Abstract
Deep-sea cephalopods are important in the bathyal ecosystems in terms of both abundance and diversity, but are seriously understudied. One of the most intriguing groups among the deep-sea cephalopods are Cirrata, relatively primitive octopods. Opisthoteuthis is the largest genus among the Cirrata. The least studied species of Opisthoteuthis in the Atlantic, Opisthoteuthis borealis Collins, 2005 was known from nine specimens only prior to our study, and nothing was described about its biology. Four males, all larger than the previously known maximum size (mantle length 78–96 mm cf. 75 mm), are described and COI sequence of the species provided to ease the identification of the Atlantic Opisthoteuthis. Our findings expand the known geographical (North Atlantic from 60° N northward and up to the Davis and Denmark Straits and the Iceland–Faroe Ridge), depth (878–1321 m) and temperature (3.0–3.6 °C) ranges of O. borealis. Arm bifurcation is reported in Cirrata for the first time, suggesting well-developed regeneration is present even in this ancient taxon of cephalopods. Ontogenetic increase of spermatophore length, i.e., when the spermatophores produced later during ontogenesis are larger than those produced earlier, is reported in Cirrata for the first time. The stomachs in all the studied specimens were at least one-third full, suggesting that O. borealis continues to feed and grow after reaching maturity. Polychaetes dominated over crustaceans in the stomach contents. Contrary to the assumption that Cirrata feed on relatively small prey only, large mature males of O. borealis consume polychaetes reaching 41.5–45.9% ML of the specimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The bathypelagic zone of the world ocean, i.e., at or below 1000 m depth, is the least explored and at the same time the largest biotope on the planet (Ramirez-Llodra et al. 2010; Robison et al. 2010). Cephalopods are among the successful taxa in deep-sea ecosystems; the majority of the families include deep-sea representatives (review: Hoving et al. 2014), achieving large biomass and abundance values and thus occupying important roles in food webs as prey and predators (reviews: Clarke 1996; Klages 1996; Rodhouse and Nigmatullin 1996; Smale 1996). Deep-sea cephalopods are largely understudied in comparison to their shallow-living relatives (Hoving et al. 2014).
One of such understudied group of cephalopods are the Cirrata, the finned octopods (Collins and Villanueva 2006; Hochberg et al. 2013). As the name indicates, they have paired fins and paired cirri between the suckers (Collins and Villanueva 2006; Hochberg et al. 2013). Other key characters are a semi-gelatinous body consistency, a well-developed web, a large cartilaginous internal shell, a single row of suckers on arms, and an absence of a radula in the majority of cirrates (Collins and Villanueva 2006; Hochberg et al. 2013). It has been suggested, based on their morphology, that this taxon is relatively primitive and close to ancestral forms of octopods (Young et al. 1998). Later, the monophyly of cirrates was proved by molecular study (Lindgren et al. 2012), with the separation of cirrate and incirrate octopods estimated to have happened in the early Jurassic, about 180 Ma (Tanner et al. 2017). The cirrates are among the largest deep-sea invertebrates, with total length (TL) reaching 1.7 m in directly measured specimens (Collins et al. 2001) and 4 m in underwater photos (Voss 1988). They inhabit meso- and bathypelagic layers worldwide (Collins and Villanueva 2006; Hochberg et al. 2013) and have recently been proven to be the deepest-dwelling cephalopods, reaching depths of 6957 m (Jamieson and Vecchione 2020). Due to their semi-gelatinous consistency and deep-sea habitat, the cirrates are often damaged by the catching gear and, following pressure decrease, while being lifted from the depths. They are easily damaged when onboard and by subsequent fixation or freezing, which leads to further confusion in their taxonomy (Vecchione and Collins 2002; Collins and Villanueva 2006).
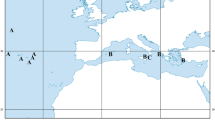
The majority of cirrates belong to the family Opisthoteuthidae, the flapjack octopods, especially in the more conservative sense, i.e., including the genus Grimpoteuthis, following Hochberg et al. (2013) and Vecchione et al. (2016) (cf. Piertney et al. 2003; Collins and Villanueva 2006). Opisthoteuthis is the largest genus among the cirrates (Hochberg et al. 2013). It includes the shallowest-dwelling cirrates, found at depths of 130–3400 m and are relatively more bottom-associated than the rest of the taxon (Villanueva et al. 2002; Collins and Villanueva 2006; Hochberg et al. 2013). Six species of Opisthoteuthis inhabit the Atlantic Ocean (Villanueva et al. 2002; Collins 2005; Collins et al. 2010): 1) O. agassizii Verrill 1883, north-western Atlantic from ~ 40° N to the Caribbean Sea; 2) O. borealis Collins 2005, northern North Atlantic, off West Greenland and the south of Iceland (Fig. 1); 3) O. calypso Villanueva et al. 2002, eastern Atlantic, from southwest Ireland (~ 50° N) to Namibia, and the Mediterranean Sea; 4) O. grimaldii (Joubin 1903), eastern Atlantic, from the Rockall Trough (~ 56° N) to Namibia; 5) O. hardyi Villanueva et al. 2002, south-western Atlantic, from South Georgia to the Falkland Islands and the Patagonian slope; and 6) O. massyae (Grimpe 1920), eastern Atlantic, from the Rockall Trough (~ 56° N) to Namibia. Among these, O. borealis is the least-known species, of which only nine specimens (sampled in 1902–1997) were known (Collins 2005) prior to our study. Three specimens of O. borealis were captured recently during annual bottom-trawl surveys in West Greenland, and one specimen was identified in the collection of the Icelandic Institute of Natural History. All specimens were male and larger than the previously known maximum size, which had a mantle length (ML) of 75 mm (Collins 2005). Thus, the main goals of our study were as follows: (1) to describe the morphology of these specimens and sequence the cytochrome oxidase subunit I (COI) gene, providing the DNA barcode of the species; and (2) to describe the reproductive biology and trophic ecology of the species based on newly available samples, as nothing is currently known of the species biology and ecology.
Distribution of Opisthoteuthis borealis in the North Atlantic. Our samples (larger circles) and all other known specimens (smaller circles; from Collins 2005)
Materials and methods
Sample collection, onboard treatment, fixation and storage
Greenlandic specimens were all captured near the type locality of the species in 2015–2019 by R/V ‘Paamiut’ and F/V ‘Helga Maria’: HM-2019-4-16, specimen ID GRL_1; PA-2017-7-68, specimen ID GRL_2; and PA-2015-6-44, specimen ID GRL_3. The Icelandic specimen (NI-21, specimen ID ICE_1) was captured on the European slope of Reykjanes Ridge in 1994 by F/V ‘Venus HF 519’ (Fig. 1; Online Resource 1). The specimens are referred to by their specimen IDs from now on. Exact locations of the stations, sampling details, depths, temperatures and dates are provided in Online Resource 1. All specimens were captured as bycatch in bottom trawls during the annual halibut monitoring surveys. Specimens GRL_1 and GRL_3 were fixed in 6% formalin onboard and stored in 6% formalin; specimen GRL_2 was frozen onboard (− 20 °C), defrosted and relocated to formalin 6% in 2019; and specimen ICE_1 was fixed in formalin 6% onboard, and relocated to ethanol 70% in 2017. All specimens, except ICE_1, were weighed onboard prior to fixation/freezing. A sample for DNA barcoding was taken from specimen GRL_1 prior to fixation and stored in 96% ethanol in the freezer (− 20 °C). All specimens, except GRL_1, were already dead once aboard. Specimen GRL_1 came onboard alive, was kept alive for 2 h and subsequent gradual cooling was used to euthanise it afterwards, following Fiorito et al. (2015). Specimens GRL_1 and GRL_2 are kept at the Greenland Institute of Natural Resources under their respective numbers. Specimen ICE_1 is kept at the Icelandic Institute of Natural History (cat. no. IINH37690); specimen GRL_3 was discarded after the analysis.
Morphological and statistical analysis
Definition of counts and measurements generally followed the guideline for cephalopods (Roper and Voss 1983) and previous major studies on cirrate morphology (Voss and Pearcy 1990; Guerra et al. 1998; Collins 2003), with the exceptions being: (a) the beaks (rostrum and hood measurements followed Clarke 1962); (b) the shells (length taken as a maximum, not as a medial (cf. Guerra et al. 1998), i.e., a direct line from the central lowest point to the level of wing tips); and (c) the male reproductive systems (see below). Counts of gill lamellae refer to the total number of lamellae on each gill; right and left gills are separated by ‘/’ in Online Resource 1. All indices are related to ML and are always detailed when used, except for weight indices of the male reproductive system, which were: (a) RSI, reproductive system index—weight of the reproductive system relative to fixed animal weight, %; (b) GSI, gonadosomatic index—weight of the testis relative to fixed animal weight, %; and (c) SCI, spermatophoric complex index—weight of the spermatophoric complex relative to fixed animal weight, %.
Proximal parts of the spermatophoric complex are referred to as spermatophoric complex parts I–III, following Nigmatullin et al. (2003) and Sabirov (2010), despite other opinions regarding this terminology (detailed and explained in Discussion). Accessory glands are referred to as accessory glands 1–3 following the order previously used for Opisthoteuthis (Villanueva et al. 2002; Collins 2005; Collins et al. 2010). Other opinions are also detailed and explained in the Discussion. Specimens with large numbers of spermatophores in the spermatophoric complex part III and spermatophoric sac were considered mature (corresponding to V2 maturity stage cf. Nigmatullin et al. 2003); a specimen with just two spermatophores in the spermatophoric complex was considered pre-mature; this was also based on smaller size and proportions of its reproductive system (corresponding to V1 maturity stage cf. Nigmatullin et al. 2003) (Online Resource 2). Spermatophores of specimens GRL_1, GRL_3 and ICE_1 were studied by scanning electron microscopy (SEM), which was performed at the Department of Zoology, Kazan Federal University,, using a Hitachi TM Series SEM. In order to prepare for the analysis, the spermatophores were washed carefully with distilled water, dehydrated using ascending ethanol concentrations (70, 80, 90, 96 and 100%), then CO2 critical-point dried.
Stomach contents were analysed and prey items identified to the lowest possible taxonomic level in all specimens. Stomach fullness was ranged from 0 (completely empty) to 4 (completely full) following Breiby and Jobling (1985).
Mann–Whitney U test (with a significance value of α = 0.05) was used to access the differences among groups of spermatophores. Statistical analyses were performed in PAST 3.25 (Hammer et al. 2001).
Barcoding COI DNA sequences and analyses
Total DNA extraction was performed on muscle tissue using the standard extraction kit protocol from the DNeasy Blood & Tissue kit (Qiagen GmbH, Hilden, Germany). Primers used for the COI barcode were forward FishF2 (5′-TCGACTAATCATAAAGATATCGGCAC-3′) and reverse FishR2 (5′-ACTTCAGGGTGACCGAAGAATCAGAA-3′) (Folmer et al. 1994). PCR was performed according standard thermal cycle protocols (e.g., Ward et al. 2005) with the protocol as follows: total reaction mixture volume was 25 mL, including 18.75 mL of ultrapure water, 2.25 mL buffer, 1.25 mL of MgCl2 (50 mM), 0.25 mL of each primer (0.01 mM), 0.125 mL dNTP (0.05 mM), 0.625 U Taq polymerase, and 1 mL of DNA template. Thermal cycling consists of an initial step of 2 min at 94 °C followed by 35 cycles of 20 s at 94 °C, 15 s at 52 °C, and 40 s at 72 °C, followed by 3 min at 72 °C, and then held at 4 °C. PCR products were UV-visualised on 1% agarose gels stained with ethidium bromide. Successful PCR products were purified using the EXOSAP method with Exonuclease 1 (EXO 10 units/μL) and Shrimp Alkaline Phosphatase (SAP 1 unit/μL, USB©) in 10-μL reactions. Reactions were carried out on a thermal cycler at 37 °C (incubation) for 30 min followed by 15 min at 80 °C (enzyme inactivation). Subsequently, 25 rounds of direct cycle-sequencing with dye-labelled terminators BigDye terminator 3.1 (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were performed following the manufacturer’s protocol.
The Kimura’s two-parameter substitution model (K2P) (Kimura 1980) was used to obtain a COI molecular taxonomic cladogram. However, we recognise that the K2P is not necessarily an appropriate model choice for barcoding data (Srivathsan and Meier 2012), and use it only to show the differences among species.
Results
Distribution
All Greenlandic specimens were captured at depths greater than 1100 m and within temperature range 3.5–3.6 °C (Online Resource 1). Only three specimens were caught in 356 deep-sea trawl stations (depth 500–1514 m) conducted in Greenland waters in 2015–2019: one specimen each in 2015, 2017 and 2019. The Icelandic specimen is the shallowest and southernmost-known record of O. borealis, at 878 m depth (Fig. 1; Online Resource 1). Opisthoteuthis borealis was not recorded during the extensive surveys of the BIOICE programme in 1991–2004, despite 132 deep-sea trawl stations (depth 500–3007 m) being sampled.
Morphology and DNA barcoding
The studied specimens had ML 78–96 mm, TL 242–399 mm and weighed 1252–2950 g with 1.5–3.6-fold weight shrinkage due to fixation (Online Resource 1). The semi-gelatinous consistency of the body increased in the defrosted specimen compared to the fixed ones. The only live specimen (GRL_1) was of a brick-orange colour, turning reddish and further to brown and oral surface of the web to dark violet due to fixation (Fig. 2). No pigment-free spots were found on the skin, which was smooth.
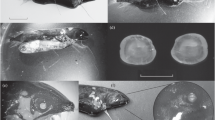
External view of Opisthoteuthis borealis; a–d Specimen HM-2019-4-16: dorsal (a), ventral (b), aboral (c) and oral (d) views; e, f Specimen PA-2017-7-68: aboral (e) and oral (f) views; g, h Specimen PA-2015-6-44: dorsal (g) and ventral (h) views; i, j Specimen NI-21: aboral (i) and oral (j) views. Legends: aI–aIV first–forth arm pair, df distal enlarged sucker field, e eye, fi fin, fu funnel, m mouth, pf proximal enlarged sucker field. Scale bars = 20 mm
The mantle in our specimens was relatively longer than previously known, i.e. 22–32% TL (cf. Table 1 and references therein). The head was wider than the mantle, with large eyes that occupied the whole side of the head (Fig. 2; Online Resource 1). The funnel was long and darker than the rest of the body (Fig. 2; Online Resource 1), with an indiscernible funnel organ inside. The fins were postero-lateral in location on the mantle and of moderate size (Online Resource 1); those on the Icelandic specimen being longer than previously known, at 86% ML (cf. Collins 2005).
Arms were relatively shorter than previously known (cf. Collins 2005), of subequal length in specimens GRL_2 and GRL_3 (Online Resource 1). The first pair was clearly the longest, and the fourth or the second and fourth were the shortest in specimens GRL_1 and ICE_1, respectively (Online Resource 1). The first pair in males was mean 5.6% ML thicker than the next thickest pair. There were no web supports present on the arms (Fig. 2). Web depth reached 1/3–2/3 arm length. Web formula matched the species description (Collins 2005) in specimen GRL_1 and differed in the rest, with the web always being more developed from the dorsal side (Online Resource 1). Arm sucker count was 76–86 (Online Resource 1). Two fields of enlarged suckers existed on all arms of every specimen studied (Fig. 2). The proximal field comprised 5–8 enlarged suckers, located between suckers 4–11, with the largest suckers being between suckers 5–8 (Online Resource 1). The distal field comprised 9–15 enlarged suckers, located between suckers 23–38, with the largest suckers being between suckers 26–32 (Online Resource 1). The largest suckers were always in the proximal field and on all arms except the first pair (Fig. 2). The first cirrus was located between suckers 2 and 3 or suckers 3 and 4 (Online Resource 1). Cirri were relatively short (Fig. 2; Online Resource 1).
The third left arm in specimen GRL_3 was bifurcated after sucker 41, at 108 mm length (Online Resource 3k–m). The main appendage of the bifurcated arm had 39 suckers and a length of 42 mm. The secondary appendage had 36 suckers and a length of 32 mm.
Pallial aperture, olfactory organs and the majority of internal characters coincided with the species description (Collins 2005), but a few things needed to be specified. The gills had seven lamellae in all specimens. The beaks clearly showed signs of wear from the smaller pre-mature specimen to the large mature ones (Online Resource 3g–j). The broad U-shaped shells were in different states of distortion (Online Resource 3a–f), and the shell from specimen GRL_1 was the one with a (relatively) undamaged natural form (Online Resource 3a, b).
The slightly oval, almost rounded testis was attached to the sperm duct in the manner typical for cirrates (Online Resource 4a–e). Among the proximal parts of spermatophoric complex, the sperm duct and part III were the longest (Online Resource 2). Among the accessory glands, gland 2 was the largest and gland 1 the smallest (Online Resource 2). The spermatophoric sac was clearly presented, visible from the dorsal side of the spermatophoric complex (Online Resource 4b, d). The terminal organ was pigmented in all the specimens studied, and of either reddish or light brownish colour (Online Resource 4a–e). The spermatophores were kept in the spermatophoric complex part III, the distal part of which had a wide lumen that occupied almost its entire volume, with thin glandular walls, and in the spermatophoric sac (Online Resource 4a, b, e). Spermatophore length was 0.8–2.1 mm. Each spermatophore was surrounded by a transparent gelatinous covering, and in the fixed or defrosted specimens, this gelatinous covering united into a continuous gelatinous mass occupying the whole volume of the respective part of the spermatophoric complex. Under the transparent gelatinous covering, the spermatophores were oval in shape, white with greenish metallic iridescence, this being seen only under stereomicroscopy (Online Resource 4f, g). Relatively short thin tapering ends were present on both sides of each spermatophore (Online Resource 4f, g). No pores existed on the surface of spermatophores (Online Resource 4g, h). The spermatophore membrane had rough surface under SEM (Online Resource 4h–j); its mean width was 9.9 μm. The spermatozoa were oriented with their heads towards the membrane and their tails towards the centre of the spermatophore (Online Resource 4j, k). The spermatozoa had thin and elongated nuclei with axially located flagella (Online Resource 4k); the mean nucleus length was 10.7 μm.
The COI sequence of O. borealis generated in this study was deposited in GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under accession number LC573902. There were no sequences of O. borealis in the GenBank or BOLD (https://www.boldsystems.org/) databases prior to our study. Analyses of COI sequences of Opisthoteuthis available from these databases showed O. borealis was highly supported as a species, being different from other Opisthoteuthis based on K2P model distances (Fig. 3).
COI molecular taxonomic cladogram including all species of Cirrata with available COI sequence in open access in BOLD (https://www.boldsystems.org/) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/) databases. Based on the Kimura’s two-parameter substitution model, rooted using Vampyroteuthis infernalis as the outgroup. Our sequence of O. borealis (specimen HM-2019-4-16) is in red. GenBank accession numbers are shown for all the specimens, except for Opisthoteuthis sp. (AAW9280), which is absent from GenBank: BOLD ID given instead. aGrimpoteuthis glacialis is a synonym of Cirroctopus glacialis (Collins and Villanueva 2006)
Reproductive biology
The testis was 2.3 times heavier than the spermatophoric complex in the pre-mature male, and in the mature males, the testis was 0.8–1.4 times the weight of the spermatophoric complex (Online Resource 2). In the pre-mature male, there were no spermatophores present in the spermatophoric complex part III, and only two spermatophores were present in the spermatophoric sac. In the mature males, there were 84–158 spermatophores: 49–110 in the spermatophoric complex part III and 25–61 in the spermatophoric sac (Online Resource 2).
Ontogenetic increase of spermatophore length was found: the spermatophores located in spermatophoric complex part III, i.e., those produced later during ontogenesis, were significantly longer than the spermatophores located in spermatophoric sac, i.e., those produced earlier in ontogenesis, in all the mature specimens studied (Online Resource 2). The ontogenetic increase varied from 13.3 to 15.4% spermatophore length among the specimens.
Trophic ecology
Stomach fullness varied from 1 to 4 points of the scale. Crustacean and polychaete remains, unidentifiable digested organic material and sand were found in all the stomachs studied. Crustaceans were isopods (one specimen in each GRL_3 and ICE_1), copepods (one specimen in GRL_3) or unidentifiable fragments (GRL_1, GRL_2 and ICE_1). Crustacean length was never greater than 12 mm, i.e., 14.0–15.4% ML of the respective specimens of O. borealis. Many chaetae were found in all stomachs studied, and jaws of Polynoidae (Polychaeta) were found in all specimens except GRL_1. One, two and three sets of jaws were found in specimens ICE_1, GRL_2 and GRL_3, respectively. The length of polychaetes was back-calculated from their jaw length using the equation from Abdullina et al. (2015): it was 15.0–24.8 mm (19.2–31.9% ML) in the pre-mature specimen and 35.7–37.7 mm (41.5–45.9% ML) in the mature ones.
Discussion
Our findings expand the hitherto-known geographical range (Fig. 1), as well as depth and temperature ranges (i.e., 878–1321 m and 3.0–3.6 °C, respectively) of O. borealis (cf. Collins 2005). Still, the majority of available specimens, i.e., 11 out of 13, were collected near the type locality in West Greenland at depths below 1000 m (Fig. 1), whereas two specimens were caught above 1000 m near Iceland.
All the specimens studied largely fitted the type description of O. borealis (cf. Collins 2005). All specimens were male and were larger than the maximum known ML (cf. Collins 2005), and some counts (i.e., number of suckers, overall and enlarged) and measurements (i.e., fins and cirri length) were increased (Table 1; Online Resource 1). Three more species of Opisthoteuthis inhabit the North Atlantic: O. calypso, and O. grimaldii and O. massyae, but these have a more southerly distribution (Villanueva et al. 2002). It is currently unknown whether they reach the distributional range of O. borealis. Only mature males of the genus Opisthoteuthis can be identified with reasonable accuracy (Villanueva et al. 2002; Collins 2005; Collins et al. 2010); moreover, cirrates in general are prone to damage during catching, fixation and storage (Vecchione and Collins 2002; Collins and Villanueva 2006). To ease the identification of the Atlantic species of Opisthoteuthis in the future, we provided the COI sequence (i.e., DNA barcode), selected the morphological characters to be used for species identification, and updated diagnostic characters for O. borealis (Table 1). Increased thickness of the first arm pair in mature males, previously described as ‘absent/slight/marked’ (Villanueva et al. 2002; Collins 2005), is one of the morphological identification features of Opisthoteuthis. It is expressed as differences in relative thickness (to ML, %) between the first arm pair and the next thickest arm pair for the Atlantic species (Table 1). Increased arm thickness has not before been found in O. agassizii, O. calypso and O. grimaldii: the mean difference between the first and the next thickest arm pair was 1.4–2.9% ML in these species (based on available data from Villanueva et al. 2002). Species with ‘slight’ arm thickness (O. borealis and O. hardyi) have 7.0–8.8% ML (our data and data from Villanueva et al. 2002, Collins 2005 and Collins et al. 2010), and species with ‘marked’ arm thickness (O. massyae) have 11.9% ML (data from Villanueva et al. 2002) (Table 1). Overall, phylogenetic relationships among the species of Opisthoteuthis worldwide, and even among Atlantic species, are poorly known. All species of Cirrata with a COI sequence available are included in Fig. 3; the resulting tree is congruent with a current conservative morphological phylogenetic hypothesis (Hochberg et al. 2013; Vecchione et al. 2016). However, we note that the COI sequence analysed by K2P is not unproblematic concerning the resolution of species included (Srivathsan and Meier 2012) with more taxa and DNA characters needed at this point.
Opisthoteuthis borealis is the second largest of the Atlantic Opisthoteuthis species in terms of ML and TL, exceeded only by O. calypso (Villanueva and Guerra 1991; Villanueva 1992; Villanueva et al. 2002). In terms of observed weights, it is smaller than both O. massyae and O. calypso (Boyle et al. 1998; Villanueva and Guerra 1991; Villanueva 1992; Villanueva et al. 2002). Arm bifurcation, well known in incirrate octopods (reviews: Toll and Binger 1991; Imperadore and Fiorito 2018), is reported in cirrates for the first time. Arm bifurcation has been related to regeneration processes (Toll and Binger 1991; Imperadore and Fiorito 2018), and our finding strongly suggests well-developed regeneration is present even in this relatively primitive and ancient taxon of cephalopods.
The reproductive system in O. borealis has a lower relative weight than that of incirrates, as previously reported for O. grimaldii (Daly et al. 1998; Boyle and Daly 2000). The proximal parts of the spermatophoric complex in cirrates have long been considered homologous with the respective parts in incirrates (Ebersbach 1915). Based on that assumption, it seems logical to consider these parts as spermatophoric complex parts I–III, following Nigmatullin et al. (2003) and Sabirov (2010), to unify the terminology of homologous parts of the spermatophoric complex. However, these parts have been referred to as ‘seminal vesicles’ I–III, following Meyer (1906) (cf. Ebersbach 1915; Aldred et al. 1983; Voss and Pearcy 1990; Guerra et al. 1998; Villanueva et al. 2002; Collins 2003, 2005; Collins et al. 2010) or spermatophore glands I–III (Young et al. 2001). There is confusion in the literature regarding the number and order of the accessory glands in the spermatophoric complex of cirrates (Meyer 1906; Ebersbach 1915; Voss and Pearcy 1990; Young et al. 2001; Villanueva et al. 2002; Collins 2003, 2005; Collins et al. 2010). In Opisthoteuthis, they are usually referred to as accessory glands 1–3 (Villanueva et al. 2002; Collins 2005; Collins et al. 2010). Other ordering of the accessory glands has been proposed (Meyer 1906; Ebersbach 1915; Young et al. 2001) where glands 1 and 3 are sometimes considered a single bilobed gland (Meyer 1906). The spermatophoric sac, which was largely overlooked in the studies of Opisthoteuthidae describing their male reproductive system (cf. Villanueva 1992; Villanueva et al. 2002; Collins 2003, 2005; Cuccu et al. 2009; Collins et al. 2010), was found in all the specimens studied. This was also reported in older studies on Opisthoteuthis (Meyer 1906), as well as in treatments of other cirrates (Voss and Pearcy 1990; Young et al. 2001).
The number of spermatophores found in our studied specimens (2–158) corresponds to other studied Opisthoteuthis (2–172) (Villanueva 1992; Villanueva et al. 2002), as well as to other cirrates (100–196) (Guerra et al. 1998; Collins and Henriques 2000). In two of the three specimens studied, more spermatophores were present in the spermatophoric complex part III than in the spermatophoric sac. Spermatophore length in O. borealis (0.8–2.1 mm) was also within known limits for cirrates (Villanueva 1992; Guerra et al. 1998; Collins and Henriques 2000; Villanueva et al. 2002; Collins 2005; Cuccu et al. 2009; Collins et al. 2010). Ontogenetic increase of spermatophore length, i.e., when the spermatophores produced later during ontogenesis are larger than those produced earlier, is reported in cirrates for the first time. Similar ontogenetic increase is known in squids and bobtail squids (Sabirov 1995; Nigmatullin et al. 2003; Hoving et al. 2010; Golikov 2015; Golikov et al. 2013, 2019). The presence of the same pattern in cirrates can suggest that the ontogenetic increase in spermatophore length may have been present in the ancestral forms of coleoids.
No additional structures or pores were found in the spermatophores (cf. O. calypso and O. massyae in Villanueva 1992). The membrane of the spermatophore in O. borealis was much thinner (mean 9.9 μm), than in O. calypso (45 μm) and Cirrothauma murrayi (23 μm) (Aldred et al. 1983; Villanueva 1992). The spermatozoa with long tail and thin elongated nucleus resemble those in O. persephone (Healy 1993), Stauroteuthis syrtensis, S. gilchristi (Collins and Henriques 2000) and C. murrayi (Aldred et al. 1983): cf. O. calypso with a thick oval nucleus and O. massyae with a very small one (Villanueva 1992). The nucleus length in spermatozoa in O. borealis (mean 10.7 μm) was close to those of S. syrtensis and S. gilchristi (Collins and Henriques 2000), shorter than in O. persephone (Healy 1993) and C. murrayi (Aldred et al. 1983), and longer than in O. calypso and O. massyae (Villanueva 1992).
The stomachs in all the specimens studied were at least one-third full, suggesting O. borealis continues to feed and grow after reaching maturity, which has been suggested for other cirrates (Collins and Villanueva 2006). The genus Opisthoteuthis, unlike other cirrates, has a relatively well-known food spectrum dominated mostly by crustaceans and sometimes by polychaetes (review: Collins and Villanueva 2006). Polychaetes dominated the stomach contents of the studied specimens of O. borealis according to size of food items (thus weight) and sometimes to number. Contrary to the assumption that cirrates prey upon relatively small prey only (Collins and Villanueva 2006), large mature males of O. borealis consume polychaetes reaching 41.5–45.9% ML of the specimens.
In summary, we studied four specimens of O. borealis, the only specimens known since the type description of the species. All were larger than the previously known maximum size. The study (1) expanded the species’ known geographical, depth and temperature ranges; (2) provided a COI sequence (i.e., DNA barcode) and expanded the morphological description of the species; (3) prepared a summary table (Table 1) which can be used to identify the Atlantic representatives of the genus Opisthotheuthis based on updated morphological characters; (4) described the species’ reproductive biology and trophic ecology, which were previously unknown; and (5) reported arm bifurcation and ontogenetic increase of spermatophore length for the first time in Cirrata.
References
Abdullina LI, Gabidullina RI, Golikov AV, Sabirov RM (2015) Age structure of Lepidonotus squamatus and Harmothoe imbricata (Polychaeta, Polynoidae) population in sublittoral kelp ecosystems of Keret Archipelago. In: Golubkov SM (ed) Abstracts of 5-th International Scientific «Dynamics and functioning of aquatic ecosystems under the impact of climate change and antropogenic stress». Lema, St. Peterburg, pp 5–6
Aldred RG, Nixon M, Young JZ (1983) Cirrothauma murrayi Chun, a finned octopod. Philos T Roy Soc B 301:1–54
Boyle PR, Daly HI (2000) Fecundity and spawning in a deep-water cirromorph octopus. Mar Biol 137:317–324
Boyle PR, Collins MA, Williamson GR (1998) The cephalopod by-catch of deep-water trawling on the Hebrides slope. J Mar Biol Assoc UK 78:1023–1026
Breiby A, Jobling M (1985) Predatory role of the flying squid (Todarodes sagittatus) in north Norwegian waters. NW Atl Fish Organ Sci Coun Stud 9:125–132
Clarke MR (1962) The identification of cephalopod “beaks” and the relationship between beak size and total body weight. Bull Br Museum (Natural History) 8:419–480
Clarke MR (1996) Cephalopods as prey. III. Cetaceans. Philos T Roy Soc B 351:1053–1065
Collins MA (2003) The genus Grimpoteuthis (Octopoda: Grimpoteuthidae) in the north-east Atlantic, with descriptions of three new species. Zool J Linnean Soc 139:93–127
Collins MA (2005) Opisthoteuthis borealis: a new species of cirrate octopod from Greenland waters. J Mar Biol Assoc UK 85:1475–1479
Collins MA, Henriques C (2000) A revision of the family Stauroteuthidae (Octopoda: Cirrata) with redescriptions of Stauroteuthis syrtensis and S. gilchristi. J Mar Biol Assoc UK 80:685–697
Collins MA, Villanueva R (2006) Taxonomy, ecology and behaviour of the cirrate octopods. Oceanogr Mar Biol 44:277–322
Collins MA, O’Dea M, Henriques C (2001) A large Cirroteuthis magna (Cephalopoda: Cirrata) caught on the Cape Verde Terrace (North Atlantic). J Mar Biol Assoc UK 81:357–358
Collins MA, Laptikhovsky V, Strugnell JM (2010) Expanded description of Opisthoteuthis hardyi based on new specimens from the Patagonian slope. J Mar Biol Assoc UK 90:605–611
Cuccu D, Mereu M, Cannas R, Follesa MC, Cau A, Jereb P (2009) Morphology, biology and molecular characterizations of Opisthoteuthis calypso (Cephalopoda: Octopoda) from the Sardinian Channel (central western Mediterranean). J Mar Biol Assoc UK 89:1709–1715
Daly HI, Boyle PR, Collins MA (1998) Reproductive status of Opisthoteuthis sp. over an annual cycle. S Afr J Mar Sci 20:187–192
Ebersbach A (1915) Zur Anatomie von Cirroteuthis umbellata Fischer und Stauroteuthis sp. Zeitschrift. Z Wiss Zool 113:361–483
Fiorito G, Affuso A, Basil J, Cole A, de Girolamo P, D’Angelo L, Dickel L, Gestal C, Grasso F, Kuba M, Mark F, Melillo D, Osorio D, Perkins K, Ponte G, Shashar N, Smith D, Smith J, Andrews P (2015) Guidelines for the Care and Welfare of Cephalopods in Research–a consensus based on an initiative by CephRes, FELASA and the Boyd Group. Lab Anim 49(S2):1–90
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Golikov AV (2015) Distribution and reproductive biology of ten-armed cephalopods (Sepiolida, Teuthida) in the Barents Sea and adjacent areas. PhD dissertation, Moscow State University [in Russian]
Golikov AV, Morov AR, Sabirov RM, Lubin PA, Jørgensen LL (2013) Functional morphology of reproductive system of Rossia palpebrosa (Cephalopoda, Sepiolida) in Barents Sea. Proc Kazan Univ Nat Sci Ser 155:116–129 [in Russian with English abstract]
Golikov AV, Blicher ME, Jørgensen LL, Walkusz W, Zakharov DV, Zimina OL, Sabirov RM (2019) Reproductive biology and ecology of the boreoatlantic armhook squid Gonatus fabricii (Cephalopoda: Gonatidae). J Molluscan Stud 85:287–299
Grimpe G (1920) Teuthologische Mitteilungen V. Zwei neue Cirraten-Arten. Zool Anz 51:230–243
Guerra A, Villanueva R, Nesis KN, Bedoya J (1998) Redescription of the deep-sea cirrate octopod Cirroteuthis magna Hoyle 1885, and considerations on the genus Cirroteuthis (Mollusca: Cephalopoda). Bull Mar Sci 63:51–81
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Paleontol Electron 4:1–9
Healy JM (1993) Sperm and spermiogenesis in Opisthoteuthis persephone (Octopoda: Cirrata): ultrastructure, comparison with other cephalopods and evolutionary significance. J Molluscan Stud 59:105–115
Hochberg FG, Norman MD, Finn JK (2013) 2.2 Cirrate octopods. In: Jereb P, Roper CFE, Norman MD, Finn JK (eds) FAO Species Catalogue for Fishery Purposes, №4: Cephalopods of the world. An annotated and illustrated catalogue of species known to date. Vol. 3. Octopods and Vampire Squids. FAO, Rome, pp. 244–267
Hoving HJT, Lipinski MR, Dam L (2010) The male reproductive strategy of a deep-sea squid: sperm allocation, continuous production, and long-term storage of spermatophores in Histioteuthis miranda. ICES J Mar Sci 67:1478–1486
Hoving HJT, Perez JAA, Bolstad KSR, Braid HE, Evans AB, Fuchs D, Judkins H, Kelly JT, Marian JEAR, Nakajima R, Piatkowski U, Reid A, Vecchione M, Xavier JCC (2014) The study of deep-sea cephalopods. Adv Mar Biol 67:235–359
Imperadore P, Fiorito G (2018) Cephalopod tissue regeneration: consolidating over a century of knowledge. Front Physiol 9:593
Jamieson AJ, Vecchione M (2020) First in situ observation of Cephalopoda at hadal depths (Octopoda: Opisthoteuthidae: Grimpoteuthis sp.). Mar Biol 167:82
Joubin L (1903) Sur quelques céphalopodes recueillis pendant les dernieres campagnes de S.A.S. le Prince de Monaco (1901–1902). Compte Rendus des Seances de l’Academic des Sciences 136:100–102
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Klages NTW (1996) Cephalopods as prey. II. Seals. Philos T Roy Soc B 351:1045–1052
Lindgren AR, Pankey MS, Hochberg FG, Oakley TH (2012) A multi-gene phylogeny of Cephalopoda supports convergent morphological evolution in association with multiple habitat shifts in the marine environment. BMC Evol Biol 12:129
Meyer WT (1906) Die Anatomie von Opisthoteuthis depressa (Ijima und Ikeda). Zeitschrift. Z Wiss Zool 85:183–269
Nigmatullin CM, Sabirov RM, Zalygalin VP (2003) Ontogenetic aspects of morphology, size, structure and production of spermatophores in ommastrephid squids: an overview. Berliner paläobiol Abh 3:225–240
Piertney SB, Huedelot C, Hochberg FG, Collins MA (2003) Phylogenetic relationships among cirrate octopods (Mollusca: Cephalopoda) resolved using mitochondrial 16S ribosomal DNA sequences. Mol Phylogenet Evol 27:348–353
Ramirez-Llodra E, Brandt A, Danovaro R, De Mol B, Escobar E, German CR, Levin LA, Martinez Arbizu P, Menot L, Buhl-Mortensen P, Narayanaswamy BE, Smith CR, Tittensor DP, Tyler PA, Vanreusel A, Vecchione M (2010) Deep, diverse and definitely different: unique attributes of the world’s largest ecosystem. Biogeosciences 7:2851–2899
Robison BH, Sherlock RE, Reisenbichler KR (2010) The bathypelagic community of Monterrey Canyon. Deep-Sea Res II 57:1551–1556
Rodhouse PG, Nigmatullin CM (1996) Role as consumers. Philos T Roy Soc B 351:1003–1022
Roper CFE, Voss GL (1983) Guidelines for taxonomic descriptions of cephalopod species. Mem Natl Museum Victoria 44:48–63
Sabirov RM (1995) Spermatophorogenesis and reproductive strategy in males of ommastrephid squids (Oegopsida: Ommastrephidae). PhD dissertation, Institute of Evolutionary Morphology [in Russian]
Sabirov RM (2010) Reproductive system in males of Cephalopoda. III. Spermatophores. Proc Kazan Univ Nat Sci Ser 152:8–21 [in Russian with English abstract]
Smale MJ (1996) Cephalopods as prey. IV. Fishes. Philos T Roy Soc B 351:1067–1081
Srivathsan A, Meier R (2012) On the inappropriate use of Kimura-2-parameter (K2P) divergences in the DNA-barcoding literature. Cladistics 28:190–194
Tanner AR, Fuchs D, Winkelmann IE, Gilbert MTP, Pankey MS, Ribeiro AM, Kocot KM, Halanych KM, Oakley TH, da Fonseca RR, Pisani D, Vinther J (2017) Molecular clocks indicate turnover and diversification of modern coleoid cephalopods during the Mesozoic Marine Revolution. P R Soc B 284:20162818
Toll RB, Binger LC (1991) Arm anomalies: cases of supernumerary development and bilateral agenesis of arm pairs in Octopoda (Mollusca, Cephalopoda). Zoomorphology 110:313–316
Vecchione M, Collins MA (2002) Systematics, ecology and biology of cirrate octopods: workshop report. Bull Mar Sci 71:79–96
Vecchione M, Mangold KM, Young RE (2016) (1922–2003). Cirrata Grimpe, 1916. Version 27 February 2016 (under construction). http://tolweb.org/Cirrata/20086/2016.02.27 in The Tree of Life Web Project (last accessed 13-Jul-2020)
Verrill AE (1883) Supplementary report on the “Blake” cephalopods. Bull Mus Comp Zool 11:105–115
Villanueva R (1992) Continuous spawning in the cirrate octopods Opisthoteuthis agassizii and O. vossi: features of sexual maturation defining a reproductive strategy in cephalopods. Mar Biol 114:265–275
Villanueva R, Guerra A (1991) Food and prey detection in two deep-sea cephalopods: Opisthoteuthis agassizii and O. vossi (Octopoda: Cirrata). Bull Mar Sci 49:288–299
Villanueva R, Collins MA, Sanchez P, Voss NA (2002) Systematics, distribution and biology of the cirrate octopods of the genus Opisthoteuthis (Mollusca, Cephalopoda) in the Atlantic Ocean, with description of two new species. Bull Mar Sci 71:933–985
Voss GL (1988) Evolution and phylogenetic relationships of deep-sea octopods (Cirrata and Incirrata). In: Clarke MR, Trueman ER (eds) The Mollusca, Volume 12, Paleontology and Neontology of Cephalopoda. Academic Press, San Diego, pp. 253–276
Voss GL, Pearcy WG (1990) Deep-water octopods (Mollusca: Cephalopoda) of the northeastern Pacific. Proc Calif Acad Sci 4th series 47:47–94
Ward RD, Zemlak TS, Innes BH, Last PR, Hebert PD (2005) DNA barcoding Australia’s fish species. Philos T Roy Soc B 360:1847–1857
Young RE, Vecchione M, Donovan DT (1998) The evolution of coleoid cephalopods and their present biodiversity and ecology. S Afr J Mar Sci 20:393–420
Young RE, Vecchione M, Mangold KM (2001) (1922–2003). Cirrate male reproductive tract. http://tolweb.org/accessory/Cirrate_Male_Reproductive_Tract?acc_id=1486 in The Tree of Life Web Project (last accessed 13-Jul-2020)
Acknowledgements
We are grateful to ‘Initiating North Atlantic Benthos Monitoring (INAMon)’ project and BIOICE programme for providing parts of the samples, to the scientific groups and crews of the mentioned vessels, to T. P. Satoh (The Kyoto University Museum) for help with molecular works. INAMon was financially supported by the Greenland Institute of Natural Resources, North Atlantic Cooperation (nora.fo; J. nr. 510-151), Sustainable Fisheries Greenland, the Ministry for Research in Greenland (IKIIN) and the Environmental Protection Agency (Dancea) of the Ministry of Environment and Food of Denmark (J. nr. mst-112-00272). This research is also part of the Danish Presidency project in Nordic Council of Ministers, mapping seabed biodiversity and vulnerability in the Arctic and North Atlantic. We thank two anonymous reviewers of the manuscript and the editor Dr. Mike Vecchione for their valuable comments and suggestions to improve the quality of the paper.
Funding
This study is part of Initiating North Atlantic Benthos Monitoring, INAMon, which was financially supported by the Greenland Institute of Natural Resources, North Atlantic Cooperation (nora.fo; J. nr. 510-151), Sustainable Fisheries Greenland, the Ministry for Research in Greenland (IKIIN) and the Environmental Protection Agency (Dancea) of the Ministry of Environment and Food of Denmark (J. nr. mst-112-00272). This research is also part of the Danish Presidency project in Nordic Council of Ministers, mapping seabed biodiversity and vulnerability in the Arctic and North Atlantic. A.V.G. and G.G. acknowledge the financial support received from the BIOICE programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national and/or institutional guidelines for animal testing, animal care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgments, if applicable.
Data availability
All relevant data are included in the paper and/or in the supplementary information. Specimens are kept in the Greenland Institute of Natural Resources (PA-2017-7-68 and HM-2019-4-16) and the Icelandic Institute of Natural History (NI-21). A sequence of COI is openly accessible from GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under accession number LC573902.
Author contributions
A.V.G. and R.M.S. designed the study; A.V.G., M.E.B., G.G., I.E.M. and D.V.Z. collected or provided the samples; A.V.G. and R.M.S. analysed the samples and respective data; J.Y.P. did molecular genetics part of the work; A.V.G., J.Y.P. and R.M.S. wrote the first draft of the paper; all authors were involved in interpreting the results and contributed to the final draft of the paper.
Additional information
Communicated by M. Vecchione
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 944 kb)
Rights and permissions
About this article
Cite this article
Golikov, A.V., Blicher, M.E., Gudmundsson, G. et al. Flapjack devilfish in the northern North Atlantic: morphology, biology and ecology of Opisthoteuthis borealis (Cephalopoda, Octopoda, Cirrata). Mar. Biodivers. 50, 108 (2020). https://doi.org/10.1007/s12526-020-01138-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-020-01138-9