Abstract
Although the Red Sea is considered as a center of endemism and a ‘hotspot’ of biodiversity, the ascidian fauna (Chordata, Ascidiacea) of the region has been poorly studied. The current study provides a review of the literature concerning the ascidian fauna of the Red Sea, including the Gulf of Aqaba and the Gulf of Suez, an updated species list based on extensive field sampling and museum data, and an analysis of the diversity and rates of endemism of the Ascidiacea of the Red Sea. The inventory lists 73 ascidian species, belonging to 13 families. The majority of species records are of colonial species, as typical of tropical regions. Representatives of 30 species are available at the National Collections of Natural History at Tel Aviv University. Only 12 species (17 %) of the Red Sea ascidians were found to be endemic to the region, whereas the majority have an extra Indo-Pacific distribution. Eight species have been recorded for the first time from this region. The Gulf of Aqaba and the Gulf of Suez represent the northern boundary of the natural distribution for the majority of tropical species recorded in this study. However, several species have successfully entered the Mediterranean Sea through the Suez Canal. This list is certainly incomplete and emphasizes the need to increase the sampling effort for this group, and for additional taxonomic studies of the ascidian fauna of this highly diverse and rich region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ascidians (Phylum: Chordata, Class: Ascidiacea), or sea squirts, are the largest and most diverse class of the sub-phylum Tunicata (also known as Urochordata). They comprise approximately 3,000 accepted species found in all marine habitats (Shenkar et al. 2012). Adult ascidians bear little resemblance to typical chordates, though their short-lived non-feeding tadpole larvae clearly exhibit the four fundamental characteristics of the phylum: a dorsal tubular nerve cord, notochord, rudimentary pharyngeal gill slits and a post-anal tail. Another important character is the presence of the endostyle in the pharynx that would evolve as the thyroid gland in vertebrates (Fujita and Nanba 1971). Following settlement, the lecithotrophic larvae undergo metamorphosis during which they lose all these characteristics except for the endostyle and the gill slit rudiments in the pharynx (Millar 1971), which become functional and multiply to form the branchial sac.
All ascidians are hermaphrodites, having both male and female organs. They generally avoid self-fertilization by developing only eggs or only sperm at any one time (Newlon et al. 2003). Most solitary ascidians release their eggs and sperm into the water for external fertilization, while colonial ascidians usually retain and brood their eggs (Lambert 2005). Under natural conditions, ascidian larvae do not normally disperse very far, often just a few meters or less (Ayre et al. 1997). The majority of ascidians filter their food from the water-column via an oral siphon that brings water into the pharyngeal chamber; a cloacal siphon then expels the water. Particles suspended in the current are trapped in a mucous net on the gill slits. The net pores range from 0.1 to 0.3 μm, allowing ascidians to filter even very small particulate matter, primarily in the range of 0.5–2 μm diameter (Bak et al. 1998; Bone et al. 2003). The substances caught in the mucous net are later transported to the stomach for digestion.
In general, ascidians constitute a minor benthic component on exposed surfaces of the natural coral reefs. They are often found in cryptic environments such as grottos, crevices and the sides or undersides of rocks and corals. In exposed sites, solitary species frequently protect themselves better than colonial species from the dangers of predation, abrasion and physical damage. In such environments, their rigid tunic is often covered by epibionts that provide camouflage and physical protection (Monniot et al. 1991). Some colonial species of the family Didemnidae that host the photosynthetic prokaryote symbiont Prochloron thrive on surfaces exposed to high irradiance on the reef flat (Kühl and Larkum 2002). Both colonial and solitary species successfully foul various artificial substrates such as jetties and other man-made substrata adjacent to the natural coral reef (Oren and Benayahu 1998; Paulay et al. 2001; Shenkar et al. 2008a). There is a growing evidence that colonial ascidians can rapidly overgrow corals and outcompete them for space (Bak et al. 1996; Shenkar et al. 2008b; Sommer et al. 2009; Vargas-Ángel et al. 2009). As ascidians are able to filter even minute particulate matter (Bak et al. 1998; Bone et al. 2003), any rise in nutrient levels and organic material will have a direct influence on their abundance. Combined with the rapid decline in coral cover around the world (Wilkinson 2004), these reports may present a growing concern (Shenkar et al. 2008b).
The Red Sea is a narrow water body, ca. 1,950 km long and averaging 280 km wide, connecting to the Indian Ocean across the narrow Bab al Mandab Straits.
At its northern end, the Red Sea terminates in two gulfs: the northern, shallow-water Gulf of Suez, ca. 30–40 m deep and with a flat bottom, opening widely into the Red Sea proper; and the north-eastern Gulf of Aqaba, with local depths of ca. 1,900 m and separated from the Red Sea proper by the narrow and shallow Straits of Tiran (Fishelson 2011).
The coral reefs of the Red Sea are well known for their extraordinary beauty and richness, and are recognized as a centre of endemism that represents a high priority for conservation (Roberts et al. 2002). The scientific explorations of the Red Sea fauna and flora began with the Danish “Arabia Felix” expedition to the Red Sea in 1772–1773, with the extremely important works of Peter Forsskäl, continuing with Savigny’s studies during Napoleon’s campaign in 1779–1801 (Fishelson 2011). Several cosmopolitan ascidian species such as Phallusia nigra and Didemnum candidum were originally described by Savigny from this area (Savigny 1816). However, not many species have been added to the original inventory list since then, and the ascidian fauna of this area remains relatively unknown. The published list of ascidians from Elat (Red Sea coast of Israel) published by Pérès (1960) contains only 19 species with an additional 6 species added by Monniot (1973). The relatively low number of ascidian species described from Elat thus appears to be a result of less research and fewer sampling efforts.
The growing awareness to the importance of the class Ascidiacea in marine ecosystems (Lambert 2001; Shenkar and Swalla 2011) emphasizes the need for additional studies of this group in diverse ecosystems such as tropical coral reefs. The current study provides a review of the literature concerning the ascidian fauna of the Red Sea, including the Gulf of Aqaba and the Gulf of Suez, an updated species list based on extensive field sampling and museum data, and an analysis of the diversity and rates of endemism of the Ascidiacea of the Red Sea.
Materials and methods
In order to establish a list of ascidian records from the Red Sea, a meticulous survey of the available literature was conducted. In addition, museum material stored at the National Collections of Natural History, Tel Aviv University (TAU), Israel, was examined. This collection includes samples from the 1950s on, with two significant periods of ascidian sampling from the Red Sea: 1962–1977 and 2003–2008.
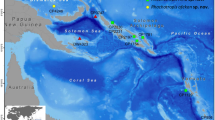
Between the years 1962 and 1977, several scientific expeditions were conducted by Israeli scientists in different areas of the Red Sea (Fig. 1; Table 1). During these expeditions, extensive sampling of marine organisms was conducted, and these were identified throughout the years by professional taxonomists, including ascidian taxonomists.
a The Red Sea showing study sites. b Study sites along the Gulf of Aqaba and Suez. Names and information of sites in Table 1
From 2003 to 2008, extensive surveys and sampling were conducted along the Red Sea coasts of Israel by the author. The ascidians sampled during these surveys were collected using scuba and snorkeling. Upon sampling, the ascidians were narcotized with menthol crystals in a closed jar in order to prevent evaporation of the menthol. Only after the samples were fully relaxed (determined by inserting a sharp probe into an open siphon and getting no response) were they transferred to a jar with seawater/formalin fixative after rinsing the menthol crystals from the animals’ body. The fixative was prepared according to the following formula: 100 ml of full-strength formaldehyde, 850 ml of seawater, and 50 ml of distilled water. One gram of sodium borate was added to the mixture and the solution was then mixed with a magnetic stirrer. In order to allow DNA analysis, a small portion of each sample was directly preserved in absolute ethanol. Dissections were stained with hemalum and mounted on permanent slides. Taxonomic identification was carried out using authoritative keys and texts (e.g., Van Name 1921, 1931, 1945; Kott 1985, 1990, 1992, 2001; Monniot et al. 1991; Monniot and Monniot 2001). The entire collection is part of the National Collections of Natural History, TAU.
Following the construction of the species list of the region, their global distribution was investigated using the Ascidiacea World Database (Shenkar et al. 2012) and verified using numerous publications by Berrril, Kott, Millar, the Monniot’s, Nishikawa and Van Name. Since ascidians play an increasing role in marine bioinvasion, the occurrence of species with known introduction records was ascertained by comparison to the list of non-indigenous ascidian species from Shenkar and Swalla (2011). Divison of colonial versus solitary species was based on the Monniot et al. (1991) keys, and species morphological descriptions.
Results
Number of species and systematic diversity
A survey of the available preserved material from the National Collections of Natural History, Tel Aviv University (TAU), Israel, combined with the number of species described from the Red Sea region in the literature, resulted in an estimation of 73 ascidian species, belonging to 13 families (Table 2; Fig. 2). The highest number of species is found in the order Stolidobranchia, with 50 % of the species recorded in this review. The Styelidae family comprised the highest number of species recorded, with the genus Polycarpa with the highest number of representatives (Table 2; Fig. 2). The majority of species records were of colonial species (41 colonial species vs. 32 solitary species records, Table 2). Their zoogeographical classification is shown in Fig. 3, while images of the most dominant species from the Gulf of Aqaba are presented in Figs. 4, 5 and 6.
Common colonial species found along the Red Sea coast of Israel. a Didemnum candidum (Savigny 1816), b Didemnum granulatum (Tokioka, 1954), c Diplosoma modestum (Michaelsen, 1920), d Botryllus eilatensis (Shenkar and Monniot 2006), e Eusynstyela latericius (Sluiter 1904) morph I (photo: A. Gur), f Eusynstyela latericius morph II
Endemism
Twelve species (17 %) were found to be endemic to the region, with publication record restricted to the Red Sea: Aplidium effusum, Ascidia savignyi, Boltenia yossiloya, Botryllus eilatensis, Botryllus rosaceus, Eucoelium hospitiolum, Polycarpa ehrenbergi, Polycarpa polycarpa, Polycitor torensis, Polyclinum hesperium, Polyclinum isiacum and Rhopalaea sp. (Table 2; Fig. 3). The majority of the endemic species belong to the order Stolidobranchia, which (as mentioned above) comprise 50 % of the total number of species.
For eight species, this is the first published record from the Red Sea (Shenkar, work in progress): Aplidium depressum (TAU- AS25417), Diplosoma modestum (TAU- AS25492, AS25493), Polycarpa cryptocarpa (TAU- AS25427), Polycarpa insulsa (TAU- AS25489), and Pyura scortea (TAU- AS25419), which were identified by the author. Leptoclinides sp. (TAU- AS18871), Polysyncraton hartmeyeri (TAU- AS19951, AS19957, AS20987), and Stolonica prolifera (TAU-AS8352; Table 2; Fig. 3), which were identified by F. Monniot.
Museum availability
Representatives of 30 species are available at TAU (collection numbers are given in Table 2). The majority of species (83 %) were collected from the Gulf of Aqaba (sites 1, 2, 3; Table 1), mainly from Elat (site 1). Only 9 species are available from the Gulf of Suez and the Red Sea (sites 4–11; Tables 1, 2).
Global distribution and introduction records
The majority of species have published Indo-Pacific distribution records (Table 2). Out of the 73 known species from the Red Sea, 14 have published records of introduction into non-native regions around the world: Ascidia cannelata, Botrylloides leachii, Botryllus schlosseri, Ciona intestinalis, Diplosoma listerianum, Distaplia stylifera, Ecteinascidia thurstoni, Herdmania momus, Microcosmus exasperatus, Phallusia nigra, Polyclinum constellatum, Rhodosoma turcicum, Styela canopus, and Styela plicata (Table 2; Fig. 3). Almost 50 % of these species have entered the Mediterranean Sea. Botryllus schlosseri, Ciona intestinalis, Styela plicata and Phallusia nigra are widely distributed and are currently considered as cosmopolitan species. The rest are considered native to the Red Sea.
Discussion
The current study provides a list of 73 ascidian species from the Red Sea, divided between 13 families (out of approximately 26 known families from around the world). The highest number of species belongs to the Styelidae family, which is the second most diverse family of the class Ascidiacea (Shenkar and Swalla 2011). The genus Polycarpa, which is well known for its diversity (Monniot et al. 1991), is represented with the highest number of species.
In tropical areas, colonial species represent approximately 80 % of the species (Kott 1981; Monniot and Monniot 1985, 2001; Primo and Vazquez 2004). It has been suggested that their indeterminate growth and a-sexual reproduction provides them with an advantage for the exploitation of tropical habitats (Bak et al. 1996; Shenkar et al. 2008b). However, in the current study, colonial species comprise only 56 % of the total species. This may be a result of the difficulty in providing accurate taxonomic identification of colonial species, and/or easier sampling in the field of solitary species by less trained researchers (as many colonial species are cryptic). It is expected that, as the sampling effort for ascidian fauna increases, the relative contribution of colonial species will also rise.
The rate of endemism found in the current study for the ascidian fauna, 16 %, is relatively low in comparison to other regions. For example, much higher rates of endemism were found in New Zealand (43 %), South Africa (45 %), the Antarctic region (44 %; Primo and Vazquez 2007, 2009), and the Eastern Mediterranean (40.9 %; Koukouras et al. 1995). As depicted in Table 2, the majority of species recorded from the Red Sea have an additional distribution in the Indo-Pacific, Mediterranean and Atlantic Ocean. It is possible, however, that the identification of some of the species that were originally described from the Red Sea in other regions of the world is not accurate. Following a comparison with the Red Sea original material, it may be discovered in the future that there are separate species involved. For example, Polyclinum saturnium, described by Savigny (1816) from the Gulf of Suez, has publication records from Japan, Australia and the Philippines, but with the lack of the original material it is difficult to ascertain whether they are indeed all attributed to the same species (Tokioka 1962). Another explanation for the relatively low endemic rates of the Red Sea ascidian fauna is that, similar to the ichthyofauna of the region, the current ascidian fauna represents the outcome of recent colonization, free of evolutionary and deep-historical signatures (Kiflawi et al. 2006). Since during the last glacial maximum water depth at Bab el Mandab was reduced to c. 15 m, water exchange with the Indian Ocean was highly limited (Siddall et al. 2003). Consequently, salinity within the Red Sea surpassed 50 ‰, decimating most reef-based organisms (Sheppard et al. 1992; Siddall et al. 2003). Ascidian adults and larva are extremely sensitive to such high salinities, and only a few species of ascidians can survive salinities above 44 ‰ (Shenkar and Swalla 2011). It is thus possible that the original endemic fauna of the Red Sea underwent extinction during this period, further contributing to the low rate of endemism.
Although the majority of ascidian species recorded in the Red Sea have an additional distribution in the Indo-Pacific oceans, the Gulf of Suez and the Gulf of Aqaba represent the northern boundary of the natural distribution of these tropical species. Following the opening of the Suez Canal, several Red Sea species (representing 8 % of the total species recorded in the current study) have successfully increased their latitudinal distribution into the East-Mediterranean basin (Shenkar and Loya 2009). Taking into account the anticipated rise in sea-water temperature, due to global warming (Rahmstorf and Ganopolski 1999), the arrival and spread of Red Sea ascidians into the Mediterranean Sea is anticipated.
The taxonomic identification of some of the species remains questionable due to the inability to investigate the original material. The ascidian collection at TAU contains less than half the species listed. Closely-related species such as Eusynstyela hartmeyeri and Eusynstyela latericius may have been confused. In addition, the validity of Boltenia ovifera, Clavelina borealis and Synoicum turgens records from the Red Sea (Savigny 1816) is questionable, as these are cold-water species with additional distributions along the North Atlantic coast and in the Arctic region (Hartmeyer 1903; Van Name 1945).
The current study is the first to provide an inventory of the ascidian fauna of the Red Sea based on the literature and museum collections. As this sea is considered a ‘hotspot’ of biodiversity (Roberts et al. 2002), this number of species strongly reflects the low sampling effort invested in ascidians of this tropical and diverse region, rather than the true number of species. For comparison, in tropical coral reefs such as those of New Caledonia, Australia’s Great Barrier Reef and the Western Pacific, where professional ascidian taxonomists have studied the local fauna, the number of species ranges from 180 to above 600 (table 2 in Shenkar and Swalla 2011).
The growing recognition of ascidians as a subject for research in the fields of ecology and evolution, and especially their promising potential for new pharmaceutical compounds, greatly emphasizes the need for future taxonomic studies of the ascidian fauna of the Red Sea.
References
Ayre DJ, Davis AR, Billingham M, Llorens T, Styan C (1997) Genetic evidence for contrasting patterns of dispersal in solitary and colonial ascidians. Mar Biol 130:51–62
Bak RPM, Lambrechts DYM, Joenje M, Nieuwland G, Van Veghel MLJ (1996) Long-term changes on coral reefs in booming populations of a competitive colonial ascidian. Mar Ecol Prog Ser 133:303–306
Bak RPM, Joenje M, de Jong I, Lambrechts DYM, Nieuwland G (1998) Bacterial suspension feeding by coral reef benthic organisms. Mar Ecol Prog Ser 175:285–288
Bone Q, Carre C, Chang P (2003) Tunicate feeding filters. J Mar Biol Assoc UK 83:907–919
Fishelson L (1971) Ecology and distribution of benthic fauna in shallow waters of Red Sea. Mar Biol 10(2):113–133
Fishelson L (2011) Red Sea explorations by Israeli Zoologists – 1951–2010. Museum of natural history, Tel-Aviv University. Available on line http://www.mnh.tau.ac.il/upload/Red%20Sea%20Explorations%2026.2.pdf
Fujita H, Nanba H (1971) Fine structure and its functional properties of the endostyle of ascidians, Ciona intestinalis: a part of phylogenetic studies of the thyroid gland. Z Zellforsch Mikrosk Anat 121:455–469
Gab-Alla AAFA (2008) Distribution of the sea squirt Ecteinascidia thurstoni Herdman, 1890 (Ascidiacea: Perophoridae) along Suez Canal and Egyptian Red Sea coasts. Oceanologia 50(2):239–253
Hartmeyer R (1903) Die Ascidien der arktis. In: Romer F, Schaudin F (eds). Fauna Arctica, 3(2):93–412
Hartmeyer R (1915) Über einige Ascidien aus dem Golf von Suez. Sitzber Ges Natturforsch Freunde Berlin 9:397–430
Kiflawi M, Belmaker J, Brokovich E, Einbinder S, Holzman R (2006) The determinants of species-richness of a relatively young coral-reef ichthyofauna. J Biogeogr 33:1289–1294
Kott P (1981) The ascidians of the reef flats of Fiji. Proc Linn Soc NSW 105:147–212
Kott P (1985) The Australian ascidiacea. Part 1: phlebobranchiata and stolidobranchiata. Mem Queensland Mus 23:1–440
Kott P (1990) The Australian ascidiacea. Part 2: aplousobranchia (1). Mem Queensland Mus 29(1):1–226
Kott P (1992) The Australian ascidiacea. Part 3: aplousobranchia (2). Mem Queensland Mus 32(2):375–620
Kott P (2001) The Australian ascidiacea. Part 4: aplousobranchia (3), didemnidae. Mem Queensland Mus 47(1):1–407
Koukouras A, Voultsiadou-Koukoura E, Kevrekidis T, Vafidis D (1995) Ascidian fauna of the Aegean sea with a check list of the Eastern Mediterranean and Black sea species. Ann Inst Oceanogr 71:19–34
Kühl M, Larkum AWD (2002) The microenvironment and photosynthetic performance of Prochloron sp. in symbiosis with didemnid ascidians. In: Seckbach J (ed) Cellular origin and life in extreme habitats vol 3: symbiosis, mechanisms and model systems. Kluwer, Dordrecht, pp 273–290
Lambert G (2001) A global overview of ascidian introductions and their possible impact on the endemic fauna. In: Sawada H, Yokosawa H, Lambert CC (eds) The biology of ascidians. Springer, Tokyo, pp 249–257
Lambert G (2005) Ecology and natural history of the protochordates. Can J Zool 83:34–50
Michaelsen W (1918) Ascidia ptychobranchia und dictyobranchia des roten meeres. Expedition Schiff Pola in das Rote Meer, nördliche und südliche Hälfte 1895/1896–1897/1898. Zool Ergebn 32:1–120, 3 pls
Millar RH (1971) The biology of ascidians. Adv Mar Biol 9:1–100
Monniot C (1973) Redescription de six ascidies du golfe d’Elat recoltées par H. Schuhmacher. Isr J Zool 22:51–62
Monniot C, Monniot F (1985) Ascidies littorales de Guadeloupe. IX: caractéristiques des populations, écologie, rapports avec la faune mondiale. Tethys 11:203–213
Monniot F, Monniot C (2001) Ascidians from the tropical western Pacific. Zoosystema 23:201–383
Monniot C, Monniot F, Laboute P (1991) Coral reef ascidians of New Caledonia. Orstom, Paris
Newlon AWI, Yund PO, Stewart-Savage J (2003) Phenotypic plasticity of reproductive effort in a colonial ascidian, Botryllus schlosseri. J Exp Zool A 297:180–188
Oren U, Benayahu Y (1998) Didemnid ascidians: rapid colonizers of artificial reefs in Eilat (Red Sea). Bull Mar Sci 63:199–206
Paulay G, Kirkendale L, Lambert G, Starmer J (2001) The marine invertebrate biodiversity of Apra Harbor: significant areas and introduced species, with focus on sponges echinoderms and ascidians. Prepared for Naval Activities Guam, under Cooperative Agreement N68711-97-LT-70001
Pérès JM (1960) Sur une collection d’ascidies de la côte israelienne de la Mer Rouge et de la péninsule du Sinai. Bull Sea Fish Res Haifa 30:39–47
Primo C, Vazquez E (2004) Zoogeography of the southern African ascidian fauna. J Biogeogr 31:1987–2009
Primo C, Vazquez E (2007) Zoogeography of the Antarctic ascidian fauna in relation to the sub-Antarctic and South America. Antarct Sci 19:321–336
Primo C, Vazquez E (2009) Antartic ascidians: an isolated and homogeneous fauna. Polar Res 28:403–414
Rahmstorf S, Ganopolski A (1999) Long-term global warming scenarios computed with an efficient coupled climate model. Clim Change 43:353–367
Roberts CM, McClean CJ, Veron JEN, Hawkins JP, Allen GR, McAllister DE, Mittermeier CG, Schueler FW, Spalding M, Wells F, Vynne C, Werner TB (2002) Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 295:1280–1284
Savigny JC (1816) Memoires sur les animaux sans vertebres. Paris 2:1–239
Shenkar N, Monniot F (2006) A new species of the genus Botryllus (Ascidiacea) from the Red Sea. Zootaxa 1256:11–19
Shenkar N, Zeldman Y, Loya Y (2008a) Ascidian recruitment patterns on an artificial reef in Eilat (Red Sea). Biofouling 24:119–128
Shenkar N, Bronstein O, Loya Y (2008b) Population dynamics of a coral reef ascidian in a deteriorating environment. Mar Ecol Prog Ser 367:163–171
Shenkar N, Loya Y (2008) Ecology and systematics of the ascidian fauna in the Gulf of Eilat (Aqaba). In: Por FD (ed) Aqaba-eilat, the improbable gulf. Environment, biodiversity and preservation. Magnes, Jerusalem, pp 197–208
Shenkar N, Loya Y (2009) Non-indigenous ascidians along the Mediterranean coast of Israel. Mar Biodiver Rec 2. doi:10.1017/S1755267209990753
Shenkar N, Lambert G (2010) A new species of the genus Boltenia (Ascidiacea) from the Red Sea. Zootaxa 2391:61–68
Shenkar N, Swalla BJS (2011) Global diversity of ascidiacea. PLoS One 6(6):e20657
Shenkar N, Gittenberger A, Lambert G, Rius, Rocha RM, Swalla BJ, Turon X (2012) World ascidiacea database. Available online at http://www.marinespecies.org/ascidiacea. Consulted on 2012-02-08
Sheppard C, Price A, Roberts C (1992) Marine ecology of the Arabian region. Patterns and processes in extreme tropical environments. Academic, London
Siddall M, Rohling EJ, Almogi-Labin A, Hemleben C, Meischner D, Schmelzer I, Smeed DA (2003) Sea-level fluctuations during the last glacial cycle. Nature 423:853–858
Sommer B, Harrison PL, Scheffers SR (2009) Aggressive colonial ascidian impacting deep coral reefs at Bonaire, Netherlands Antilles. Coral Reefs 29:245
Tokioka T (1962) Contribution to Japanese ascidian fauna. 19. Addition to Japanese ascidian fauna, with notes on two already known species. Publ Seto Mar Biol Lab 10:259–282
Van Name WG (1921) Ascidians of the West Indian region and south eastern United States. Bull Am Mus Nat Hist 44:283–494
Van Name WG (1931) New North and South American ascidians. Bull Am Mus Nat Hist 61:207–227
Van Name WG (1945) The North and South American ascidians. Bull Am Mus Nat Hist 84:1–476
Van Name WG (1952) The ‘Manihine’ expedition to the Gulf of Aqaba 1948–1949 station list and collectors’ notes. Preliminary hydrological report. Tunicata. Bull Brit Mus Nat Hist (zool) 1(8):215–220
Vargas-Ángel B, Godwin LS, Asher J, Brainard R (2009) Invasive didemnid tunicate spreading across coral reefs at remote Swains Island, American Sāmoa. Coral Reefs 28:53
Wilkinson CR (2004) Status of coral reefs of the world (Global coral reef monitoring network and Australian institute of marine science, Townsville, Australia). Available online http://www.gcrmn.org/status2004.aspx
Acknowledgments
The author would like to thank Dr. M. Goren and Prof. Emeritus L. Fishelson for their advice and helpful suggestions, and A. Shlagman and R. Ben-David Zaslow from the National Collections of Natural History at Tel Aviv University for their technical assistance. The author is grateful to G. Lambert for her assistance with the taxonomic identification. This research was supported by the Steinhardt National Collections of Natural History (Zoological Museum, Tel Aviv University) and by a fellowship of the Israel Taxonomy Initiative.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shenkar, N. Ascidian (Chordata, Ascidiacea) diversity in the Red Sea. Mar Biodiv 42, 459–469 (2012). https://doi.org/10.1007/s12526-012-0124-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-012-0124-5