Abstract
This paper presents the archaeometallurgical study of iron objects unearthed from the Niejiagou bone workshop dated between late Warring States period to the end of Qin dynasty. Through metallographic study and slag inclusion analysis, all analysed samples were confirmed to be made from cast iron as raw material, with the exception of NJG-34, which production technique cannot be positively confirmed. To convert cast iron into soft iron or steel, both annealing for decarburisation and fining techniques were applied, with the preference of annealing technique for the majority of the iron tool. The extremely fine-grained soft iron/steel microstructure, which was rarely observed in past research, represents a highly developed annealing technique with possible independent normalising operation adopted to reduce the grain size. Such a decarburisation method creates materials with exceptionally high quality, while the cost is also much higher compared to the finning process. Additionally, two iron objects with unique slag inclusions in terms of their morphology and compositional data were found, which possibly represent a new decarburisation method unknown to current research yet. Overall, this site displayed a unique technological choice on cast iron decarburisation methods, which can be argued to be the result of unlimited production cost considering this site was state controlled and served the Qin royalty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
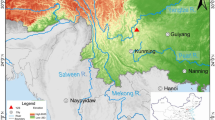
The Niejiagou bone workshop is located in Yaodian County, Xianyang city, Shaanxi province, 582 meters to the west of the foundation of the palace buildings of the Qin Xianyang city (Fig. 1). The site was first discovered during a field survey in November 2014, when two deposit pits of bone remain were found, followed by a formal excavation carried out by Shaanxi Provincial Institute of Archaeology. In the deposit pit K1, 52 iron objects, mainly small iron tools were unearthed, along with a total of 600 kg of bone artefacts and 30 bronze objects (mostly coins) as well as pottery sherds and stone tools. Based on the stratigraphy, typology of potteries and bronze coins, the K1 pit was dated between late Warring States period (475 BC–221 BC) to the Qin dynasty (221 BC–206 BC). In reference to the surrounding environment, the pit was considered as the secondary deposit of a bone artefact manufacturing workshop serving, and regulated by the Qin central government (Shaanxi Provincial Institute of Archaeology 2019).
In our previous research (see Liu et al. 2019a), a systematic study of iron production techniques in the State of Qin during the Late Warring States period has been carried out, confirming that cast iron smelting has been the dominating method to extract iron, with annealing and fining techniques adopted to malleablise or decarburise cast iron. However, since all the analysed archaeological samples were recovered from burial context, whether, or to what extent does such a result reflects the real-life technological choices in the Qin state is still open to debate. Unearthed from a bone artefact workshop, the iron products found in the Niejiagou pit K1 undoubtedly represents the use of iron in actual production activities, which gives us an opportunity to extent the study of iron production techniques adopted in the Qin State/Empire. More importantly, considering the nature of such a site, which is a workshop centrally regulated by the state and serves the Qin royalty, it will also be interesting to compare the iron production techniques with those civilian/burial iron, to see whether there is a difference in terms of the technological choices between different social hierarchies. In this sense, by collaborating with the Shaanxi Provincial Institute of Archaeology, archaeometallurgical study on a selection of the unearthed iron objects were carried out. The results and associated discussion are presented below.
Materials and analytical methods
In this research, a total number of 28 samples were taken, most of them were categorised as knives designed with different functions, including scrapping, cutting and carving. To avoid further confusion, this paper invariably named them as knives, with those having a rectangular section shape marked in Table 1. The rest of the samples include chisels, shovels, adzes, bar and unrecognisable fragments. It needs to be noted that all fragments have a clear shape, which can be confirmed to be part of a final object rather than production waste. Photos of the original objects are given in Fig. 2 (with exception of sample NJG-13, NJG-21 and NJG-35).
In order to reveal their material types and further understand the production techniques, metallographic study and slag inclusion analyses were adopted in this research. The microstructure of iron-carbon alloys conveys key technical information regarding the carbon content and shaping technique as well as possible heat-treatment process adopted during the manufacturing process. However, when studying iron products from early China, where the same type of material can be produced through either direct or indirect process, slag inclusion study will be necessary to further separate them apart as the abundance of certain oxides such as P2O5 and CaO can reflect the redox condition as well as possible additives used during the reaction stage (Dillmann and L’Héritier 2007); therefore, further compositional analysis of the slag inclusions using scanning electron microscope equipped with energy dispersive spectrometer (SEM-EDS) was carried out.
In this research, all samples were mounted in epoxy resin with a 4:1 mixing ratio between epoxy resin and resin hardener. Such resin blocks were first ground with abrasive paper from coarse (P320) to fine (P4000), then polished with diamond paste of 3 μm and 1 μm grades, to expose a polished cross-section of the metal. After polishing, metallographic study was first carried out. In this step, the samples were etched with 2 wt.% nital to reveal its microstructure. Metallography pictures were taken in the optical microscope lab, using a Leica DM 4500 P LED microscope equipped with a Leica DFC 290 HD camera. All metallography photos are provided in Supplementary Material-1.
After the metallographic study, all samples were polished again to remove the etched layer, then carbon coated for further microstructural and compositional analysis using Carl-Zeiss EVO scanning electron microscope equipped with an Oxford X-Man 80 EDS detector. The accelerating voltage were set at 20 kV with a working distance of 8.5 mm following manufacturer’s recommendations. The EDS signal collection process was set as 750,000 signal counts for each analysis. Cobalt calibration was performed every hour to control for drift. Precision and accuracy were monitored through the measurement of basalt standards. All results are presented as stoichiometric oxides and normalised to 100% and can be found in Supplementary Material-2.
Results
Metallography
Through metallographic study, the majority of the samples can be confirmed to be made from cast iron as raw material with characteristic microstructure and clean metal matrix with no slag inclusions. These samples can be further categorised as cast iron, malleable cast iron and decarburised soft iron/steel. Cast iron samples include white cast iron (NJG-22, shovel; NJG-33, fragment; see Fig. 3a) and grey cast iron (NJG-36, fragment see Fig. 3b). Malleable cast iron includes sample NJG-24 (iron bar) and NJG-35 (fragment). NJG-24 has flocculant graphite distributed on a mixture of pearlite and ferrite matrix (Fig. 3c), while NJG-35 has spherical graphite distributed on a ferrite matrix (Fig. 3d). The decarburised (through the annealing process) soft iron/steel samples have various carbon content leading to different microstructures, including pearlite, ferrite or a mixture of both (Fig. 3e, f).
The rest of the samples, including NJG-6, 7, 11, 20 and 34, have a soft iron or steel microstructure with various types of slag inclusions embedded in the metal matrix (Fig. 4). For these samples, further slag inclusion analysis was carried out to study their production techniques.
Slag inclusion study
Inclusions in sample NJG-6 are composed of SiO2, P2O5, MnO and FeO, while other elements are below detection limit. These inclusions are mostly small nodules, distributed randomly in a homogeneous pearlite matrix (Figs. 4a and 5a). Inclusions in sample NJG-7 are also small in size (Figs. 4b and 5b), mainly composed of SiO2, P2O5 and FeO, with occasional small amounts of CaO and MnO being detected. Most of the remaining components are below detection limit (Fig. 6).
Slag inclusions in sample NJG-11 and 20 are heterogeneous in terms of their morphology and composition, including glassy silicate inclusions with low FeO content and fayalitic inclusions with high FeO content (Fig. 7). The mean and median value of P2O5 and CaO are both higher than 10 wt.% (Fig. 8). To decide whether these inclusions came from the same smelting/fining practice, non-reduced compound (NRC) analysis were carried out using combinations of %MgO/%Al2O3, %Al2O3/%SiO2 and %K2O/%CaO following Dillmann and L’Héritier (2007). The results have shown the inclusions in sample NJG-11 are mostly linearly correlated. In sample NJG-20, however, the distribution is much wider, with no clear correlation or sub-groups being found (Fig. 9). This phenomenon can be attributed to multiple factors such as local concentration effect or the use of additives/flux in an inhomogeneous reaction condition (Dillmann and L’Héritier 2007; Liu et al. 2019a). Overall, this very paper will consider these inclusions from the same smelting/fining practice.
Inclusions in sample NJG-34 are much more homogeneous, mostly elongated glassy silicates with low FeO content (mostly below 10 wt.%), high SiO2 (between 40-60 wt.%) and CaO (between 10-40 wt.%) content, while no P2O5 were detected (Fig. 8).
Discussion
Technical reconstruction
Based on the analytical results, cast iron sample NJG-22, 33 and 36 can be confirmed to be made through the mould-casting process, with sample NJG-36 gone through a slow cooling process during solidification, where flake graphite instead of cementite were crystalised and formed into grey cast iron. This is most likely to be achieved by pre-heating the mould before casting, which was commonly used to make grey cast iron before the application of coke fired blast furnace, when smelted cast iron contains sufficient amount of silicon, that cold moulds can be used to produce grey cast iron (Williams 2013).
In reference to past research, sample categorised as malleable cast iron and decarburised soft iron/steel should be made by first cast into white cast iron, then went through an annealing process (Li 1975, 1976; He 1981; Hua 1982; Zhao et al. 1985; Wagner 1989). Such a technique was likely to be first applied at around sixth century BC in Central China (Chen 2014:221-228). By heating up the finished cast in an annealing kiln up to 700 °C and above, the cementite inside white cast iron will decompose into iron and carbon atoms. Through controlling the redox condition inside the kiln, these carbon atoms can be either removed or form into temper carbon (graphite), which can effectively reduce the hardness and brittleness of cast iron.
According to the analytical results, NJG-24 and NJG-35 should be made through an annealing for malleablisation process, where the finished cast were heated in a neutral or reducing atmosphere inside the annealing kiln; therefore, decomposed carbon atoms formed into graphite in various shape, and as a result, the metal matrix were also decarburised and formed into ferrite or pearlite or a mixture of both. Spherical graphite in NJG-35 can be only formed under certain condition, requiring both fine control of annealing temperature and certain alloying elements in the raw cast iron as nodularizer (Li et al. 1983; Yan and Wu 1985), yet EDS analysis on the metal matrix has shown no noticeable difference compared to sample NJG-24, which has the typical flocculant graphite. Malleable cast iron with spherical graphite has been occasionally found in Central China since Warring States period; however, evidence is not enough to suggest such a material is the result of an intentional technological choice (Han and Ko 2007: 549-555).
Samples with a soft iron or steel microstructure with no slag inclusions should be made through an annealing for decarburisation process, where the cast were heated in an oxidising condition, and decomposed carbon atoms were removed as carbon-dioxide gas, leaving the cast iron decarburised into soft iron or steel after sufficient length of time. Additionally, the majority of the samples from this category have an extremely fine pearlite matrix or a mixture of pearlite and ferrite, where the grain boundaries can be barely seen under optical microscope (see Fig. 3e, f and Supplementary Material). Such a microstructure should theoretically be the result of a normalising operation, which involves heating the iron to 30–50 °C above AC3 temperature (727–912 °C, phase transformation temperature where ferrite transforms into austenite), maintaining it for a period of time, then quickly cool down through air cooling (Cui and Tan 2000: 290-306). However, since all these samples were decarburised through the annealing process, whether such microstructures were the result of an intentional and independent normalising operation cannot be confirmed, as in essence the normalising process is very close to the annealing for decarburisation process in terms of the operation, with only minor differences in temperature control and cooling choices.
The rest five samples with slag inclusions embedded can be either made through the direct process, namely bloomery smelting, or the fining process, where cast iron was converted into soft iron/steel by remelting it and introduce the air at the same time to burn out the carbon. Both processes can create similar microstructure with very minor difference in the concentration of certain oxides of the slag inclusions.
To begin with, slag inclusions in NJG-6 and NJG-7 are predominantly composed of oxides that are sensitive to redox conditions. These oxides, including P2O5, SiO2 and MnO, can be partly or fully reduced during cast iron smelting, and will be re-oxidised in the following decarburisation process, hence they will be enriched in slag inclusions (Dillmann and L’Héritier 2007; Chen and Zhang 2016; Disser et al. 2014; Liu et al. 2019a). The presence of such oxides in high concentration indicates the iron was produced through the indirect process, with the temperature above the melting point since these elements cannot be removed in solid state. Additionally, according to the compositional data, slag inclusions in these two samples showed no significant amount of other oxides such as MgO, Al2O3 and K2O, indicating the furnace linings or fuel ash did not contribute to the formation of such inclusions, which excludes the possibility of the traditional fining process. Furthermore, the size and distribution pattern of such inclusions also differ from the inclusions seen from other indirect products (Lam et al. 2018; Liu et al. 2019a). As demonstrated in Fig. 4a, b and Fig. 5, the majority of inclusions in sample NJG-6 and NJG-7 are extremely small nodular inclusions, with radius lower than 5 μm that cannot be accurately measured by SEM-EDS. The distribution of these inclusions also appears to be more randomly when compared with other direct and indirect products. Altogether, the evidence suggests these inclusions were formed under a different mechanism rather than being the remains of smelting/fining slag, indicating a different decarburisation technique was applied for the production of these two samples. However, the actual operational details of such a process remains unclear with limited analytical evidence.
Slag inclusions in sample NJG-11 and 20 contain high amount of CaO and P2O5 which differs from typical bloomery slag inclusions. As demonstrated abundantly in past research, the presence of these two oxides in high weight percentage suggests the reaction condition was oxidising and in liquid state, with calcium rich flux such as limestone being added, which is most likely to be the fining process that were widely used in Guanzhong Plain since the late Warring States period (Liu et al. 2019a).
While sample NJG-34 also differs from typical bloomery products in terms of the low FeO and high CaO concentration, the absence of P2O5 made it difficult to speculate its production technique since Calcium alone cannot be considered as discriminating element (Dillmann and L’Héritier 2007). Additionally, during slag inclusion analysis, EDS analysis were also performed on the metal matrix of each sample to qualitatively evaluate their alloying elements. While NJG-6, 7, 11 and 20 shown no detectable amount of Phosphorous, in NJG-34, the phosphorous content is 1.1 wt.%. Combining with the absence of P2O5 in the inclusions, this indicates the phosphorous in the metal has not been removed, which in turn suggests sample NJG-34 did not gone through a fining process, since according to the Ellingham diagram, phosphorous will be oxidised prior to carbon in the fining process. Based on this evidence, there are two possible technical pathways for the production of sample NJG-34. Possibility one is that this sample was made through a direct process, with the ore rich in CaO, or flux such as limestone were used to form silicate slag rich in CaO. Another possibility is that this sample were made from cast iron decarburised through annealing for decarburisation process, and those inclusions were remains of cast iron smelting slag which was not properly separated during the smelting/melting stage.
However, either possibility can be reasonably counterargued. To begin with, all the rest analysed samples were confirmed to be made from cast iron as raw material. Therefore, it is unlikely that the craftsmen in this area adopt the bloomery smelting system for the production of samples NJG-34, especially when considered cast iron smelting and different decarburisation techniques has been widely adopted in the Guanzhong Plain during the late Warring States period, with no evidence of bloomery smelting been found (Liu et al. 2019a). On the other hand, existing research does not support the second possibility either, since no slag inclusions has been found in any previously analysed cast iron products from early China, including those cast iron, malleable cast iron and decarburised soft iron/steel samples from this research. In this sense, the production technique of sample NJG-34 cannot be confidently confirmed based on available evidence.
Decarburisation choices
Based on the analytical results and associate discussion, it can be observed that in the Niejiagou site, the majority of analysed samples were made from cast iron as raw material, with both annealing and fining technique adopted to convert cast iron into soft iron/steel. These two technical pathways serve the same objective yet are different in may spheres. To begin with, the annealing for decarburisation process is carried out with cast iron in solid state. Since the main reaction (C+CO2=2CO) is endothermic, and the migration of carbon atoms in cast iron needs continuous supply of energy, hence this process requires continuous heating until the decarburisation is complete. As for the fining process, since the reaction took place in liquid state, and the main reaction (C+O2=CO2) is exothermic; hence, this process only requires an initial heating up process to melt the cast iron, and the following decarburisation process can be kept going from the heat released from the oxidation of carbon.
The fuel consumption of the annealing process cannot be accurately measured with too many key parameters missing. For the fining process, on the other hand, it can be evaluated based on the investigation into the traditional fining process carried out in pre-modern China. In those fining practices in Henan province, China, during the 1950s, a typical charge is 7 kg of wood and 7 kg of charcoal, enough to decarburise 70 kg of cast iron (Wagner 1985: 25). As a comparison, to maintain an annealing kiln at a temperature above 700 °C for days will undoubtedly consume much more fuel. In this sense, the fining process will be much more cost-effective than the annealing process.
Additionally, the annealing process usually takes many hours to days to complete. The actual time varies depending on the annealing temperature, the initial carbon content and the amount of cast iron to be decarburised. According to the annealing practices in the early twentieth century, to fully decarburise white cast iron into soft iron or steel, the annealing duration was around 144–240 h (6–10 days) (Hua 1982). In comparison, the fining process takes much less time since the reaction is much more intense with the stirring being applied on melted cast iron. According to the traditional fining practices, a typical fining operation takes around one hour to complete, including 20 min of heating up, 20 min of stirring (puddling) and another 20 min to remove the charge and hammering (Wagner 1985: 25).
While it appears that the annealing for decarburisation process is more costly in terms of labour and fuel consumption compared to the fining process, it does have some unique technical advantages. To begin with, no slag inclusions will be introduced during annealing, and the final product will keep the clean matrix from cast iron. Conversely, the fining process will generate large amounts of slag inclusions in the final product, which cannot be expelled completely through smithing. Slag inclusions in the metal matrix are defects that will affect the overall strength of the metal, as they constitute point of stress concentration that will cause fatigue fracture, reducing the ductility while also promoting corrosions to develop (Bowman et al. 1984). Additionally, when the annealing temperature were properly controlled, the final products could have a fine and homogeneous pearlite or pearlite and ferrite structure, which yields excellent mechanical strength. In this sense, the annealing process produces soft iron/steel with a better quality at a much higher cost, while the fining process made it possible to obtain soft iron/steel more economically based on cast iron smelting with compromise in mechanical strength. Both type of techniques were widely used in early China, and the choice between them should mainly rest on the consideration between the performance and the cost.
In this research, among the analysed soft iron and steel samples, a clear preference towards applying the annealing technique for decarburisation has been displayed, where the majority of the analysed craft tools (14 out of 17) from the Niejiagou site were made through this process, with fine grains and clean metal matrix, suggesting an excellent mechanical strength. Such microstructures were rarely seen in soft iron/steel products from other civilian sites/burials dated to Warring States period or early Han dynasty. In comparison, only sample NJG-11 and NJG-20 can be confirmed to be made from the fining process. Considering the Niejiagou bone workshop locates within the Qin palace area in the capital city serving and controlled by the Qin royalty, it is possible that when making these iron tools, the production cost is less important compared to the overall quality, since these tools are unlikely to be made for profit. In comparison, previous study carried out on iron objects unearthed from civilian cemetery in the Qin state has shown no clear preference between these two types of decarburisation methods. While both techniques were adopted, the annealing process appears to be more often used to partially decarburise or malleablise farming implements rather than converting cast iron into soft iron or steel. When making craft tools such as knives and chisels, both annealing and fining process were used to decarburise cast iron; however, due to limited quantity of samples, no clear preference among them has been observed (Liu et al. 2019a, b).
Conclusion
In this research, archaeometallurgical study is carried out for the first time on iron objects unearthed from non-burial background in the Guanzhong Plain. Through metallographic study and slag inclusion analysis, the majority of the samples were confirmed to be made from cast iron, with the exception of sample NJG-34, which is unable to be determined yet. The production techniques include mould-casting at different cooling rates, annealing for decarburisation and malleablisation as well as the fining technique, yielding a wide range of materials including white and grey cast iron, malleable cast iron, steel with various carbon content and soft iron. Aiming to convert cast iron into soft iron or steel, both annealing and fining technique were adopted, while a clear preference towards the annealing for decarburisation process has been observed. By comparing the differences between these two technical pathways, this paper argues that the annealing technique is more costly compared to the fining process, yet when properly controlled, it is capable of yielding materials with excellent mechanical strength. In this sense, given the nature of such a workshop, it is possible that when making such a technological choice, the main consideration was the overall quality rather than the production cost. Without the limitation of production cost, the craftsmen chose to apply the annealing for decarburisation process for producing most of the craft tools. Metallographic study indicates the annealing temperature were properly controlled at 30–50 °C above the AC3 temperature followed by air cooling, or possibly applied an independent normalising process to produce fine-grained hypoeutectoid and eutectoid steel. Additionally, this research also found two samples with unique slag inclusions in terms of their morphology and composition. Based on limited evidence, we argued that these two samples were possibly made through indirect process, where the cast iron was decarburised above the melting temperature, yet unlike the traditional fining process, no refractory material or fuel ash contributed to the formation of slag inclusions, indicating a decarburisation unknown to the current research yet.
References
Bowman MD, Munse WH, Will W (1984) Fatigue behavior of butt welds with slag inclusions. J Struct Eng 110(12):2825–2842
Chen J (2014) New investigation into the metal production in ancient China中国古代金属冶铸文明新探. Science Press科学出版社, Beijing
Chen J, Zhang Z (2016) Discussion on the chaogang technique in early China based on the slag analysis 基于炉渣分析的古代炒钢技术判定问题. Cult Relics South China 南方文物 1:115–121
Cui Z, Tan Y (2000) Metallurgy and heat treatment金属学与热处理. Mechanical Industry Publishing House 机械工业出版社, Beijing
Dillmann P, L’Héritier M (2007) Slag inclusion analyses for studying ferrous alloys employed in French medieval buildings: supply of materials and diffusion of smelting processes. J Archaeol Sci 34(11):1810–1823
Disser A, Dillmann P, Bourgain C, l’Héritier M, Vega E, Bauvais S, Leroy M (2014) Iron reinforcements in Beauvais and Metz Cathedrals: from bloomery or finery? The use of logistic regression for differentiating smelting processes. J Archaeol Sci 42:315–333
Han R, Ko T (2007) History of science and technology in China: mining and metallurgy中国科学技术史:矿冶卷. Science Press, Beijing
He T (1981) Heat-treatment techniques in ancient China我国古代的钢铁热处理技术. Metal Heat-Treat金属热处理 03:3–6
Hua J (1982) Discussion on the high toughness cast iron during Han and Wei dynasties汉魏高强度铸铁的探讨. Stud Hist Natl Sci \自然科学史研究 1:1–20
Lam W, Chen J, Chong J, Lei X, Tam WL (2018) An iron production and exchange system at the center of the Western Han Empire: scientific study of iron products and manufacturing remains from the Taicheng site complex. J Archaeol Sci 100:88–101
Li Z (1975) The development of iron and steel technology in early feudalism China中国封建社会前期钢铁冶炼技术发展的探讨. Acta Archaeol Sin考古学报 2:1–22
Li Z (1976) The metallurgical achievements in early China based on the iron products unearthed from Mianchi从渑池铁器看我国古代冶金技术的成就. Cultural Relics文物 (08):59–61
Li S, Qian H, Li J (1983) On the formation process of spheroidal graphite and its matrixin early Han iron objects关于汉代铁器中球状石墨和基体组织成因的研究. J Chongqing Univ (Natl Sci) 重庆大学学报 (自然科学版) 1:007
Liu Y, Martinón-Torres M, Chen J, Sun W, Chen K (2019a) Iron decarburisation techniques in the eastern guanzhong plain, china, during late warring states period: an investigation based on slag inclusion analyses. Archaeol Anthropol Sci 11(12):6537–6549
Liu Y, Chen K, Mei J, Martinón-Torres M, Sun W, and Shao A (2019b) Scientific study of iron objects unearthed from Xinfeng Cemetery陕西临潼新丰秦墓出土铁器的科学分析及相关问题. 考古Archaeology (07):108–116
Shaanxi Provincial Insitute of Archaeology (2019) Excavation of the bone workshop in Niejiagou, Xianyang, Shaanxi province陕西咸阳聂家沟秦代制骨作坊清理简报. 考古与文物Archaeol Cult Relics 3:50–62
Wagner DB (1985) Dabieshan: traditional Chinese iron-production techniques practised in southern Henan in the twentieth century. Curzon Press, London and Malmö
Wagner DB (1989) Toward the reconstruction of ancient Chinese techniques for the production of malleable cast iron. East Asian Inst Occas Pap 4:3–72
Williams R (2013) A question of grey or white: why Abraham Darby chose to smelt iron with coke. Hist Metall 47:125–137
Yan G, Wu B (1985) Production technology of malleable cast iron可锻铸铁生产技术. Shanghai Scientific and Technical Literature Publishing House上海科技人文出版社, Shanghai
Zhao Q, Li J, Han R, Qiu L, Tsun K (1985) New discussion on the Tieshenggou iron smelting site in Gong county 巩县铁生沟汉代冶铸遗址再探讨. Acta Archaeol Sin考古学报 (2):157–183
Acknowledgements
We would like to express our gratitude to the Shaanxi Provincial Institute of Archaeology, for all the effort to make this research happen. We would also like to thank Professor Marcos Martinόn-Torres from University of Cambridge, Dr. Michael Charlton, Tom Gregory and Yijie Zhuang from UCL institute of Archaeology, for their guidance and help. We are also indebted to Professor Rubin Han from the Institute of Historical Metallurgy and Materials, USTB for her kind support and advices.
Funding
This research received funding from the National Social Science Fund of China (18BKG033; 18BKG011).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, Y., Xu, W. et al. Archaeometallurgical study of iron objects from a third century BC bone workshop in Xianyang, Shaanxi Province, China. Archaeol Anthropol Sci 14, 71 (2022). https://doi.org/10.1007/s12520-022-01538-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-022-01538-x