Abstract
The relative abundance of the genus Siderastrea and its relationship with temperature and irradiance was assessed around Sal Island (Cape Verde). In some of the surveyed sites, these corals accounted for 80–90 % of the living cover, making it a biotope-dominant organism. Unlike Siderastrea corals from West Atlantic and Caribbean locations, genetic analyses of the dinoflagellate symbiotic partner revealed high specificity between Siderastrea sp. in Cape Verde and the Symbiodinium type C46. Biotope restriction of the ecological success of Siderastrea in Cape Verde may be explained in part by this host–symbiont partnership, resulting locally in a small optimum ecological niche with specific light intensity regimes. Distinctively, West Atlantic and Caribbean Siderastrea associates with a much broader range of Symbiodinium diversity, suggesting that these symbioses exhibit some flexibility under differing environmental conditions where these corals occupy a wider range of ecological niches. Geographic isolation and/or long-standing environmental conditions are probably responsible for such adaptions and coral–dinoflagellate symbioses. Additional genetic analyses on Clade C Symbiodinium associated with Siderastrea were conducted with the hyper-variable plastid psbA minicircle to resolve phylogeographic patterns that indicate the relative connectivity and/or isolation of these symbionts throughout the tropical Atlantic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reef-building photosymbiotic scleractinians rely on endosymbiotic dinoflagellates (genus Symbiodinium) to meet their energy requirements and to enhance calcification rates and skeletal growth (Hallock 1981; Muscatine et al. 1981; Muller-Parker and D'Elia 1997; Hoegh-Guldberg 2004; Stanley 2006). The successful construction of massive coral reef structures greatly depends on rapid calcification (Veron and Stafford-Smith 2000) in corals, which is attributed to the photosynthesis of the endosymbiont partner (Pearse and Muscatine 1971; Muscatine et al. 1981; Stanley 2006; Colombo-Pallotta et al. 2010).
Advances in molecular tools and techniques and the generalization of the use of genetic markers have revealed that the genus Symbiodinium is much more diverse than early researchers had ascertained using morphology. The lack of diagnostic morphological traits misled early studies to identify most symbiotic dinoflagellates as a single pandemic species, Symbiodinium microadriaticum Freudenthal, 1962 (Trench 1993; Stat et al. 2006), and still hampers species recognition and validation. An interim taxonomy of the groups based on genetic evidence uses alphanumeric designations to classify Symbiodinium into evolutionarily distinct, ecologically functional entities (LaJeunesse 2002; Pochon et al. 2004; Rodriguez-Lanetty et al. 2004; Sampayo et al. 2007, 2009; LaJeunesse et al. 2010). Grouped into distinct phylogenetic “clades” (e.g., A, B, C, etc.), nucleotide sequence phylogenies based on rDNA internal transcribed spacers 1 and 2, plastid genes, and microsatellite flanker regions identify numerous lineages well below the “clade” level, corresponding to operational taxonomic units (OTUs) (Sampayo et al. 2009), and exhibiting unique ecological and biogeographic distributions (Baillie et al. 2000; LaJeunesse 2002; Sampayo et al. 2007; LaJeunesse et al. 2010).
The study of these obligate symbioses over large geographic scales can provide important data regarding how isolation, environment, and host biology have influenced their coevolution (LaJeunesse 2005; Finney et al. 2010) and also reveal the variation and/or persistence of particular partner combinations from region to region. Such studies also provide insight into mechanisms underlying the hosts’ resilience and ecological restrictions to their distribution and abundance. Furthermore, the study of coral–dinoflagellate associations and diversity are crucial to assess the possibility that partner recombination, involving changes in host–symbiont specificity, offer a mechanism for rapid physiological adjustment to changing environmental conditions (Baker et al. 2004; Berkelmans and van Oppen 2006).
Atlantic siderastreids (Siderastreidae: Siderastrea) can be major reef builders (Foster 1980; Oigman-Pszczol and Creed 2004; Castillo et al. 2011) and are the primary contributor to reef frameworks found in Cape Verde (Moses et al. 2003; Monteiro et al. 2009). They have proven to be remarkably resilient and resistant to a number of stress factors such as low salinity, sediment deposition, and burial (Lewis 1989; Lirman et al. 2002; Lirman and Manzello 2009).
Considering the importance of the coral–dinoflagellate associations, it is likely that the diversity of Symbiodinium associating with Siderastrea is, at least partially, responsible for their resilience and resistance to stressors. Atlantic Siderastrea species are known to associate with different Symbiodinium partners (LaJeunesse 2002; Thornhill et al. 2006a, b; Costa et al. 2008; Finney et al. 2010), which may be linked to their geographic distributions and ecological abundance.
This study investigates the diversity of Symbiodinium associating with Siderastrea radians (Pallas, 1766) in Cape Verde, the most abundant scleractinian coral in the archipelago, and explores the ecological and phylogeographic implications of such diversity. In Sal Island (Cape Verde), local environmental conditions such as depth, temperature, and light exposure were monitored to determine if local factors influence relative abundance and distribution of S. radians and/or Symbiodinium sp. diversity. Finally, the diversity from Cape Verde is compared to associations found in siderastreids from Brazil (new data) and from locations in the Caribbean (comprising published and unpublished data). Biogeographic patterns and Symbiodinium diversity associated with Siderastrea spp. is discussed from the perspective of environmental conditions, geographic distance, potential for dispersal, and host species identity.
Methods
Cape Verde study sites and environmental monitoring
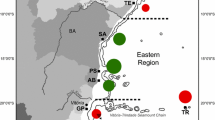
Conducted between April and December 2009, fieldwork was restricted to Sal Island (Cape Verde), where well-established coral communities, over a rocky (basaltic) reef and sedimentary terraces (hereafter referred to as ‘bedrock’), had been previously reported (Laborel 1974; Morri and Bianchi 1995; Moses et al. 2003; Monteiro et al. 2009). In order to compare variability surrounding Sal, the island was divided into 10 sections by imposing a 7-km grid, and each section was assigned a code (Fig. 1). The two most northern sections (NW and NE) were not easily accessible and were not included in this study. At each remaining section, we have deployed an Onset HOBO-Pendant® Temperature/Light Data Logger at 5–6 m and at 10–11 m depth, to collect information on temperature and light intensity every 15 min. Every 15–20 days, data were downloaded and loggers were cleaned to avoid fouling. One additional logger was set up inland for surface light intensity monitoring where no structures would shade it and influence its readings. Assuming light intensity values of the logger set at the surface as 100 % of light intensity reaching the surface, one can calculate the average percentage of light reaching the several logger sets. This “relative light intensity” excludes daily and seasonal fluctuations and allows a better comparison between sites. The logger set deployed at section E3 was lost, which, together with the lack of coral cover in initial surveys, led to the exclusion of this section from the study.
On a larger geographical scale, remote sensing data were used to assess and compare broad environmental conditions across several locations where Siderastrea corals occur. Sea surface temperature (SST) and aerosol mass concentration time series were generated in Giovanni, a web-based application developed and maintained by the NASA GES DISC, that provides a simple and intuitive way to visualize, analyze, and access vast amounts of Earth science remote sensing data. Monthly SST data (11 μm/day) and aerosol mass concentration (μg.cm−2) between January 2001 and December 2009 was compiled for Cape Verde, south and north Brazil, and several other locations in the Greater Caribbean (where samples had been collected). For each location, compiled data was averaged by month and by year for comparison.
Siderastrea radians relative abundance assessment
Siderastrea radians relative abundance in Sal Island was assessed over hard nature, horizontal and near horizontal substratum, where reef-building corals occur, using photoquadrat surveys. Two depth categories were considered: 4–6 m and 10–12 m. In each of the seven remaining sections of the island (W1, W2, W3, SW, SE, E1, and E2), three randomly distributed survey dives were conducted for each depth category in locations where coral communities were present, making a total of 54 survey dives. Each survey had three sets of five photoquadrats taken (a total of 15 quadrats with >1 m2 each), using an underwater photography system (Nikon D80 with 10–20 mm lens inside an Aquatica aluminum housing), assembled on a custom-made “quadpod” structure, to assure constant distance and angle at which the images were captured. All quadrats were randomly placed, following a random number table (adapted from Wallenstein et al. 2008) and depth-tagged. All images were white balance-adjusted and labeled according to their sampling station. Compiled photoquadrat imagery was analyzed with Coral Point Count with Excel extensions (CPCe) software (Kohler and Gill 2006) using a custom-made list of categories and subcategories. This list compiled observed taxa plus other, necessary, non-biological categories/subcategories, such as ‘sand’, ‘pavement’ and ‘rubble’. Each photoquadrat was scaled (using the “quadpod” structure as reference) and had a total of 144 points overlaid in random-stratified manner—six random points over 24 (4 rows × 6 columns) equal cells (20,5 × 20,5 cm), over an area of 101 cm2. Taxa and other subcategories (e.g., sand, pavement) were assigned to each point over the CPCe interface. Random placements of points result in points overlaying the quadrat structure or shade areas where valid categorical taxon assignment was impossible. Non-valid categories were excluded from subsequent analysis. Live cover (%) and the relative abundance of S. radians (in relation to living coverage) was averaged and estimated for both depth categories in each section.
Symbiodinium diversity associating with Siderastrea
S. radians samples (n = 17) were collected from eastern and western sites surrounding Sal Island (Cape Verde) in 2007 and 2009. Corals were identified in the field and pivot samples were collected to confirm taxonomic identification following Veron and Stafford-Smith (2002). In Brazil, Siderastrea samples (n = 24) were collected in intertidal and subtidal zones ,by mask and snorkel and SCUBA in late November 2008, at several sites in the province of Paraíba (north) and in the province of Bahia (south). Brazilean siderastreids comprise S. stellata and S. radians, two closely related species, which can be morphologically very similar (Neves et al. 2008). Sampled corals were identified in the field and have been grouped under Siderastrea spp. (radians/stellata) due to their similarity and difficulty in making a reliable identification to species level.
Samples from small fragments of tissue from each individual were preserved in 20 % DMSO preservation buffer (Seutin et al. 1991) and stored at −20 °C (in Mueller Lab, Department of Biology, Pennsylvania State University, USA). Skeletal fragments (approximately 10–20 mm2 tissue) were mechanically disrupted using a Biospec Beadbeater (Bartlesville, OK, USA) for 1 min at maximum speed. Nucleic acids were then extracted using a modified Wizard DNA extraction protocol developed by Promega (Madison, WI, USA); see LaJeunesse et al. (2003, 2010) for protocol details. The ITS2 region was analyzed by denaturing gradient gel electrophoresis (DGGE) fingerprinting as previously described (LaJeunesse 2002; Sampayo et al. 2009; LaJeunesse et al. 2010). Amplified ITS2 fragments were separated on a CBScientific DGGE system (Del Mar, CA, USA) using 8 % poly-acrylamide gels (40:1 acrylamide/bis) with an internal gradient 45–80 % (formamide and urea were used as denaturants). Gels were electrophoresed at 110 V for 15–16 h, stained for 20 min with 1× SYBR Green (Molecular Probes, Eugene, OR, USA) in 1× TAE running buffer, and photographed using a Fotodyne (Hartland, WI, USA) imaging system. For identification and/or identity confirmation of a symbiont type, diagnostic bands from denaturing gels were excised, eluted, re-amplified using the non-GC reverse primer, and directly sequenced as described in LaJeunesse (2002). Chromatograms were visually inspected for accuracy in base calling (Sequence Navigator) and edited sequences aligned by eye. Symbiodinium ITS2 diversity associating with Siderastrea from Cape Verde and Brazil was compared with that found in other locations (described in LaJeunesse 2002; Thornhill et al. 2006a; Warner et al. 2006; Finney et al. 2010).
Fine-scale differences among Symbiodinium diversity associating with Sideastrea was assessed using sequences of the non-coding region of the psbA minicircle (psbA ncr), following the protocol and primers specified by Moore et al. (2003), and included archived samples from various hosts and regions in the Caribbean including Carry Bow Cay, Belize; Lee Stocking and Abaco, Bahamas; St Croix; Barbados, Curacao, and the Flower Garden Banks in the Gulf of Mexico (LaJeunesse 2002; Thornhill et al. 2006a, b; Finney et al. 2010; unpublished collections). All samples were sequenced using Big Dye 3.1 reagents (Applied Biosystems, USA) at the DNA Core Facility (Pennsylvania State University). Chromatograms were visually inspected for accuracy in base calling (Sequence Navigator) and the edited sequences aligned by eye.
Phylogenetic reconstructions using PAUP (v.4.0b10; Swofford 2000) under the criterion of Maximum Parsimony (MP) and Distance under default settings were generated using psbA ncr partial sequences. Insertions and deletions (indels) were analyzed as a 5th character state. All bootstrap values testing the statistical support of internal branches were calculated based on 1,000 replicates.
Results
Local environmental conditions
Local monitoring of temperature and light intensity was only possible in 7 of the 10 sections of Sal Island. Data loggers were unable to be deployed on the NE section, they were unable to be recovered on the NW section, and they disappeared from section E3. Light intensity (lux) and temperature (°C) data from the remaining sites were collected for 21 weeks, from July to December 2009. Light intensity for the daily period between 1030 and 1530 hours, excluding the night period and periods of low-angled light incidence, was compiled for each depth category at each section and averaged over each month (July–December). Temperature data were compiled and monthly averaged for each section for the full 21 weeks period (Fig. 2).
Monthly average of light intensity (top), relative light intensity (middle) and water temperature (bottom) for the period July–December in sections W1, W2, W3, SW, SE, E1 and E2 of Sal Island. Top monthly average light intensity (lux) and standard deviation at depths of 5 and 10 m. Middle relative light intensity (5 and 10 m), percentage of light intensity at the surface. Bottom monthly average water temperature (°C) and standard deviation (columns), and maximum ( red line) and minimum (
red line) and minimum ( blue line) temperatures (averaged)
blue line) temperatures (averaged)
Comparison of monthly average light intensity between sections does not reveal a clear and obvious pattern or gradient in relation to N–S or E–W (Fig. 2). However, relative light intensity values suggest higher intensities on west sections (Fig. 2), and average light intensity of all west sections (over the 21 weeks) is slightly higher than that of the east sections (data not shown).
Water temperature data, collected over the 21-week period, revealed no major differences between depth categories, with differences between depth categories only ranging between 0.0 and 0.2 °C. Average, maximum, and minimum temperature data reveals northern sections of the island have slightly lower temperatures than southern sections (Fig. 2)
Environmental conditions: comparing geographic regions
Environmental conditions were not monitored across locations, allowing a broad geographical assessment. A comparison of different conditions over different geographic locations relied on remote sensing data. Compiled Sea Surface Temperature and Aerosol Mass Concentration time series from the “Giovanni online data system” (Acker and Leptoukh 2007), were averaged (yearly and monthly) for Cape Verde, Brazil (north and south) and several locations in the Greater Caribbean region (Fig. 3). Yearly averaged SST in Cape Verde is 1–3 °C lower than other locations; however, minimum average temperatures (February–March) are above minimum average temperatures from Florida Banks. Aerosol mass concentration (the total aerosols mass in a vertical column of atmosphere) has a proxy indicator for light intensity attenuation, assuming the effect of aerosols in dispersing and scattering light (in air) and the increase of water turbidity from aerosol fallout (Bohren and Huffmann 1983; Li et al. 1996). Peaking between July and August, Cape Verde yearly and monthly average values are consistently higher than other locations, probably due to its proximity to the Sahara desert and its seasonal dust plumes (Garrison et al. 2003).
Sea surface temperature: 11 μm day (a) and aerosol mass concentrations (b) regimes in Cape Verde (red lines) and other locations (black lines). Yearly and monthly averages (left and right, respectively) compiled from MTMOSST.001 using the Giovanni online data system (Acker and Leptoukh 2007)
Siderastrea radians relative abundance in Sal Is. (Cape Verde)
Photoquadrat surveys and imagery analysis results reveal different coral live coverage and S. radians relative abundances across the sections of Sal Island (Figs. 1, 4), with lower values in the eastern shoreline (windward). Siderastrea radians abundance in relation to the total coral live cover (Fig. 4) varies between 23 and 91 %. In some sites, S. radians colonies form a pavement morphology (similar to the one described in Moses et al. 2003), and are the dominating organism. No further statistical analysis was performed, however, comparing S. radians relative abundance and its variation between depth categories reveals no clear patterns. In some sections, S. radians is higher at −10 m and in other sectionsat −5 m. Higher abundances were observed in sections/depths where the average light intensity (between 10:30 and 15:30 hours) was between 5,500 and 6,500 lx (Fig. 5).
Siderastrea radians and living cover (left) and S. radians relative abundance (right) at −5 and −10 m over horizontal and near horizontal hard substratum in sections W1, W2, W3, SW, SE, E1, and E2 of Sal Island. Values estimated from imagery using CPCe (Kohler and Gill 2006), from 3 sets of 5 photoquadrats (1 m2 each)
Average light intensity versus Siderastrea radians relative abundance: average light intensity values estimated from 1030–1530 hours readings for the period July–December; relative abundance and quintic polynomial trendline estimated from photoquadrats at each dive survey site (see above for description)
Symbiodinium diversity associating with Siderastrea
All ITS2-DGGE fingerprints of Siderastrea radians samples collected in Cape Verde (n = 17) had the same single prominent band, typical of an association with a single, dominant, Symbiodinium type. Unlike Siderastrea corals from Brazil and the Great Caribbean, which can associate with different types of Symbodinium, all samples from Cape Verde associated with a single type: Symbiodinium C46.
In Brazil, Siderastrea samples were identified as Siderastrea sp. (radians/stellata). Sampled colonies associated with Symbiodinium B5, C3, C3 + B5, C46, C46 + B5 and C1 (the former only found in samples collected in tidal pools). There were some differences in the diversity of Symbiodinium found in the two regions of Brazil. Except for those in intertidal pools, Siderastrea colonies sampled in south Brazil associated with Symbiodinium C46. In Paraíba (north), colonies could also associate with C3, B5, and some of sampled colonies co-hosted different Symbiodinium types: C3 + B5 and C46 + B5. Such results reveals that: (1) C46 occurs in both East and West side of the Atlantic, and (2) that it can be found in Siderastrea corals co-hosting multiple symbiont types or as a single dominant type.
Previous studies (described in LaJeunesse 2002; Thornhill et al. 2006a; Warner et al. 2006; Finney et al. 2010) in several locations in the Caribbean also report associations of S. radians with C1, C3, C46, B5, and D1a. In fact, the analysis of the symbiosis displayed by S. radians in the Atlantic shows a complex “mosaic” of associations with different symbiont types (Fig. 6).
Geographic patterns of Symbiodinium types associated with the genus Siderastrea in regions throughout the Atlantic. The numbers of colonies sampled in each location are listed next to each pie graph. Some Siderastrea colonies in Brazil hosted combinations of Symbiodinium types (C3 + B5 in black and B5 + C46 in gray) The existence of a particular type may also depend on depth. For example, B5/B5a occur only in shallow water colonies (1–5 m)
Phylogenetic analyses
Because of its high mutational rate, the phylogeny based on the non-coding region of the psbA minicircle (psbA ncr) allows the exploration of the relationships of closely related species (Barbrook et al. 2006; LaJeunesse and Thornhill 2011). The psbA non-coding region sequences for type C46 (from Cape Verde and Brazil) and for type C3 (from Brazil and the Greater Caribbean) each form divergent monophyletic lineages (Fig. 7). The Symbiodinium C46 lineage comprises psbA haplotypes whose phylogenetic relationships suggest that populations in Brazil and Cape Verde may be intermixed, whereas C3 lineage exhibits substantial sequence divergence between Brazil and the Greater Caribbean locations (Fig. 6). Sequences of Symbiodinium samples from Cape Verde (C46) could not be aligned with sequences from other clades (A, B, and D) or with C1 sequences from Siderastrea hosts of other geographic locations, suggesting they have diverged to such an extent that, to study this relationship, a more conservative region of the genome should be explored.
Neighbor-joining tree using maximum parsimony inference of Symbiodinium C3 and C46 associated with Siderastrea from locations in the Atlantic based on partial sequences (∼450–550 bases) of the psbA ncr. Symbiodinium C3 comprises several geographically distinct lineages and indicates divergence between Brazil and the Caribbean. C46 from Cape Verde and Brazil comprise an independently evolved lineage specific to Siderastrea stellata/radians. Bootstrap values (>70), based on 1,000 replicates, are labeled above each branch
Discussion
Atlantic siderastreids (S. radians, S. siderea, and S. stellata) associate with different Symbiodinium types depending on geographic location and depth (Finney et al. 2010; this study). In Cape Verde, however, S. radians was found to associate only with Symbiodinium C46, a host specialist known to occur only with colonies from this genus (LaJeunesse 2005; Finney et al. 2010). The interplay of factors shaping these coral–algae associations is complex (Iglesias-Prieto et al. 2004; LaJeunesse et al. 2004a, 2010; Sampayo et al. 2007; Goulet et al. 2008), and the exclusive association of S. radians with Symbiondinium C46 may be influenced by environmental conditions, biogeographic barriers, host specificity, or some combination of these factors. Diversity and distribution patterns of Symbiodinium in Atlantic Siderastreids (this study; LaJeunesse 2002; Thornhill et al. 2006a; Warner et al. 2006; Finney et al. 2010) indicate that: (1) C46 occurs exclusively in Siderastrea colonies, in both the Eastern and Western Atlantic; (2) Siderastrea colonies from Western Atlantic locations can harbor several different Symbiodinium “types”; and (3) Symbiodinium C46 is the only species found in Siderastrea colonies from the Tropical Eastern Atlantic.
Niche restrictions
The success of S. radians in Cape Verde suggests that this slow-growing scleractinian coral is well adapted to the region’s environmental conditions. Unlike what is usually observed in other geographic regions, S. radians in Cape Verde can have diameters >100 cm across, and in some locations they can form a pavement-like morphology and become a dominant organism (this study; Moses et al. 2003; Monteiro et al. 2009). However, data suggest that S. radians dominance in Sal Is. is site-restricted (Figs. 1, 4).
S. radians association with a single Symbiodinium type (Fig. 6) suggests that this host specialist is also well adapted to the region’s environmental conditions, but the low diversity of Symbiodinium associating with S. radians in the region may be contributing to the restriction of the animal’s realized niche. Symbiodinium can be differently adapted to various ranges of irradiance and temperature (Iglesias-Prieto and Trench 1994; Rowan et al. 1997; Iglesias-Prieto et al. 2004; LaJeunesse et al. 2004a; Thornhill et al. 2006b). In such cases, it is not only Symbiodinium types that can be better adapted to specific ranges, as they have different tolerances to changes in irradiance and temperature. Conclusions from previous studies (Iglesias-Prieto et al. 2004; LaJeunesse et al. 2004a; Warner et al. 2006; Bongaerts et al. 2010; Finney et al. 2010) have suggested that depth (and irradiance attenuation) influences the partitioning of compatible Symbiodinium to certain coral colonies. In Brazil and in the Caribbean (see above), differences in predominant assemblages suggest a relation between changes in Symbiodinium diversity associating with Siderastrea and the hosts’ ability to thrive in different ecological niches. In contrast, the exclusive association between Siderastrea and Symbiodinium C46 in Cape Verde may limit the hosts’ distribution to a narrow range of habitat. Variation in the relative abundance of S. radians around Sal Island (Cape Verde) appears to be influenced by irradiance (Figs. 4, 5), decreasing where average light intensity is lower or higher than an optimum range. Harboring a single predominant Symbiodinium type, this coral thrives at different depths, but preferably where average light intensity (between 1030 and 1530 hours, when the Sun is closer to its azimuth) is between 5,500 and 6,500 lx (Fig. 5). The preference of this coral host for locations with a specific range of light intensity suggests that Symbiodinium C46 may be better adapted to a specific light (intensity and/or quality) regime and may influence the hosts’ local distribution and restrict the host’s ecological niche. Furthermore, the exclusivity of this association in Cape Verde may constrain S. radians tolerance and resilience to changing environmental conditions. Corals with a higher diversity in Symbiodnium associations are expected to better withstand changing conditions, as symbionts differ in their tolerance thresholds (Fitt et al. 2001; Kemp et al. 2006; Goulet et al. 2008; Oliver and Palumbi 2010). Physiological experimentation is required to verify this hypothesis.
Phylogeographic patterns
The geographic and ecological distributions of Symbiodinium are sometimes complex, because they are affected by local and regional environmental factors and by their dispersal capability (as “free-living” cells or with the host, either carried by rafting colonies or by the brooded larvae released to the water column). Sea surface temperature and aerosol mass concentration in the Cape Verde geographic region are consistently different than other locations where S. radians associates with other symbionts (Fig. 3). Lower average water temperatures could be one of the factors contributing to the exclusion of other Symbiodinium associations with S. radians in Cape Verde. However, despite the overall lower temperature, SST monthly averages (Fig. 3a) reveal a reasonably low seasonal temperature fluctuation and within the range of variation of other locations. Average photosynthetically active radiation, the amount of light available for photosynthesis (data not shown), is not significantly different between locations (Acker and Leptoukh 2007); however, aerosol mass concentration is considerably higher in Cape Verde (Fig. 3b). This high concentration of aerosols in the region is a reflection of its proximity to the African continent. Seasonal dust plumes rise from the Sahara desert (Shinn et al. 2000; Garrison et al. 2003; Gill et al. 2006) and are pushed west by prevailing and harmatan winds (Braby 1913; Wilke et al. 1984; Griffin et al. 2001; Zazo et al. 2007). Dust and aerosols fall out across the Atlantic influencing light reaching the surface and increasing water turbidity (Braby 1913; Bohren and Huffmann 1983). The prevailing longitudinal gradient in aerosol mass concentration is an indicator of different light regimes that can be contributing to the shaping of coral–symbiont associations.
Geographic isolations may explain in part the absence of other Symbiodinium types or species (ITS2) associated with S. radians from Cape Verde. Unlike fishes with amphi-Atlantic distribution (Floeter et al. 2007; Monteiro et al. 2008; Rocha et al. 2008), coral larvae and “free-living” cells are dispersed passively, which limits their ability to disperse across the mid-Atlantic Barrier. The geographic distance separating regions and local currents, such as the Amazonas outflow, act as barriers to reef-coral dispersal and gene flow. Indeed, populations of Siderastrea sideraea and S. radians show some genetic partitioning between the Caribbean, Brazil, and Africa (Nunes et al. 2011). However, the presence of Symbiodinium C46 in coral hosts from Cape Verde, Brazil, and Caribbean locations indicates that there is some connectivity between these major Atlantic provinces.
Previous studies (Baillie et al. 2000; LaJeunesse et al. 2004a, b, 2010) have suggested that certain host–generalist and host–specialist symbionts may disperse long distances and across inhospitable environments while others are unable to do so. The absence of strong phylogeographic differentiation in the psbA ncr phylogeny among haplotypes of Symbiodinium C46 (Fig. 7) between Cape Verde and Brazil suggests occasional long-distance dispersal events. The non-coding region of psbA minicircle exhibits greater sequence divergence and considerably less overlap between intragenomic, inter-individual, and inter-specific variation found in ITS sequence data (LaJeunesse and Thornhill 2011). The significant sequence divergence between C3 and C46 associating with siderastreids (Fig. 7) clearly distinguishes these as independently evolving lineages (Avise and Wollenberg 1997), despite the sequence similarity in ITS rDNA (LaJeunesse 2001; LaJeunesse et al. 2004a; Sampayo et al. 2009; Finney et al. 2010). Phylogenetic analysis of the non-coding psbA minicircle (Fig.7) indicates that Symbiodinium C3 found in Siderastrea comprises cryptic lineages, not resolved by ITS sequence data, that exist in Brazil and various locations within the Caribbean. Geographic separation has clearly influenced the genetic make-up and psbA haplotype coalescence between C3 populations. In contrast, the psbA haplotypes of Symbiodinium C46 from the Cape Verde and Brazil cluster together suggesting periodic mixing between populations of this symbiont from each region. In the light of laboratory experiments (Neves et al. 2008), in which Siderastrea radians planulae remained competent for only 24–48 h, it is unlikely that S. radians planulae would survive the journey across the Atlantic. Similarly, “free-living” cells of endosymbiotic Symbiodinium types are mainly benthic and usually absent from the water column or in very low densities (Littman et al. 2008; Pochon et al. 2010). Hoeksema et al. (2012) indicate that brooding corals can survive for periods that are long enough to enable trans-Atlantic dispersal through rafting (attached to drifting objects). The possibility that S. radians disperses by rafting remains speculative, but may explain in part its genetic similarity across the Atlantic (Nunes et al. 2011) and the broad distribution of Symbiodinium C46.
Summary
Cape Verde has a single Siderastrea species, which seems to associate with a single species of Symbiodinium. Besides very high relative abundance, S. radians colonies can reach sizes of over 100 cm across and dominate an entire area (Moses et al. 2003; Monteiro et al. 2009), in comparison with the 30 cm across seen in the Caribbean and Brazil (Veron and Stafford-Smith 2000). The local success of this coral suggests that this coral–dinoflagellate combination is well adapted to the environmental conditions in the region. In Sal Island, the distribution of S. radians is restricted by light intensity and possibly temperature.
The phylogenetic resolution provided by the psbA non-coding region enhances our understanding and appreciation of the ecological and geographic partitioning and suggests that Symbiodinium C46 undergoes periodic or sporadic exchange between Cape Verde and Brazil (Moore et al. 2003). These different patterns suggest that dispersal and/or gene flow may be considerable for some Symbiodinium types and therefore may significantly influence the ecology and evolution of particular host–symbiont combinations including rates of response to changing environmental conditions
References
Acker J, Leptoukh G (2007) Online analysis enhances use of NASA earth science data. Eos Trans Am Geophys Union 88:14–17
Avise JC, Wollenberg K (1997) Phylogenetics and the origin of species. Proc Natl Acad Sci USA 94:7748–7755
Baillie B, Belda-Baillie C, Silvestre V, Sison M, Gomez AV, Gomez ED, Monje V (2000) Genetic variation in Symbiodinium isolates from giant clams based on random-amplified-polymorphic DNA (RAPD) patterns. Mar Biol 136:829–836
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Coral reefs: corals' adaptive response to climate change. Nature 430:741
Barbrook A, Visram S, Douglas A, Howe C (2006) Molecular diversity of dinoflagellate symbionts of cnidaria: the psbA minicircle of Symbiodinium. Protist 157:159–171
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a “nugget of hope” for coral reefs in an era of climate change. Proc R Soc Lond B 273:2305–2312
Bohren CF, Huffmann DR (1983) Absorption and scattering of light by small particles. Wiley, New York
Bongaerts P, Riginos C, Ridgway T, Sampayo EM, van Oppen MJH, Englebert N, Vermeulen F, Hoegh-Guldberg O (2010) Genetic Divergence across Habitats in the Widespread Coral Seriatopora hystrix and Its Associated Symbiodinium. PLoS ONE 5:e10871
Braby H (1913) The Harmattan wind of the Guinea Coast. Q J R Meteorol Soc 39:301–306
Castillo KD, Ries JB, Weiss JM (2011) Declining coral skeletal extension for forereef colonies of Siderastrea siderea on the Mesoamerican Barrier Reef System, Southern Belize. PLoS ONE 6:e14615
Colombo-Pallotta MF, Rodríguez-Román A, Iglesias-Prieto R (2010) Calcification in bleached and unbleached Montastraea faveolata: evaluating the role of oxygen and glycerol. Coral Reefs 29:899–907
Costa CF, Sassi R, Gorlach-Lira K (2008) Zooxanthellae genotypes in the coral Siderastrea stellata from coastal reefs in northeastern Brazil. J Exp Mar Biol Ecol 367:149–152
Finney JC, Pettay DT, Sampayo EM, Warner ME, Oxenford HA, LaJeunesse TC (2010) The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb Ecol 60:250–263
Fitt W, Brown B, Warner M, Dunne R (2001) Coral bleaching: interpretation of thermal tolerance limits and thermal thresholds in tropical corals. Coral Reefs 20:51–65
Floeter SR, Rocha LA, Robertson DR, Joyeux JC, Smith-Vaniz WF, Wirtz P, Edwards AJ, Barreiros JP, Ferreira CEL, Gasparini JL, Brito A, Falcón JM, Bowen BW, Bernardi G (2007) Atlantic reef fish biogeography and evolution. J Biogeogr 35:22–47
Foster AB (1980) Environmental variation in skeletal morphology within the Caribbean Reef Corals Montastraea annularis and Siderastrea siderea. Bull Mar Sci 30:678–709
Garrison VH, Shinn EA, Foreman WT, Griffin DW, Holmes CW, Kellogg CA, Majewski MS, Richardson LL, Ritchie KB, Smith GW (2003) African and Asian dust: from desert soils to coral reefs. Bioscience 53:469–480
Gill JA, Watkinson AR, McWilliams JP, Cote IM (2006) Opposing forces of aerosol cooling and El Nino drive coral bleaching on Caribbean reefs. Proc Natl Acad Sci USA 103:18870–18873
Goulet T, LaJeunesse TC, Fabricius K (2008) Symbiont specificity and bleaching susceptibility among soft corals in the 1998 Great Barrier Reef mass coral bleaching event. Mar Biol 154:795–804
Griffin D, Garrison V, Herman J, Shinn E (2001) African desert dust in the Caribbean atmosphere: microbiology and public health. Aerobiologia 17:203–213
Hallock P (1981) Algal symbiosis: a mathematical analysis. Mar Biol 62:249–255
Hoegh-Guldberg O (2004) Coral reefs in a century of rapid environmental change. Symbiosis 37:1–31
Hoeksema BW, Roos PJ, Cadée GC (2012) Trans-Atlantic rafting by the brooding reef coral Favia fragum on man-made flotsam. Mar Ecol Prog Ser 445:209–218
Iglesias-Prieto R, Trench R (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Iglesias-Prieto R, Beltrán VH, LaJeunesse TC, Reyes-Bonilla H, Thomé PE (2004) Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc Lond B 271:1757–1763
Kemp D, Cook C, LaJeunesse TC, Brooks W (2006) A comparison of the thermal bleaching responses of the zoanthid Palythoa caribaeorum from three geographically different regions in south Florida. J Exp Mar Biol Ecol 335:266–276
Kohler K, Gill S (2006) Coral Point Count with Excel extensions (CPCe): a visual basic program for the determination of coral and substrate coverage using random point count methodology. Comput Geosci 32:1259–1269
Laborel J (1974) West African Reef Corals, an hypothesis on their origin. Proc 2nd Int Coral Reef Symp 1:425–443
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiotic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a“species” level marker. J Phycol 37:866–880
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
LaJeunesse TC (2005) “‘Species’” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581
LaJeunesse TC, Thornhill DJ (2011) Improved resolution of reef-coral endosymbiont (Symbiodinium) species diversity, ecology, and evolution through psbA non-coding region genotyping. PLoS ONE 6:e29013
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O (2003) Low symbiont diversity in Southern Great Barrier Reef Corals, relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
LaJeunesse TC, Bhagooli R, Hidaka M, de Vantier L, Done T, Schmidt GW, Fitt WK, Hoegh-Guldberg O (2004a) Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar Ecol Prog Ser 284:147–161
LaJeunesse TC, Thornhill DJ, Cox EF, Stanton FG, Fitt WK, Schmidt GW (2004b) High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23:596–603
LaJeunesse TC, Pettay D, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK (2010) Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J Biogeogr 37:785–800
Lewis J (1989) Spherical growth in the Caribbean coral Siderastrea radians (Pallas) and its survival in disturbed habitats. Coral Reefs 7:161–167
Li X, Maring H, Savoie D, Voss K (1996) Dominance of mineral dust in aerosol light-scattering in the North Atlantic trade winds. Nature 380:416–419
Lirman D, Manzello D (2009) Patterns of resistance and resilience of the stress-tolerant coral Siderastrea radians (Pallas) to sub-optimal salinity and sediment burial. J Exp Mar Biol Ecol 369:72–77
Lirman D, Manzello D, Maciá S (2002) Back from the dead: the resilience of Siderastrea radians to severe stress. Coral Reefs 21:291–292
Littman RA, van Oppen MJH, Willis BL (2008) Methods for sampling free-living Symbiodinium (zooxanthellae) and their distribution and abundance at Lizard Island (Great Barrier Reef). J Exp Mar Biol Ecol 364:48–53
Monteiro P, Ribeiro D, Silva JA, Bispo J, Gonçalves J (2008) Ichthyofauna assemblages from two unexplored Atlantic seamounts: Northwest Bank and João Valente Bank (Cape Verde Archipelago). Sci Mar 72:133–143
Monteiro J, Almeida C, Freitas R, Delgado A, Porteiro F, Santos RSS (2009) Coral assemblages of Cape Verde: preliminary assessment and description. Proc 11th Int Coral Reef Symp 2:1416–1419
Moore RB, Ferguson KM, Loh WKW, Hoegh-Guldberg O, Carter D (2003) Highly organized structure in the non-coding region of the psbA minicircle from clade C Symbiodinium. Int J Syst Evol Microbiol 53:1725–1734
Morri C, Bianchi C (1995) Cnidarian Zonation at Ilha do Sal (Arquipélago de Cape Verde). Beitr Paläontol 20:41–49
Moses C, Helmle K, Swart P, Dodge E, Merino SE (2003) Pavements of Siderastrea radians on Cape Verde reefs. Coral Reefs 22:506. doi:10.1007/s00338-003-0346-x
Muller-Parker G, D'Elia C (1997) Interactions between corals and their symbiotic algae. In: Birkeland C (ed) Life and death of coral reefs. Chapman and Hall, New York, pp 96–113
Muscatine L, McCloskey L, Marian R (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26:601–611
Neves E, Andrade S, Silveira F, Solferini V (2008) Genetic variation and population structuring in two brooding coral species (Siderastrea stellata and Siderastrea radians) from Brazil. Genetica 132:243–254
Nunes F, Norris RD, Knowlton D (2011) long distance dispersal and connectivity in Amphi-Atlantic Corals at regional and basin scales. PLoS ONE 6:e22298
Oigman-Pszczol S, Creed J (2004) Size structure and spatial distribution of the corals Mussismilia hispida and Siderastrea stellata (Scleractinia) at Armação dos Búzios, Brazil. Bull Mar Sci 74:433–448
Oliver TA, Palumbi SR (2010) Many corals host thermally resistant symbionts in high-temperature habitat. Coral Reefs 30:241–250
Pearse VB, Muscatine L (1971) Role of symbiotic algae (Zooxanthellae) in coral calcification. Biol Bull 141:350–363
Pochon X, LaJeunesse TC, Pawlowski J (2004) Biogeographic partitioning and host specialization among foraminiferan dinoflagellate symbionts (Symbiodinium; Dinophyta). Mar Biol 146:17–27
Pochon X, Stat M, Takabayashi M, Chasqui L, Chauka LJ, Logan DDK, Gates RD (2010) Comparison of endosymbiotic and free-living Symbiodinium (dinophyceae) diversity in a Hawaiian reef environment. J Phycol 46:53–65
Rocha LA, Rocha CR, Robertson DR, Bowen BW (2008) Comparative phylogeography of Atlantic reef fishes indicates both origin and accumulation of diversity in the Caribbean. BMC Evol Biol 8:157–173
Rodriguez-Lanetty M, Krupp DA, Weis VM (2004) Distinct ITS types of Symbiodinium in Clade C correlate with cnidarian/dinoflagellate specificity during onset of symbiosis. Mar Ecol Prog Ser 275:97–102
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Sampayo E, Franceschinis L, Hoegh-Guldberg O, Dove S (2007) Niche partitioning of closely related symbiotic dinoflagellates. Mol Ecol 16:3721–3733
Sampayo E, Dove S, LaJeunesse TC (2009) Cohesive molecular genetic data delineate species diversity in the dinoflagellate genus Symbiodinium. Mol Ecol 18:500–519
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–90
Shinn E, Smith G, Prospero J, Betzer P (2000) African dust and the demise of Caribbean Coral Reefs. Geophys Res Lett 27:3029–3032
Stanley GD Jr (2006) Photosymbiosis and the evolution of modern coral reefs. Science 312:857–858
Stat M, Carter D, Hoegh-Guldberg O (2006) The evolutionary history of Symbiodinium and scleractinian hosts - Symbiosis, diversity, and the effect of climate change. Perspect Plant Ecol Evol Syst 8:23–43
Swofford D (2000) PAUP*, Phylogenetic analysis using parsimony (*and other methods). Sinauer, Sunderland
Thornhill DJ, Fitt WK, Schmidt GW (2006a) Highly stable symbioses among western Atlantic brooding corals. Coral Reefs 25:515–519
Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW (2006b) Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar Biol 148:711–722
Trench R (1993) Microalgal-invertebrate symbioses-a review. Endocytob Cell Res 9:135–175
Veron JEN, Stafford-Smith M (2000) Corals of the world. 1:490. Australian Institute of Marine Science, Townsville
Veron JEN, Stafford-Smith M (2002) Coral ID (CD-Rom, first release). Australian Institute of Marine Science, Townsville
Wallenstein FFMM, Neto AI, Álvaro NV, Tittley I (2008) Subtidal rocky shore communities of the Azores: developing a biotope survey method. J Coast Res 24:244–249
Warner ME, LaJeunesse TC, Robison JD, Thur RM (2006) The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr 52:1887–1897
Wilke B, Duke B, Jimoh W (1984) Mineralogy and chemistry of Harmattan dust in northern Nigeria. Catena 11:91–96
Zazo C, Goy J, Dabrio CJ, Soler V, Hillaire-Marcel C, Ghaleb B, González-Delgado JA, Bardají T, Cabero A (2007) Quaternary marine terraces on Sal Island (Cape Verde archipelago). Quat Sci Rev 26:876–893
Acknowledgements
We wish to thank Cape Verde Diving, Dunas de Sal and Scuba Caribe dive operators who provided help and logistic support to all fieldwork in Cape Verde. We thank the Brazilian students Tatiana P. de Leon Amorim and Carolina da Rocha Simões for help with collections and processing of samples. D. Thornhill provided several psbA sequences from colonies of Siderastrea monitored near Lee Stocking Island, Bahamas. We also thank all reviewers for their comments and contributions. This research was also part of J.M.’s doctoral research program (SFRH/BD/27869/2009), funded by the Portuguese Foundation for Science and Technology (FCT). Funding for this research was also provided by the IOC-UNESCO-World Bank Targeted working group on coral bleaching and Pennsylvania State University. IMAR-DOP/UAz (Research and Development Unit no. 531) and LarSyS-Associated Laboratory are supported by the Portuguese Foundation for Science and Technology (FCT) under a strategic project and DRCTC-GR Azores through a Pluriannual Funding scheme (COMPETE and PRO-Convergência). Exchange of specimens was made possible by a Material Transfer Agreement between Penn State University and the Universidade Federal da Paraiba and by CITES permit 02/2008 issued by Direcção Geral do Ambiente, Cape Verde.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Monteiro, J.G., Costa, C.F., Gorlach-Lira, K. et al. Ecological and biogeographic implications of Siderastrea symbiotic relationship with Symbiodinium sp. C46 in Sal Island (Cape Verde, East Atlantic Ocean). Mar Biodiv 43, 261–272 (2013). https://doi.org/10.1007/s12526-013-0153-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-013-0153-8