steels. Metall. Trans. A 9, 671–679 (1978)
P. Movahed, S. Kolahgar, S.P.H. Marashi, M. Pouranvari, N. Parvin, The effect of intercritical heat treatment temperature on the tensile properties and work hardening behavior of ferrite–martensite dual phase steel sheets. Mater. Sci. Eng. A 518, 1–6 (2009)
M. Alibeyki, H. Mirzadeh, M. Najafi, A. Kalhor, Modification of rule of mixtures for estimation of the mechanical properties of dual-phase steels. J. Mater. Eng. Perform. 26, 2683–2688 (2017)
M. Balbi, I. Alvarez-Armas, A. Armas, Effect of holding time at an intercritical temperature on the microstructure and tensile properties of a ferrite–martensite dual phase steel. Mater. Sci. Eng. A 733, 1–8 (2018)
R. Tyagi, S.K. Nath, S. Ray, Mater. Sci. Technol. 20, 645–652 (2004)
Y. Mazaheri, N. Saeidi, A. Kermanpur, A. Najafizadeh, Correlation of mechanical properties with fracture surface features in a newly developed dual-phase steel. J. Mater. Eng. Perform. 24, 1573–1580 (2015)
M. Calcagnotto, D. Ponge, D. Raabe, On the effect of manganese on grain size stability and hardenability in ultrafine-grained ferrite/martensite dual-phase steels. Metall. Mater. Trans. A 43, 37–46 (2012)
T. Takayama, M. Wey, T. Nishizawa, Grain growth in dual-phase steel. Tetsu-to-Hagané 68, 1016–1023 (1982)
J.-H. Park, Y. Tomota, M.-Y. Wey, Suppression of grain growth in dual phase steels. Mater. Sci. Technol. 18, 1517–1523 (2002)
N. Nakada, Y. Arakawa, K.S. Park, T. Tsuchiyama, S. Takaki, Dual phase structure formed by partial reversion of cold-deformed martensite. Mater. Sci. Eng. A 553, 128–133 (2012)
K. Mukherjee, S.S. Hazra, M. Militzer, Grain refinement in dual-phase steels. Metall. Mater. Trans. A 40, 2145–2159 (2009)
M. Calcagnotto, D. Ponge, D. Raabe, Mater. Sci. Eng. A 527, 7832–7840 (2010)
A. Karmakar, M. Mandal, A. Mandal, Md Basiruddin Sk, S. Mukherjee, D. Chakrabarti, Effect of starting microstructure on the grain refinement in cold-rolled low-carbon steel during annealing at two different heating rates. Metall. Mater Trans. A 47, 268–281 (2016)
M. Papa Rao, V. Subramanya Sarma, S. Sankaran, Processing of bimodal grain-sized ultrafine-grained dual phase microalloyed V-Nb steel with 1370 MPa strength and 16 pct uniform elongation through warm rolling and intercritical annealing. Metall. Mater. Trans. A 45, 5313–5317 (2014)
H. Azizi-Alizamini, M. Militzer, W.J. Poole, Formation of ultrafine grained dual phase steels through rapid heating. ISIJ Int. 51, 958–964 (2011)
Z. Nasiri, H. Mirzadeh, Enhancement of work-hardening behavior of dual phase steel by heat treatment. Materialwiss. Werkstofftech. 49, 1081–1086 (2018)
H. Mirzadeh, M. Alibeyki, M. Najafi, Unraveling the initial microstructure effects on mechanical properties and work-hardening capacity of dual phase steel. Metall. Mater. Trans. A 48, 4565–4573 (2017)
S. Ghaemifar, H. Mirzadeh, Enhanced mechanical properties of dual phase steel by repetitive intercritical annealing. Can. Metall. Q. 56, 459–463 (2017)
S. Ghaemifar, H. Mirzadeh, Refinement of banded structure via thermal cycling and its effects on mechanical properties of dual phase steel. Steel Res. Int. 89, 1700531 (2018)
A. A. Gorni, Steel Forming and Heat Treating Handbook (2012). www.gorni.eng.br. Accessed November 2018
J. Trzaska, L.A. Dobrzański, Modelling of CCT diagrams for engineering and constructional steels. J. Mater. Process. Technol. 192, 504–510 (2007)
S. Saadatkia, H. Mirzadeh, J.M. Cabrera, Hot deformation behavior, dynamic recrystallization, and physically-based constitutive modeling of plain carbon steels. Mater. Sci. Eng. A 636, 196–202 (2015)
H. Mirzadeh, J.M. Cabrera, J.M. Prado, A. Najafizadeh, Hot deformation behavior of a medium carbon microalloyed steel. Mater. Sci. Eng. A 528, 3876–3882 (2011)
B. Pourbahari, H. Mirzadeh, M. Emamy, R. Roumina, Enhanced ductility of a fine-grained Mg–Gd–Al–Zn magnesium alloy by hot extrusion. Adv. Eng. Mater. 20, 1701171 (2018)
M. Naghizadeh, H. Mirzadeh, Elucidating the effect of alloying elements on the behavior of austenitic stainless steels at elevated temperatures. Metall. Mater. Trans. A 47, 5698–5703 (2016)
B. Pourbahari, H. Mirzadeh, M. Emamy, Elucidating the effect of intermetallic compounds on the behavior of Mg–Gd–Al–Zn magnesium alloys at elevated temperatures. J. Mater. Res. 32, 4186–4195 (2017)
D.H. Wen, Y.C. Lin, Y. Zhou, A new dynamic recrystallization kinetics model for a Nb containing Ni–Fe–Cr-base superalloy considering influences of initial δ phase. Vacuum 141, 316–327 (2017)
F.J. Humphreys, M. Hatherly, Recrystallization and Related Annealing Phenomena, 2nd edn. (Elsevier, Amsterdam, 2004)
M.S. Chen, Z.H. Zou, Y.C. Lin, H.B. Li, W.Q. Yuan, Effects of annealing parameters on microstructural evolution of a typical nickel-based superalloy during annealing treatment. Mater. Charact. 141, 212–222 (2018)
X.M. Chen, Y.C. Lin, F. Wu, EBSD study of grain growth behavior and annealing twin evolution after full recrystallization in a nickel-based superalloy. J. Alloys Compd. 724, 198–207 (2017)
H. Hu, B.B. Rath, On the time exponent in isothermal grain growth. Metall. Trans. 1, 3181–3184 (1970)
H.J. Frost, M.F. Ashby, Deformation-Mechanism Maps: The Plasticity and Creep of Metals and Ceramics (Pergamon Press, Oxford, 1982)
G.R. Speich, V.A. Demarest, R.L. Miller, Formation of austenite during intercritical annealing of dual-phase steels. Metall. Mater. Trans. A 12, 1419–1428 (1981)
J. Roesler, H. Harders, M. Baeker, Mechanical Behaviour of Engineering Materials: Metals, Ceramics, Polymers, and Composites (Springer, Berlin, 2010)
P. Thibaux, A. Métenier, C. Xhoffer, Carbon diffusion measurement in austenite in the temperature range 500 °C to 900 °C. Metall. Mater. Trans. A 38, 1169–1176 (2007)
E.A. Brandes, G.B. Brook, Smithells Metals Reference Book (Butterworth-Heinemann, Oxford, 1992)
Acknowledgements
The authors would like to greatly thank the members of the Advanced Steels and Thermomechanically Processed Engineering Materials Laboratory for their help and support. Financial support by the University of Tehran is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najafkhani, F., Mirzadeh, H. & Zamani, M. Effect of Intercritical Annealing Conditions on Grain Growth Kinetics of Dual Phase Steel. Met. Mater. Int. 25, 1039–1046 (2019). https://doi.org/10.1007/s12540-019-00241-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-019-00241-2