Abstract
Background
The study examined the association between prenatal tobacco or co-exposure to tobacco and cannabis and children’s cortisol reactivity at kindergarten age and the role of child sex, maternal negative mood (depression/perceived stress), and parenting behavior during play interactions as moderators of this association.
Methods
The sample was 238 mother-child dyads (67 tobacco users, 83 co-users of tobacco and cannabis, and 88 non-users). Data used were obtained from pregnancy assessments and six postnatal assessments at 2, 9, 16, 24, and 36 months and kindergarten age. Infant cortisol was measured in response to two laboratory stress paradigms.
Results
Co-exposed children had a significantly greater decrease from pre-stressor to post-stressor and overall lower cortisol response compared with non-exposed children. This association was moderated by maternal harshness during play interactions across early childhood. Co-exposed children had flatter cortisol responses regardless of the mother’s level of harshness or stress/depression. However, non-exposed children who experienced low harshness had the normative cortisol peak 20 min post-stressor, while non-exposed children with high maternal harshness had a flatter cortisol pattern. Similarly, non-exposed children with more depressed/stressed mothers had higher pre-stressor cortisol levels, while those who experienced low maternal depression/stress had lower pre-stressor cortisol but peaked post-stress.
Conclusions
Results suggest that prenatal polysubstance exposure is associated with greater risk for lower cortisol response in children and highlight the role of parenting behavior for non-exposed but not the co-exposed children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tobacco and cannabis are two of the most commonly used substances among pregnant women, with rates of tobacco use ranging from 18 to 27% with even higher rates among young, low-income women [1]. Tobacco is often used with cannabis, with rates of co-use as high as 45% [2], but the literature on the effects of co-use on child outcomes is limited. This is a critical gap given recent changes in cannabis legalization across a number of states in the USA and increases in cannabis potency in recent years [3, 4].
As suggested by several theoretical frameworks such as the developmental origins of health and disease [5,6,7] and the adaptive calibration model [8], the prenatal period is a sensitive period in development. Thus, given high fetal stress posed by prenatal tobacco and cannabis exposure and associated risks such as fetal hypoxia-ischemia, there are likely to be alterations in the structure and function of various organs to promote survival. This fetal programming may result in life long changes in neuroendocrine and metabolic dysfunction [9]. The adaptive calibration model of stress response [8] suggests that infants are born with stress and immune systems that are predisposed to respond in particular ways based on prenatal experiences and genetic associations (see also [10]), includes the possibility of over and under-activation of the hypothalamic-pituitary-adrenal (HPA) system, and emphasizes sex differences. The general model of allostatic load [11] also has some application in the context of prenatal adversity. This model suggests that the constant need to respond to ongoing stressors produces an allostatic load that wears on stress response systems over time resulting in dysregulation of these systems.
In response to stress, the HPA system activates a slow cascade of signals resulting in release of cortisol, the primary glucocorticoid hormone produced by the HPA system in humans [12, 13]. Cortisol has wide-ranging impact on regulating metabolic and immune functions and responding to increased stress [14]. The diurnal pattern of cortisol has been well characterized, with a peak about 30 min after waking, a sharp decrease in concentrations across midday, and a slow decrease later in the day. Under acute stress, cortisol increases about 15–20 min after onset of stressor followed by a return to pre-stressor levels about 30–40 min post-stress.
The literature on HPA functioning among children who were prenatally exposed to tobacco is small, and mostly limited to infancy. In addition, we were unable to find any literature on co-exposure to tobacco and cannabis and acute or diurnal HPA responses in children. Among tobacco exposure studies, the direction of the association is positive in the neonatal period, but becomes more mixed thereafter. For instance, higher concentrations of epinephrine and norepinephrine in the amniotic fluid of pregnant smokers compared with non-smokers were reported in the fetal period [15]; higher ACTH but not cortisol among tobacco exposed compared with non-exposed infants was reported in the neonatal period [16]; and higher cortisol in cord blood for tobacco exposed compared with non-exposed neonates was reported in two additional studies [17, 18]. In contrast, in a well-characterized sample with multi-method assessment of prenatal exposure and a prospective design, Stroud and colleagues [19] reported flatter basal and acute cortisol response to infant exam for tobacco exposed compared with non-exposed infants across the first month of life. Beyond the first month of life, there were reports of lower cortisol response due to high pre-stressor cortisol among exposed compared with non-exposed infants at 2 but not 6 months of infant age [20]; one report of higher cortisol reactivity among exposed compared with non-exposed infants at 7 months of age [21]; lower cortisol response among tobacco exposed infants at 9 months of age in the current sample, especially among boys [22]; and one study with a small sample size indicating no associations at about 11 years of child age [23]. Although most studies focused on prenatal exposure, one of the few studies of postnatal tobacco exposure reported no associations at about 6 months of infant age [24].
In summary, there is large variability among studies in age of measurement, sample size, the nature of the comparison group (degree to which comparison group was similar to exposure group in demographic risk), research design (prospective vs. retrospective measurement of exposure), and how exposure was measured. Studies also varied in measurement of HPA functioning, with measurements taken from amniotic fluid and cord blood, to reactivity in response to frustration or pain (from inoculations). In addition, there are few studies beyond infancy. Taken together, the results reflect a pattern of hyper-reactivity of stress hormones in the fetal period followed by more studies reporting a flatter or blunted response pattern or no associations in later childhood. This changing pattern from neonatal to later childhood may reflect a changing stress regulation system as a function of age related maturation or accumulating postnatal stressors [8, 25]. Indeed, theoretical frameworks such as the adaptive calibration model are supportive of such changes [8]. Finally, although there were a number of studies on other drug exposures, there were none focusing on co-exposure to tobacco and cannabis without other drugs.
In addition to prenatal exposure, conflicting findings may be a function of ongoing postnatal stressors that have not been adequately considered in previous studies of tobacco exposure. Primary among them are maternal negative affect and parenting. Mothers who are at greater demographic risk such as low-income, younger age, and single status are more likely to continue using tobacco and cannabis in pregnancy [1, 2]. These mothers are also more likely to experience greater negative affect or mood due to higher perceptions of stress and symptoms of depression [26, 27]. The combination of higher prenatal risk due to substance exposure and higher postnatal risk as a result of maternal negative affect such as depression and stress may be associated with the greatest risk for disruptions in children’s stress response systems [22, 28].
Maternal substance use and associated psychosocial stressors may also be associated with increased risk for disruptions in parenting. The stress buffering hypothesis supports the hypothesis that caregiving experiences characterized by high levels of nurturance may buffer the effects of stress exposure. Thus, children who experience positive parenting even in a context promoting greater biological vulnerability due to substance exposure may be protected, while those who experience more negative parenting in the context of prenatal risk may be more likely to display risk [22, 29]. Indeed, children’s capacity to regulate emotions and behavior is strongly rooted in dyadic parent-child interactions [30]. Among aspects of parenting, maternal harshness characterized by greater negative affect toward the child and intrusive behaviors is known to increase risk in the context of adversity [31]. A number of studies noted the important protective role of maternal sensitivity and low harshness in promoting children’s stress regulation [31, 32]. Theories such as differential susceptibility [33] suggest that children with highly reactive stress response systems posed by tobacco and cannabis exposure may benefit the most from the buffering effects of sensitive parenting with low harshness, and diathesis stress models suggest that these infants may be the most vulnerable to negative parenting such as high maternal harshness.
Finally, in addition to maternal negative affect and parenting, child sex may moderate the association between prenatal exposure and stress reactivity. At 9 months of age, child sex moderated the association between prenatal tobacco exposure and infant stress reactivity, with tobacco exposed boys demonstrating lower cortisol response to stress compared with non-exposed boys [22]. There were no differences among exposed and non-exposed girls. Sex differences have also been suggested by the adaptive calibration model [8], indicating that a shift toward a lower cortisol response would be more prevalent among males given the evolutionary benefits of such a pattern under conditions of high environmental risk. Others have noted that boys may be more vulnerable to stressors such as fetal hypoxia associated with substance exposure [34].
The main goal of this study was to examine the association between prenatal tobacco or co-exposure to tobacco and cannabis and cortisol reactivity at early school age. An additional goal was to examine the role of maternal depression/stress, parenting, and child sex as moderators of this association. We hypothesized that children exposed to both tobacco and cannabis and those exposed to tobacco alone would have flatter cortisol responses to acute laboratory stressor compared with non-exposed children. We also hypothesized that the association between prenatal exposure and child cortisol would be stronger among children of mothers with higher levels of depression/stress, those who displayed harsh, less sensitive parenting, and for boys. Finally, we conducted exploratory analyses to examine the role of continued postnatal tobacco and cannabis exposure on children’s cortisol responses, examining unique effects of postnatal exposure after accounting for associations due to prenatal exposure.
Method
Sample Selection and Procedures
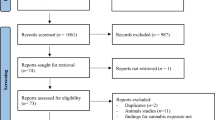
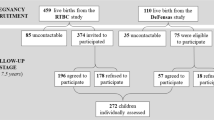
All women presenting for prenatal care completed a self-report health screener that included questions about substance use when they presented for their first prenatal appointment at a large urban prenatal clinic. Women who met initial eligibility criteria based on the screener were invited to participate, with final eligibility determined after the birth of the child. Women were initially excluded if they were more than 20 weeks in gestation, younger than 18 years of age, or pregnant with multiple fetuses. Women were also excluded if they used illicit substances other than cannabis (based on calendar based self-report, salivary assays at the end of each trimester, and infant meconium after delivery) or were heavy drinkers after pregnancy recognition (more than 1 drink/day on average or 4 or more drinks on one occasion based on the detailed calendar based interview). Eligible and interested women were interviewed once at the end of each trimester, at 2, 9, 16, 24, and 36 months of child ages, and after the child entered kindergarten. The original aim of the study was to examine prenatal tobacco exposure effects on developmental outcomes. Thus, women who were current smokers were recruited first. At the end of each month of recruitment, the closest matching non-smoker (based on age and education) was included, with smokers over-sampled to allow for a full range of light to heavy smokers. An additional 33 mother-child dyads were recruited at kindergarten age through Facebook advertisements for mothers with kindergarten aged children to participate in a child health study. Maternal substance use was measured through retrospective self-reports for these additional women, and all previous exclusion criteria applied, with the exception of more than 20 weeks gestation. Child birth outcome and other medical data were extracted from medical records for 94% (n = 31) of the women. The final sample included in the current study consisted of 238 mother-child dyads (67 tobacco users, 83 co-users of tobacco and cannabis, and 88 non-tobacco and cannabis users) with cortisol data at kindergarten age (see Fig. 1 for flow of recruitment), with 115 boys (48.3%). As indicated in Table 1, the sample was diverse with mostly low-income mothers with relatively low education.
Informed consent was obtained from individual participants included in the study at the first prenatal appointment, with the 33 Facebook recruits providing consent at the kindergarten appointment. Mothers were paid for each pre- and postnatal assessment on an escalating scale (ranging from $20 for each prenatal assessment to $150 for the kindergarten assessment) and children received toys and gifts. A Federal Certificate of Confidentiality was obtained from the National Institutes of Health to protect maternal substance use data even from the threat of subpoena.
Measures
Prenatal Substance Use
Multiple methods were used to measure maternal substance use in pregnancy as noted above, including self-report and biomarker analyses. The Timeline Follow-Back Interview (TLFB; [35]) was used once at the end of each trimester and yielded daily data on maternal substance use. The TLFB is a well validated and reliable calendar based method for substance use data (see [36] for details). Final measures included average number of cigarettes, joints, and alcoholic drinks per day across pregnancy and within each trimester. Mothers recruited at kindergarten age (n = 33) were administered the TLFB for postnatal substance use and asked to report average number of cigarettes and joints per day smoked prenatally in each trimester of pregnancy, as well as reporting on prenatal alcohol use before and after pregnancy recognition. Medical record data were checked for n = 31 of 33 women. Maternal oral fluid samples were collected once in each trimester (n = 205), frozen, and shipped to the US Drug Testing Laboratory for cotinine assays (a metabolite of nicotine) for all participants (see [36] for details). Finally, infant meconium samples were collected after birth from soiled diapers, twice a day until the appearance of milk stool. These samples were frozen and shipped in batches to the National Institute on Drug Abuse, where they were assayed with a validated LC-MS/MS method [see [37, 38] for details]. Women who reported smoking on the health screener or the TLFB or were positive for cotinine in oral fluid or had infants with nicotine metabolites in meconium were assigned to the tobacco group; those who were positive on any of these indices for both tobacco and cannabis use were assigned to the co-exposure group.
Postnatal Substance Use
Children’s oral fluid samples were collected and assayed for cotinine at 2, 9, 16, and 24 months and kindergarten visits. At the infant/toddler ages, these samples were collected by placing two eye spears (BD Opthalmology “Visispears” [product #581089], marketed by Salimetrics as “Sorbettes” [product #5029]) into the mouth of each child at each laboratory visit. At kindergarten age, samples were collected using passive drool method. Samples were collected in a storage vial, immediately moved to the − 80 °C freezer, and shipped to Dr. Granger’s laboratory for assay. Total postnatal tobacco exposure was calculated by averaging the number of visits with positive child cotinine from 2 months to kindergarten age for a measure reflecting cumulative child exposure (from maternal or other household members) across time. Maternal postnatal tobacco and cannabis exposure were measured by averaging number of cigarettes/week and joints/week from the TLFB across all ages.
Maternal Depression and Perceived Stress
Maternal depression was assessed at all the time points with the Beck Depression Inventory (BDI-II; [39]). The BDI-II consists of 21 statements reflecting depressive mood (e.g., loss of pleasure). The BDI-II had good internal consistency (Cronbach’s α ranged from α = 0.86 at kindergarten age to α = 0.92 at 16 months) and was fairly stable across time (Pearson correlation coefficients range from r = 0.46 to 0.58 for adjacent time points, p < 0.001). Maternal stress was assessed using the global measure of perceived stress (PSS; [40]) at 2, 9, 16, and 24 months. The PSS was internally consistent at each time point (Cronbach’s α ranged from α = 0.81 at 2 months to α = 0.83 at 9 months) and was fairly stable across time, with Pearson correlation coefficients ranging from r = 0.65 to r = 0.74, p < 0.001, at adjacent time points. The two measures were highly correlated within time (r = 0.51 to 0.55, p < 0.001), standardized, and averaged across time to create a composite measure of maternal depression/stress (Cronbach’s α = 0.91).
Maternal Warmth and Harshness
Both maternal warmth and harshness were assessed during a 10-min period of free play with their infants at the 9, 16, and 24-month laboratory visits. Mothers were asked to spend time with their infants as they normally would at home in a room filled with age-appropriate toys. These interactions were videotaped and coded subsequent to the appointment by two coders blind to exposure status using a collection of global 5-point rating scales called the Parent-Child Early Relational Assessment [41]. Both coders were trained by the first author until inter-rater reliability criterion was reached (exact agreement or within 1 scale point for each item on the composite). A composite maternal warmth/sensitivity scale was created based on observed maternal warmth and sensitivity, such as a parent’s enjoyment and pleasure in interacting with their infant, positive affect, and sensitivity to their child’s state. A composite maternal harshness scale was created based on observed displays of maternal affect such as angry hostile tone of voice, expressed negative affect, and angry hostile mood. The internal consistencies for both scales were excellent across all time points, with Cronbach’s α ranging from 0.94 to 0.96 for warmth/sensitivity and from 0.92 to 0.94 for harshness. Inter-rater reliability conducted on a random selection of 10–13% of the tapes ranged from intra-class correlation coefficients of 0.94 to 0.95 for warmth/sensitivity and 0.84 to 0.97 for harshness. Disagreements between two coders were resolved by consensus and these were the final scores used in analyses. Both maternal warmth/sensitivity and harshness were significantly correlated over time (correlations ranged from r = 0.53 to 0.58 for warmth, p < 0.001, and r = 0.36 to 0.46 for harshness, p < 0.001). Maternal warmth and harshness scores were averaged over time to obtain cumulative measures of parenting across early childhood.
Infant Cortisol
At kindergarten age, infant emotion regulation was assessed using two frustration paradigms from the school age version of the Laboratory Temperament Assessment Battery (LABTAB; [42]). The first 4-min paradigm was the “Impossibly Perfect Stars” during which children were repeatedly asked to draw the “most perfect star” they could, and each star they drew was critiqued (that one is too flat, too pointy, too long, etc.) for 4 min. Children were praised for their drawing at the end of 4 min. The second paradigm was the “Wrong Gift” during which children were asked to rank order five prizes from best to worst (e.g., slinky, yo-yo, top, rubber toy, worn broken white crayon) and told that they would get to keep one of the prizes, left in the room by themselves for 2 min while the gift was wrapped, and presented with the broken white crayon (rated as the worst prize by most children). After a minute, children were told we had made a mistake and were asked to pick out the prize they liked the best. Children relaxed, watched neutral videos, read books, or engaged in drawing/coloring for the next 40 min.
The order of the procedures was as follows: The time 1 (T1) sample was collected after children arrived at the laboratory, were weighed and measured, and spent some time settling in (the last 51 participants were given an extra time to settle in—about 40 min before the pre-task sample was collected after the first batch of assays indicated high initial (T1) cortisol response for the sample as a whole); children were then hooked up to electrodes for collection of autonomic data and watched an emotionally neutral video for 5 min. Time 2 (T2) sample was collected at the end of this relaxation period. The two stress paradigms were conducted next (about 9 min in total), and time 3 saliva sample was collected 20 min after the end of the stress paradigms (T3), and time 4 saliva sample was collected 40 min after the end of the stress paradigms (T4).
Saliva samples were collected using passive drool method into a cryovial (product # 5004.01-06) and a saliva collection aid (product # 5016.02) purchased from Salimetrics. Samples were placed in – 80 °C freezer and sent to the Institute for Interdisciplinary Salivary Bioscience Research at the University of California, Irvine. Samples were assayed for cortisol using a highly sensitive enzyme immunoassay cleared by the US Food and Drug Administration (510 k) for use as an in vitro diagnostic measure of adrenal function. The test uses 25 μL of saliva and has a lower limit of sensitivity < 0.007 μg/dL and assay range of 0.012–3.00 μg/dL. All samples were assayed in duplicate and averaged scores were used in analyses.
Covariates
Covariates included demographic risk, birth outcomes, hours of sleep the night before saliva sample was collected, medication use, and maternal age in the first trimester. A demographic cumulative risk score was computed from 4 variables: maternal race, maternal education, maternal occupation, and maternal partner status. Minority status (69%), below high school education (29.1%), and single status (not married or living with partner (54.7%) were assigned to the risk category. Maternal occupation was coded using the Hollingshead scale (M = 2.06, SD = 1.6, range = 1–8) and divided by the maximum value of 9 in order to create a proportion. The final demographic cumulative risk variable was created by averaging the 4 items described above, with a possible maximum score of 1 (M = 0.67, SD = 0.27, range = 0.07–0.96). Measures of fetal growth (i.e., birth weight (M = 3.23 kg, SD = 0.52), birth length (M = 50.10 cm, SD = 2.75), birth head circumference (M = 34.45 cm, SD = 6.85) were taken by trained obstetrical nurses in the delivery room. Gestational age was calculated by trained study staff using conception and birth dates (M = 38.93 weeks, SD = 1.75). Hours of sleep were calculated for the night prior to saliva collection based on maternal reports of child bedtime and wake time (M = 10.03, SD = 1.29). Mothers also reported whether or not their children were currently taking any medications (15.9% were taking medications, none were taking NSAIDs). Maternal age in the first trimester of pregnancy (M = 24.37, SD = 4.75) was used as a covariate. Finally, procedure order (less vs. more time before T1 sample) and average number of standard drinks/day in pregnancy were additional covariates considered in initial analyses.
Data Transformations
Cortisol data were examined for outliers (defined as + 3 SD from the mean; [43]) or physiologically improbable values above 4 μg/dL. There were three cases with values above 4 for T1 cortisol, one case with a value above 4 for T2 cortisol, two cases with values above 4 for T3 cortisol, and 3 cases with values above 4 for T4 cortisol. Data were checked for outliers after removing these cases from the data file. There were four outliers for T1 and T2 cortisol, three outliers for T3 cortisol, and two outliers for T4 cortisol. Following recommendations by [44], these values were winsorized by replacing values that were 3 SD above the mean with the value of 3 SD above the mean, as in previous studies [22, 45].
Analytic Strategy
We first examined potential covariates using correlations or ANOVAs as appropriate. Confounds with significant bivariate associations at p < 0.10 with both the substance use variables and child cortisol were included as covariates in initial model testing. HLM 7 [46] was used to estimate a growth model in cortisol response to the acute laboratory stressor. The two-level model included repeated measures of cortisol at level 1, and time invariant predictors at level 2, and modeled both linear and quadratic trends. Time was coded as: T1 cortisol = 0, T2 cortisol = 1, T3 cortisol = 2, and T4 cortisol = 3 as the intervals between collections were approximately even. We first ran an unconditional means model, followed by an unconditional growth model, a growth model conditional on child sex, and finally, the fully conditional model. In all models, the intercept was set to time 0 (baseline cortisol). Group status was dummy coded as tobacco only vs. not and co-exposed vs. not following guidelines by Aiken and West [47] so that each exposure group was compared with the referent group of non-exposed children.
The first pass of the fully conditional model included all of the covariates described above, and significant covariates were retained. Analyses examining potential interaction of prenatal exposure and child sex on child cortisol were conducted by including the interaction term of sex and prenatal exposure in addition to their main effects and significant covariates. Finally, interaction of prenatal exposure and parenting effects on child cortisol responses were conducted by including the interaction terms of exposure and parenting in the model. All components of interaction terms were centered according to the recommendations of Aiken and West [47].
Missing Data
As expected in any longitudinal study, there were some incomplete data for some of the participants at one or more of the five assessment points included in this study. All participating dyads had prenatal substance use data and kindergarten cortisol data; however, there were 57 participants that were missing data from at least one of the postnatal appointments resulting in missing data on one or more of the parenting variables. When compared with women with complete data, there were no significant differences in birth weight, gestational age, birth length, average cigarettes per day prenatally and postnatally, average joints per day prenatally and postnatally, average drinks per day prenatally and postnatally, maternal education, and days the infant was breastfed. Women with missing data were older in the first trimester of pregnancy than women without missing data, t(235) = − 2.71, p = 0.008, and their children had larger birth head circumferences t(197) = − 3.25, p = 0.001. Little’s test indicated that data were missing completely at random, χ2(2) = 1.637, p = 0.441, and Restricted Information Maximum Likelihood was used in the estimation of all model parameters.
Results
Descriptive data for demographics and substance exposure are presented in Table 1 and correlations among variables are presented in Table 2.
Consideration of Covariates
Demographic risk was associated with T1 and T4 cortisol values (r = 0.15 and 0.16 respectively, p < 0.05), birth length was associated with T2 cortisol at r = 0.19, p < 0.01, and hours of sleep was marginally associated with T1 cortisol at r = − 0.11, p < 0.10. There were no associations between child medication use and maternal age in the first trimester with either substance exposure or cortisol concentrations. Thus, demographic risk, procedure order, number of standard drinks/day in pregnancy, and hours of sleep were included as covariates in initial model testing. Child sex was also included given the potential for sex differences in cortisol concentrations.
Model Testing
Estimation of the unconditional means model revealed that 70% of the variation in cortisol was attributable to differences among children. The variance components of the unconditional growth model indicated that there was significant variation in both initial status and rate of change over time (see Table 3). Order of assessment was associated with both intercept (β = − 0.32, SE = 0.06, p < 0.001) and linear slope of cortisol (β = 0.15, SE = 0.05, p = 0.025). Among covariates, participants who had a longer initial wait time to get acclimated to the lab had lower baseline cortisol and a greater increase in cortisol, suggesting that those who had a shorter wait time were still reacting to the stress of preparing to arrive for the session. There were no sex differences or sex by exposure group interactions.
Co-exposure to both tobacco and cannabis prenatally was a significant predictor of linear (β = − 0.07, SE = 0.04, p = 0.04) and quadratic slope (β = 0.02, SE = 0.01, p = 0.02). Co-exposed children had overall lower cortisol levels and a sharp decline in cortisol in contrast to the slight increases exhibited by children in the non-exposed group (see Fig. 2). There was a significant interaction between co-exposure and maternal harshness on linear slope of cortisol (β = − 0.06, SE = 0.03, p = 0.046; see Fig. 3). Finally, there was a significant interaction of tobacco exposure and maternal mood (β = 0.02, SE = 0.01, p = 0.032) as well as co-exposure and maternal mood (β = 0.03, SE = 0.01, p = 0.025) on quadratic slope (Figs. 4 and 5). Cortisol response among co-exposed children did not vary by level of maternal harshness. However, non-exposed children with low levels of maternal harshness showed a more normative cortisol response with a peak at 20 min post-stressor, while non-exposed children with high levels of maternal harshness exhibited a flatter cortisol response (Fig. 4). Similarly, compared with tobacco exposed or co-exposed children who did not exhibit changes in cortisol in response to high or low levels of maternal stress/depression, non-exposed children with depressed/stressed mothers had higher pre-stressor cortisol levels while those who experienced low maternal depression/stress had lower pre-stressor cortisol but peaked post-stress. Contrary to expectations, there was no main effect of maternal sensitivity or interactions between exposure status and maternal sensitivity on intercept or slope.
Discussion
The main goal of this study was to examine the association between prenatal tobacco or co-exposure to tobacco and cannabis and cortisol reactivity at early school age and to examine the role of maternal negative mood, parenting, and child sex as potential moderators of this association. Results indicated a significant main effect of co-exposure on cortisol response to an acute laboratory stressor such that co-exposed children displayed overall lower levels of cortisol and a sharp decrease in cortisol from pre- to post-stressor while non-exposed children exhibited a slight increase before the decline. There may be two explanations for this pattern in the co-exposed group. The first is that the laboratory stressors were not stressful enough for the co-exposed group. Children exposed to both tobacco and cannabis may be exposed to higher postpartum stress (see [48]), and the laboratory stressors did not elicit a stress response from these children. A second interpretation is that these children had a blunted response pattern due to hypo-activation of the stress response system. As noted by the Adaptive Calibration Model [8], hypo-activation of the stress response system would be the pattern expected in response to severe stress. Children exposed to both tobacco and cannabis may have experienced more severe prolonged stress beginning in the prenatal period due to fetal ischemia or hypoxia and postnatal stressors associated with continued maternal polysubstance use. While there are no studies of co-exposure to both tobacco and cannabis, recent studies of tobacco exposure with careful multi-method and prospective assessments of exposure (e.g., [19]) have reported flatter cortisol response to infant examinations across the first month of life for tobacco exposed compared with non-exposed infants, similar to results obtained in the current sample in later infancy [22]. Other studies of tobacco exposure reporting no associations between exposure and cortisol had exposure measures based on self-report and assessed after delivery [21] or several years later [23] and small sample sizes. Our results indicate that in addition to differences in methods, some of these discrepancies may be due to other co-occurring substance use such as cannabis.
An alternative explanation for mixed results may be the presence of other moderators that may ameliorate or exacerbate risk. Our results indicated a significant interaction between co-exposure and maternal harshness on linear slope. Co-exposed children had blunted cortisol responses regardless of the mother’s level of harshness, but non-exposed children exposed to high level of maternal harshness did not have the normative increase in cortisol in response to the stressor. In addition, there was no interaction of exposure group and maternal sensitivity. Thus, the stress buffering hypothesis was not supported by the results of the current study. While we hypothesized that caregiving experiences characterized by high levels of nurturance would buffer the effects of substance exposure, it seems that parenting was not able to overcome the detriments to the stress response system presented by fetal co-exposure to tobacco and cannabis. Indeed, co-exposed children exhibited flatter cortisol responses in the context of both low and high levels of maternal harshness. The pattern of these interactions are different from those obtained in infancy in the current sample where we reported a significant additive effects of tobacco exposure and maternal insensitivity or intrusiveness [22]. It is possible that by kindergarten age, the cortisol values of co-exposed children were too low for additive effects of additional risk variables to be apparent.
Few studies examined if cortisol outcomes among tobacco exposed or co-exposed children vary as a function of parenting. This is surprising given studies indicating interactions of pregnancy smoking and parenting on other child outcomes (e.g., [49]). For instance, in a study with multi-method, prospective measurement of prenatal tobacco exposure, results indicated a significant interaction of exposure and maternal responsiveness (a construct very similar to warmth/sensitivity) on children’s behavioral disinhibition at 5 years of age. The pattern of interaction indicated additive effects of exposure and low maternal responsiveness on behavioral disinhibition and protective effects of high responsiveness among exposed children [49]. Such protective effects of positive parenting obtained in infancy but not school age on cortisol with the current sample suggest loss of plasticity in the stress response system with accumulating pre- and postnatal stressors with increasing age. It has long been understood that prolonged exposure to toxic stress may result in the dysregulation of the stress response system [50]. Our findings are in line with extant work that suggests that as stress response patterns are continually disrupted by exposure to chronic adversity the system becomes desensitized, resulting in blunted or non-responsive stress responses to acute stressors. This chronic adversity may take many forms, including fetal exposure to substances and postnatal exposure to poor parenting.
Contrary to expectations, the association between exposure and cortisol was not moderated by child sex. This is in contrast to results obtained in infancy with only tobacco exposed boys, but not girls, demonstrated hypo-activation of the stress response system [22]. However, results are similar to those obtained by [49] for behavioral disinhibition, with no interaction of tobacco exposure and child sex on measures of behavioral disinhibition at 5 years of age. Finally, there were no associations between postnatal exposure and child cortisol. These results are similar to those reported by [24], indicating no associations between postnatal exposure (current maternal smoking in the past 48 h) and child salivary cortisol at 4–6 months of infant age.
Limitations and Future Research
The present study had many strengths, including a longitudinal design that incorporated prospective assessments of substance use with biological verification and a multi-method, multi-informant approach that included observational measures of maternal parenting behaviors across infancy and toddlerhood. However, there were also limitations. One limitation was that our sample size restricted our potential to examine whether the hypothesized model differed for boys and girls. Another limitation was that the sample consisted primarily of women with relatively low income and low education levels, and our inclusion criteria at recruitment excluded mothers who drank or consumed illicit drugs other than cannabis during their pregnancy, so the results may not be generalizable to tobacco and cannabis using families with higher income or higher education. However, this limit to generalizability should be considered in the context that maternal tobacco and cannabis use in pregnancy occurs more often in the context of low income or low education. We were also unable to speak to dose-response associations. In order to use the continuous measures of cigarettes and marijuana and interaction and examine an interaction of maternal mood/stress or harshness by exposure in a multi-level framework with repeated measures over time, we would have to run a 4-way interaction of cigarettes × marijuana × maternal harshness × time. The power to do so was severely limited. In addition, these continuous measures were based on maternal report only and did not take advantage of the multi-method assessment of substance use for the majority of the sample (with the exception of the small sample of Facebook recruits)—maternal reports, maternal saliva in each trimester, and infant meconium. The grouping of participants into non-exposed, tobacco only, and co-exposed to tobacco and marijuana was based on all measures of substance use. An additional limitation was that while TLFB data is valid and reliable when used longitudinally and is comparable with repeated biomarkers [51], it may not be as reliable for the subsample of Facebook recruits as data collected in the longitudinal sample. However, it is worth noting that the pattern of results were similar with and without the Facebook recruits in the model. In addition, maternal warmth/sensitivity or mood measures collected at earlier time points (before kindergarten assessments) were not available for this subsample. Finally, it is unclear how acute stress reactivity in the laboratory may relate to diurnal cortisol patterns. Indeed, one interpretation of the results may be that co-exposed children are exposed to higher level of stressors as noted in previous data [48], and the two laboratory frustration paradigms were not stressful enough to initiate a cortisol response for these children.
Future studies may examine if prenatal tobacco and co-exposure to tobacco and cannabis results in a depressed or flat diurnal pattern of cortisol in addition to responses to acute stressors. Future research may also consider dose-response effect of both tobacco and cannabis exposure. Given that the group assignment was based on multiple methods of ascertainment of exposure, while dose-response associations would be limited to single measures, we chose to examine our data with exposure group as the primary independent variable, thus not examining timing or dose-response associations. It is possible that children exposed to both tobacco and cannabis had a flatter cortisol response compared with non-exposed children due to higher levels of tobacco exposure. Finally, future studies measuring cortisol in the laboratory may allow a longer wait time before taking the first saliva sample. Despite these limitations, the current results add to the sparse literature on tobacco and co-exposure to tobacco and cannabis on child cortisol reactivity in early school age.
References
Services UDoHaH. The health consequences of smoking—50 years of progress: a report of the surgeon general, vol. 17. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014.
Chabarria KC, Racusin DA, Antony KM, Kahr M, Suter MA, Mastrobattista JM, et al. Marijuana use and its effects in pregnancy. Am J Obstet Gynecol. 2016;215:506 e1–7.
Calvigioni D, Hurd YL, Harkany T, Keimpema E. Neuronal substrates and functional consequences of prenatal cannabis exposure. Eur Child Adolesc Psychiatry. 2014;23:931–41.
Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci. 2010;55:1209–17.
Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;327:1077–81.
Barker DJ, Osmond C, Winter P, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;334:577–80.
Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938.
Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neurosci Biobehav Rev. 2011;35:1562–92.
Buss C, Entringer S, Wadhwa PD. Fetal programming of brain development: intrauterine stress and susceptibility to psychopathology. Sci Signal. 2012;5:pt7–pt.
Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: past, present, and future. Dev Psychopathol. 2013;25:1359–73.
McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153:2093–101.
Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D. Focus on methodology: salivary bioscience and research on adolescence: an integrated perspective. J Adolesc. 2012;35:1081–95.
Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–69.
Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: a systematic review and meta-analysis. Psychoneuroendocrino. 2017;83:25–41.
Divers WA Jr, Wilkes MM, Babaknia A, Yen SS. An increase in catecholamines and metabolites in the amniotic fluid compartment from middle to late gestation. Am J Obstet Gynecol. 1981;139:483–6.
McDonald SD, Walker M, Perkins SL, Beyene J, Murphy K, Gibb W, et al. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. BJOG. 2006;113:1289–95.
Varvarigou AA, Petsali M, Vassilakos P, Beratis NG. Increased cortisol concentrations in the cord blood of newborns whose mothers smoked during pregnancy. J Perinat Med. 2006;34:466–70.
Varvarigou AA, Liatsis SG, Vassilakos P, Decavalas G, Beratis NG. Effect of maternal smoking on cord blood estriol, placental lactogen, chorionic gonadotropin, FSH, LH, and cortisol. J Perinat Med. 2009;37:364–9.
Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, et al. Maternal smoking during pregnancy and infant stress response: test of a prenatal programming hypothesis. Psychoneuroendocrino. 2014;48:29–40.
Ramsay DS, Bendersky MI, Lewis M. Effect of prenatal alcohol and cigarette exposure on two- and six-month-old infants' adrenocortical reactivity to stress. J Pediatr Psychol. 1996;21:833–40.
Schuetze P, Lopez FA, Granger DA, Eiden RD. The association between prenatal exposure to cigarettes and cortisol reactivity and regulation in 7-month-old infants. Dev Psychobiol. 2008;50:819–34.
Eiden RD, Molnar DS, Granger DA, Colder CR, Schuetze P, Huestis MA. Prenatal tobacco exposure and infant stress reactivity: role of child sex and maternal behavior. Dev Psychobiol. 2015;57:212–25.
Huijbregts SCJ, van Berkel SR, Swaab-Barneveld H, van Goozen SHM. Neurobiological and behavioral stress reactivity in children prenatally exposed to tobacco. Psychoneuroendocrino. 2011;36:913–8.
Granger DA, Kivlighan KT, El-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research - recent developments and applications. Ann N Y Acad Sci. 2007;1098:122–44.
Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23:881–96.
Eiden RD, Leonard KE, Colder CR, Homish GG, Schuetze P, Gray TR, et al. Anger, hostility, and aggression as predictors of persistent smoking during pregnancy. J Stud Alcohol Drugs. 2011;72:926–32.
Massey SH, Backes KA, Schuette SA. Plasma oxytocin concentration and depressive symptoms: a review of current evidence and directions for future research. Depress Anxiety. 2016;33:316–22.
Laurent H. Early calibration of the HPA axis by maternal psychopathology. Psychoneuroendocrino. 2017;78:177–84.
Bada HS, Bann CM, Whitaker TM, Bauer CR, Shankaran S, LaGasse L, et al. Protective factors can mitigate behavior problems after prenatal cocaine and other drug exposures. Pediatrics. 2012;130:e1479–e88.
Schore AN. Affect regulation and the origin of the self: the neurobiology of emotional development. Hillsdale: Lawrence Erlbaum Associates; 1994.
Laurent HK, Harold GT, Leve L, Shelton KH, Van Goozen SH. Understanding the unfolding of stress regulation in infants. Dev Psychopathol. 2016;28:1431–40.
Feldman R, Dollberg D, Nadam R. The expression and regulation of anger in toddlers: relations to maternal behavior and mental representations. Infant Behav Dev. 2011;34:310–20.
Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;135:885–908.
Coles CD, Kable JA, Lynch ME. Examination of gender differences in effects of tobacco exposure. In: Lewis M, Kestler L, editors. Gender differences in prenatal substance exposure. Washington, DC: Am Psychol; 2012. p. 99–120.
Sobell L, Sobell M. Timeline followback: a technique for assessing self reported ethanol consumption, vol. 17. Totowa: Humana Press; 1992.
Eiden RD, Schuetze P, Shisler S, Huestis MA. Prenatal exposure to tobacco and cannabis: effects on autonomic and emotion regulation. Neurotoxicol Teratol. 2018;68:47–56.
Gray TR, Eiden RD, Leonard KE, Connors G, Shisler S, Huestis MA. Nicotine and metabolites in meconium as evidence of maternal cigarette smoking during pregnancy and predictors of neonatal growth deficits. Nicotine Tob Res. 2010;12:658–64.
Gray TR, Eiden RD, Leonard KE, Connors GJ, Shisler S, Huestis MA. Identifying prenatal cannabis exposure and effects of concurrent tobacco exposure on neonatal growth. Clin Chem. 2010;56:1442–50.
Beck AT, Steer RA, Brown GK. Beck depression inventory-II. San Antonio: The Psychological Corporation; 1996.
Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96.
Clark R. The parent-child early relational assessment: a factorial validity study. Educ Psychol Meas. 1999;59:821–46.
Gagne JR, Van Hulle CA, Aksan N, Essex MJ, Goldsmith HH. Deriving childhood temperament measures from emotion-eliciting behavioral episodes: scale construction and initial validation. Psychol Assess. 2011;23:337–53.
Gunnar M, Broderson L, Krueger K, Rigatuso J. Dampening of adrenocortical reactivity during earliy infancy: normative changes and individual differences. Child Dev. 1996;67:877–89.
Tukey JW. Exploratory data analysis. Reading: Addison-Wesley; 1977.
Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–64.
Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Thousand Oaks: Sage Publications; 2002.
Aiken LS, West SG. Multiple regression: testing and interpreting interactions. Newbury Park: Sage; 1991.
Schuetze P, Eiden RD, Colder CR, Huestis MA, Leonard KE. Prenatal risk and infant regulation: indirect pathways via fetal growth and maternal prenatal stress and anger. Child Dev. 2018;89:e123–e37.
Clark CAC, Massey SH, Wiebe SA, Espy KA, Wakschlag LS. Does early maternal responsiveness buffer prenatal tobacco exposure effects on young children's behavioral disinhibition? Dev Psychopathol. 2018:1–14.
Shonkoff JP, Garner AS, Committee on Psychosocial Aspects of C, Family H, Committee on Early Childhood A, Dependent C, et al. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129:e232–46.
ewis-Esquerre, JM, Colby, SM, O’Leary T, Eaton CA, Kahler CW, Monti, PM. Validation of the timeline follow-back in the assessment of adolescent smoking, Drug and Alcohol Dependence. 2005;79:33–43.
Acknowledgements
The authors are grateful to the families who participated in the study and to Research Technicians for data collection and coding. Special thanks goes to Dr. Amol Lele at Women and Children’s Hospital of Buffalo for her collaboration on data collection.
Funding
The study was supported by the National Institute on Drug Abuse at the National Institutes of Health under award number R01DA019632 and the Intramural Research Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
In the interest of full disclosure, DAG is founder and scientific and strategy advisor at Salimetrics LLC and Salivabio LLC. These relationships are managed by the policies of the committees on conflict of interest at the Johns Hopkins University School of Medicine and the University of California at Irvine.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eiden, R.D., Shisler, S., Granger, D.A. et al. Prenatal Tobacco and Cannabis Exposure: Associations with Cortisol Reactivity in Early School Age Children. Int.J. Behav. Med. 27, 343–356 (2020). https://doi.org/10.1007/s12529-020-09875-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12529-020-09875-8