Abstract
The paper aims to point out the subsistence in Eneolithic Central Italian communities by Stable Isotope Analysis. This period marked a tipping point in the food strategies because it was characterized by economic changes and several technological improvements leading to enhance land exploitation and livestock breeding. Carbon and nitrogen stable isotope analysis has been used to analyze the food consumption of 54 people belonging to 5 Eneolithic communities scattered throughout Central Italy, where no data have yet been published. The estimation of the main protein intake has been achieved in order to quantify the differences among these communities. The results are consistent with a diet mainly based on terrestrial resources, with no exclusive marine sources consumption, although their occasional usage cannot be ruled out, especially for selected funerary contexts. The data suggest an overall subsistence based on greater local resource procurement, supported by regional productivity maximization. A roughly homogeneous landscape could be outlined in Tuscany and Marche communities witnessing a shared diet preference that could be modified by local preferences. The fully developed trade routes between the two sides of the Apennines could address the overall dietary homogeneity of the studied communities, especially between Fontenoce di Recanati and the southern Tuscan human groups such as Grotta del Fontino and Buca di Spaccasasso, with lesser influence for Le Lellere and Podere Cucule that seem to suggest a more locally based subsistence, even though the funerary affinities do not match this overall diet homogeneity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Copper Age scenario
The Copper Age (or Eneolithic) is a period ranging ca. 4000–2100 cal. BCE and despite the radiometric dates are limited for each archeological context, the measurements shed light on the relationships between the Neolithic and Eneolithic (Manfredini et al. 2013; Dolfini 2010; Leonini et al. 2013; Cazzella et al. 2013; Cocchi Genick 2014). The term Copper Age emphasizes the introduction of the first metal items and their use alongside lithic products: the period is also characterized by economic changes and several technological innovations leading to improved land exploitation and demographic increases. Thus, the Eneolithic marks the tipping point for the transformations in human groups: the emergence of copper processing corresponds to a significant increase in trade, both for the retrieval of the raw material and for manufactured objects (Kostov 2005). This trade accounts for the appearance in Europe of metal objects and exotic items, such as ivory and ostrich eggs, imported from North Africa and the Middle East (Chapman 1990; García Sanjuán et al. 2013). The human groups become more and more complex, creating a variety of funerary practices for selected people (Leonini and Sarti 2006; Cazzella and Guidi 2011; Recchia and Baroni 2011), and somewhere the funerary practices have linked to the megalithic structures, especially in Western European regions (Rubinetto et al. 2013). The Copper Age is also characterized by innovations in productive activities: livestock breeding increases, resulting into what has been called “The Secondary Products Revolution” (Greenfield 2013). This phenomenon is linked to changes in faunal exploitation: domestic animals were initially used solely for meat consumption, but in the Copper Age this management mode is replaced by the optimizing of their secondary products or applications. Secondary animal products can be obtained without slaughtering the animals, and the same animal can be repeatedly exploited over its lifetime (Greenfield 2010): thus, domestics are used for milk, wool, and textile productions. These products enable a significant shift in the economic development of Copper Age communities: the increase in both hunting and productivity leads to mobility, resulting in the occupation of a range of several environments (Sherratt 1993). The wide use of cattle as traction animals enables the cultivation of more land and the transportation of products for greater distances. These factors allow for the accumulation of wealth, and consequently for social differentiation (Champion et al. 2009).
Copper Age in Italy
In Italy, the Copper Age exhibits itself in some cultural fractions (facies) having in common the use of natural cavities as funerary areas, though the sites may be manipulated (Leonini and Sarti 2006). These funerary spaces are distributed in all the peninsular areas, where complex funerary practices are scattered (artificial and natural caves, open air spaces, and tomb enclosures could be addressed). Even the rituals start to be more complex with the coexistence of single and multiple burials as well as violations or disturbance for secondary burial and several funerary goods left to the people. Though several archeological researches have been conducted in Italy about Copper Age communities (Martini 2006; Barfield et al. 2010; Cazzella and Guidi 2011), few studies have broadened the knowledge about how the communities used their alimentary resources, enabling an approach to the socioeconomic organization of human communities. Archeological human remains contain a wealth of information about not only the biology but also the cultural adaptations of past populations, including their dietary supply. It should be noted that to reconstruct the lifestyle of prehistoric communities, human bone analysis is sometimes the only available material and without it a holistic interpretation is not possible.
Thus, in the present paper, a bio-molecular approach based on stable isotope analysis (Katzenberg 2008) will be used to analyze the food consumption of several Eneolithic communities scattered throughout Central Italy, where to the best of our knowledge no data have yet been published. The samples have been chosen to analyze putative cultural connections between the western and eastern regions in central Italy, as previously suggested for archeological records (Sarti 2005): dietary patterns could be hypothesized as one of the most retained markers of the people and societies’ cultural identity. This bio-molecular approach could support an anthropological point of view about an overall homogeneity of Copper Age populations, likely a consequence of extensive gene and cultural flows among human groups (Borgognini Tarli 1992; Vargiu et al. 2009).
Contexts: archeological areas
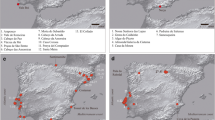
The Eneolithic sites selected for the present study are scattered throughout Central Italy and are mainly located in Tuscany and Marche: Fontenoce di Recanati (Marche), Podere Cucule, Le Lellere, Buca di Spaccasasso, Poggialti Vallelunga, and Grotta del Fontino (Tuscany) (Fig. 1). These funerary areas have been archeologically and chronologically evaluated (Silvestrini et al. 1993; Pacciani 1993; Cencetti and Pacciani 1994; Chilleri and Pacciani 2002; Vigliardi 2002; Volante 2014), but currently lack a bio-molecular characterization of the human remains that have been recovered there. The burials are extremely heterogeneous in every area, and the chronology of the funerary assets is reported in Table 1.
The selected samples pertain to not ritually homogeneous funerary contexts: it is well known that collective burial caves such as Grotta del Fontino and Buca di Spaccasasso hosted a huge number of bone remains in secondary burial deposition, while in rock cut tombs (for instance in Fontenoce di Recanati) the skeletons are found in primary deposition mode.
Stable isotopes background
The analysis of carbon and nitrogen stable isotopes of bone collagen over the last 35 years has been extensively used to study the diets of ancient communities, especially concerning their protein intake (Braun et al. 2013). Indeed, modeling studies based on the results of controlled animal feeding experiments proposed relatively fixed contribution of carbon from protein and energy fractions of diet to collagen (Fernandes et al. 2012). The method is based on the estimation of the ratios of 13C/12C and 15N/14N, detectable from the bone collagen that reflect the diet concerning the last decades of life (Ambrose 1990; Hedges et al. 2007). The natural abundance of 13C and 15N is expressed as per mil (‰) deviation from international standards: δ13C or δ15N (Rsample/Rstandard − 1) × 1000, where R in δ13C or δ15N is 13C/12C or 15N/14N, respectively.
The carbon standard is represented by V-PDB (Vienna PeeDee Belemnite), while atmospheric nitrogen (ambient inhalable reservoir, AIR) (Mariotti 1983) is used as the nitrogen standard.
Dietary reconstruction is based on the concept that the carbon and nitrogen isotope values in consumers’ bone collagen are higher than the corresponding values of their prey (Schwarcz and Schoeninger 2012). For this purpose, the carbon isotope composition (δ13C) is suitable to determine the consumption of plants with different photosynthetic pathways (C3 and C4) (Vogel 1980; Gannes et al. 1998), even though this marker is also useful in distinguishing terrestrial and marine sources (Walker and DeNiro 1986; O'Brien 2015), and freshwater ecosystems are also identified because they often display lower δ13C values than C3 terrestrial plants (Dufour et al. 1999; Doppler et al. 2011). Additionally, the difference in values between two species at adjacent trophic levels is about 1‰ (Bocherens and Drucker 2003; Lee-Thorp 2008).
The nitrogen signature (δ15N) provides information on overall trophic levels because an average growth between 3 and 5‰ in the food net is observed (Tykot 2004; Hedges and Reynard 2007). Thus, it is possible to detect low levels in plants, and greater levels in herbivores, omnivores, and carnivores (DeNiro and Epstein 1978). Moreover, the marine ecosystem sources could be ascertained through nitrogen stable isotope data: this phenomenon also accounts for the marine denitrification process that is associated with the fractionation of the heavy isotope and with the presence of 15N-rich compounds (Dahnke and Thamdrup 2013).
Materials and methods
Sample
A total of 75 human bones were analyzed for the carbon and nitrogen stable isotopes. These samples reported in Table 2 represent the whole human remains sample recovered in each funerary contexts. Despite the sample size in each community is heterogeneous and some of them seem to be trivial, they represent the first cumulative sample of Eneolithic Italian populations and its isotopic characterization comes to fill a critical gap for the region and the Eneolithic timeframe bringing new and reliable evidence regarding prehistoric human populations in the Italian peninsula. For each funerary context, information on sex and age at death were partially available from previous studies (Silvestrini et al. 1993; Pacciani 1993; Cencetti and Pacciani 1994; Chilleri and Pacciani 2002; Vigliardi 2002; Volante 2014).
The baseline for the terrestrial protein component in the diet has been accounted for through nine faunal remains from Fontenoce di Recanati in the Marche region: three are bone fragments of Canis familiaris, two are of Sus scrofa, and four are related to unknown herbivore species found in association with human skeletal remains.
No faunal remains were available for the Tuscany area, so we considered the values from a neighboring area (Latium) for a roughly coeval site (Casetta Mistici, in the area around Rome, De Angelis et al. 2016) that should be consistent with faunal values from Fontenoce di Recanati, supporting the use of the latter as reference ecologic data, while the baseline from the aquatic resources was estimated using information in literature related to different prehistoric time frames due to the lack of local fishbone findings (Richards and Hedges 1999; Drucker and Bocherens 2004; Herrscher and Le Bras-Goude 2010).
Analytical methods
The extraction of collagen was performed following the Longin’s protocol (1971) modified by Brown et al. (1988). In addition, the extraction was performed simultaneously on a modern bovine sample used as a reference. To obtain a satisfactory yield of collagen, the extraction must be performed on about 500 mg of bone powder. The ultrafiltration step, using > 30 kDa Amicon® Ultra-4 Centrifugal Filter Units with Ultracel® membranes (Millipore), was also performed for samples showing a low preservation appearance, in order to maximize the collagen concentration.
Each collagen extract was weighed 0.8–1.2 mg and analyzed in triplicate using continuous-flow isotope ratio mass spectrometry (CF-IRMS) in IGAG (Istituto di Geologia Ambientale e Geoingegneria, CNR) facilities.
To test reliability and exclude contamination from exogenous carbon and nitrogen sources, the samples were compared against established criteria to ascertain the percentages of carbon and nitrogen, atomic C/N ratios, and collagen yields (Ambrose 1990; DeNiro 1985; van Klinken 1999). Analytical precision is ± 0.2‰ for δ13C, reported with respect to the PDB standard, and ± 0.3‰ for δ15N, reported with respect to AIR.
Past v.3.14 software (Hammer et al. 2001) was used to perform descriptive statistics and comparison tests (the Mann-Whitney U test and the Kolmogorov-Smirnov test).
The linear mixing model firstly proposed by Fraser et al. (2013) and recently developed also by Fontanals-Coll et al. (2016) has been employed in order to quantify the fraction of animal protein exploitation out of total dietary protein consumption. As stated by the authors, this model quantifies the percentage of animal protein intake, starting from the midpoint of the usual δ15N offset of + 3‰ and + 5‰ between consecutive trophic levels. Thus, the information based upon the plant, faunal, and human δ15N values could be used to build up several models based on dissimilar trophic chains lying on selected resources δ15N values. These models could proficiently use the plant δ15N values to infer the faunal (herbivore) ones as well as the recorded herbivore data might be significant in achieving sympatric plants values: plant and herbivore values determine the starting point for the esteem of the percentage of animal protein in human diet. Specifically, a first attempt for estimating the animal protein fraction (percentage) of total dietary protein was performed starting from the herbivore δ15N values recorded in Fontenoce di Recanati: the herbivore average δ15N value allows to infer the plant δ15N value by subtract 4‰. Afterwards, several attempts were focused on the single plant group contribution in food web and in the definition of the starting point of the model: the significance of wheat, barley, and pulses was evaluated, whose increasing of 4‰ allows to account for the herbivore δ15N average value that is due to 0% animal protein–based diet. This δ15N magnification was iteratively performed to detect the average human δ15N value whether their diet would be totally due to herbivore-derived protein (100% animal protein–based diet). The interception of the line connecting herbivore- and human-estimated values with the real δ15N values of each community matches their estimated percentage of animal protein in the diet. Unfortunately, few archaeobotanical investigations have been carried out in these areas, despite the fact that numerous archeological surveys have been performed. To the best of our knowledge, no isotopic data are currently available on coeval domestic plants in the considered areas, making hard the application the proposed model with local plant resources. Therefore, the plant δ15N values to draw up the model come from Vaiglova et al. (2014), where floral and faunal isotope data have been determined for reconstructing early farming practices at Kouphovouno, a Late Neolithic village in southern Greece (ca. 5950–4500 cal. BC): this is the best time period and topographical comparison that could be used to constrain the “regional” plant isotopic features. Furthermore, a restricted sample of actual crops (5 grain samples) from central Italy has been isotopically analyzed by Brescia et al. (2002) so as these data were recruited in IsoArcH repository (Salesse et al. 2018) to be used for modeling.
Results
Collagen quality indicators
The collagen extraction was performed for all the samples, but the yield of the organic component was properly obtained for only a fraction of the human bones (Table 3).
The results of the analysis of the human bones for carbon and nitrogen isotopes are listed in Table 4.
The C:N ratio and percent collagen yield are listed as an indication of the reliability of the δ13C and δ15N sample measurements. In order to assess the preservation state of the extracted collagen, different criteria were used: carbon content greater than or equal to 30%, nitrogen content greater than or equal to 10% (Ambrose 1990), and C/N ratios between 2.9 and 3.6 (DeNiro 1985). Data from collagen with an elemental composition below these criteria and C/N ratios outside this range were excluded. The extraction yield was not used as a criterion (Ambrose 1990) because the ultrafiltration technique was used: only samples with yield of 0% were ruled out.
The faunal remains from Fontenoce di Recanati yielded enough collagen to be analyzed in five cases (Table 5): a bone fragment of Canis familiaris, two pig samples, and two unidentified herbivore fragments. The obtained faunal δ13C values are consistent with a C3 European ecosystem (Schwarcz and Schoeninger 1991) where the δ15N signature suggests the proper trophic level for the identified species. The compared faunal samples from Latium (Casetta Mistici, Latium, De Angelis et al. 2016) were proven to be consistent with values from Fontenoce di Recanati, supporting the use of the latter as ecological reference data.
The higher trophic level of the humans (compared to the fauna) ensures quality results and suggests that the livestock might be considered prey stock for the humans.
Out of 75 samples, only 54 fitted the quality criteria (72%). Remarkably, Poggialti Vallelunga does not allow us to perform the analytical process due to the lack of suitable extracted collagen.
Isotopic results
Taking all 54 human individuals (Table 4), δ13C ranges from − 17.5 to − 21.2‰, whereas δ15N values are between 6.9 and 11.6‰. The general distribution indicates a certain heterogeneity (Fig. 2) both in the δ13C and δ15N values, which have a range of more than 4‰.
The sample stratification according to the site allows us to evaluate putative differences in food exploitation, even though they do not reach the level of statistical significance (coupled Mann-Whitney U test, Table 6).
The mean δ13C and δ15N for the five funerary areas were estimated (Table 7, Fig. 3).
The values are consistent with a diet mainly based on terrestrial resources, and all the samples rely on a higher trophic level than the faunal samples, with no clear indication of exclusive marine source consumption. However, their occasional usage cannot be ruled out, due to the high δ15N values in Buca di Spaccasasso and Grotta del Fontino (δ15N = 9.6‰) and their not-so-negative δ13C data.
The previously accomplished anthropological evaluation of the remains (Silvestrini et al. 1993; Pacciani 1993; Cencetti and Pacciani 1994; Chilleri and Pacciani 2002; Vigliardi 2002; Volante 2014) allowed us to determine the age class of all individuals except for those at Grotta del Fontino, where no anthropological evaluation is available. This information led us to dissect the variability in food consumption between adults and children in Fontenoce di Recanati (8 adults and 7 children) and Podere Cucule (2 adults and 1 child).
The mean values of the infants in Fontenoce di Recanati (δ13C = − 19.7‰ and δ15N = 9.4‰) are quite similar to the adult ones (δ13C = − 19.4‰ and δ15N = 8.9‰), suggesting a diet similar to that of the adult population, which means that in childhood no dietary differences caused by physiological growth-related processes (breastfeeding/weaning) can be ascertained (Mann-Whitney U test for δ13C, U = 95.5; Z-score = 0.67; p > 0.05 and Mann-Whitney U test for δ15N, U = 101.5; Z-score = 0.42; p > 0.05). Instead, despite the uniqueness, the sole child in Podere Cucule shows a lower δ15N signature than the adults (7.9‰ vs 9.5‰), suggesting different food consumption, even though the δ13C is quite similar (− 18.4‰ vs − 18.1‰). No differences could be ascertained between males and females in Le Lellere (3 females and 5 males) and Fontenoce di Recanati (4 females and 4 males) although the limited sample size could affect the reliability of this estimation (Le Lellere males/females δ13C U = 30, Z-score = 0.05; p > 0,05; δ15N U = 22, Z-score = 0.81; p > 0,05; Fontenoce di Recanati males/females δ13C U = 31, Z-score = − 0.10; p > 0.05; δ15N U = 30, Z-score = − 0.10; p > 0.05).
Based on the model proposed by Fraser et al. (2013), we tried to distinguish the fraction of animal protein from the total protein intake. It seems to be significantly high in all the considered sites (δ15N mean value, 9.3‰) except for Le Lellere (δ15N value, 7.6‰). If the plain observation leads to an interpretation of these values as due to large amounts of animal protein, this conclusion might be due to an overestimation of this consumption because these values could be ascribed also to a regular intake of plant proteins (Fraser et al. 2013). We can define within this framework a model wherein the inferred plant δ15N value has been determined by taking this offset from the herbivore collagen δ15N values (4.3‰), assuming that humans and herbivores consumed the same plant resources. The standard model can thereby be drawn suggesting how the mean δ15N values for every site indicate a diet exclusively (100%) based on animal protein consumption, and Le Lellere seems to be partially at odds with an animal protein consumption of > 80% of the total protein exploitation (Fig. 4).
Modeled scenario estimating the animal protein fraction (percentage) of total dietary protein in the Central Italy human diet starting from the herbivore values recorded in Fontenoce di Recanati. The herbivore average δ15N value allows to infer the plant δ15N value by subtract 4‰. Avg, average value
Other scenarios could be designed using the real plant values instead of the inferred ones.
The aforementioned literature data for wheat, barley, and peas have been considered a starting point for the models used to quantify the animal protein fraction.
If only wheat (Fig. 5) was the origin of the plant protein intake, every site would be characterized by a vegetarian eating program (fraction of animal protein in total dietary proteins = 0%).
Modeled scenario estimating the animal protein fraction (percentage) of total dietary protein in the Central Italy human diet starting from wheat values from Vaiglova et al. (2014). Herbivore value is obtained by the increase of 4‰ of the starting point of the model
Conversely, if barley (Fig. 6) is set as the starting point of the model, the communities under consideration would have had a mixed diet composed of around 80–90% of animal proteins for almost all the Tuscan sites and Fontenoce di Recanati, while Le Lellere would sustain a more equilibrated landscape (50% of animal proteins consumption).
Modeled scenario estimating the animal protein fraction (percentage) of total dietary protein in the Central Italy human diet starting from barley values from Vaiglova et al. (2014). Herbivore value is obtained by the increase of 4‰ of the starting point of the model
A comparable scenario should be outlined with the pulses (Le Lellere lies below 60%, whereas the other communities exceed 90%; Fig. 7).
Modeled scenario estimating the animal protein fraction (percentage) of total dietary protein in the Central Italy human diet starting from pulses value from Vaiglova et al. (2014). Herbivore value is obtained by the increase of 4‰ of the starting point of the model
The use of Central Italian diachronic sample from Brescia et al. (2002) allows us to reach a credible scenario where Podere Cucule, Buca di Spaccasasso, Grotta del Fontino, and Fontenoce di Recanati communities seem to be featured by a remarkable but not exclusive percentage of animal proteins in their nutritional habits. Indeed, they are characterized by 60–80% of the fraction of animal proteins out of total dietary ones whereas Le Lellere does not exceed 30% (Fig. 8).
Modeled scenario estimating the animal protein fraction (percentage) of total dietary protein in the Central Italy human diet starting from actual grains values from Brescia et al. 2002. Herbivore value is obtained by the increase of 4‰ of the starting point of the model
Discussion
The stable isotope analysis performed for Italian eneolithic human remains in five sites suggests the people consumed a diet mainly based on C3 plant and C3 consumer backbone resources.
The value distribution appears consistent with an overall subsistence based on greater local resource procurement, supported by regional productivity maximization, than the hunting commitments typical of the earlier populations (Lelli et al. 2012; Mannino et al. 2015; Pickard 2016; Crittenden and Schnorr 2017; Scorrano et al. 2018). Even though a global land supply could be highlighted in all the considered areas, appreciable differences seem to exist at the local/regional level that could be ascertained by a single evaluation.
At first glance, there is no evidence of C4 burning up in the whole sample, supporting the notion of widespread Bronze Age consumption in Italy moving from the northeastern regions (Varalli et al. 2016). In spite of the lack of botanical records for these areas, we are able to identify wheat, barley, and some legumes as the plants normally consumed by these communities, on the basis of the archeological information.
The data allows us to state that there is no direct evidence of exclusive marine resource intake, although their occasional consumption (along with freshwater consumption) cannot be ruled out at all. In fact, up to 20% of the consumed protein could conceivably come from the marine ecosystem without any δ13C shift in collagen-derived values (Milner et al. 2004); thus, a mixed diet could be easily misidentified as an exclusively terrestrial diet (Jim et al. 2006). The seashore vicinity of some sites, such as those at Grotta del Fontino and Buca di Spaccasasso, might suggest the putative role marine resources could play in the diet; this is further supported by the discovery of shellfish remains attributable to selective Tuscan coastal environments (Vigliardi 2002; Cavanna 2007) even though the presence of shellfish remains alone cannot be taken to prove their edible usage due to their ornamental role (Micheli 2004). This suggests the existence of a process of collecting and supplying such resources from different coastal types in these areas. Buca di Spaccasasso is currently 10 km away from the current coastline, whereas Grotta del Fontino is located at the edge of the current Grosseto plain, but diachronic differences in the geographic conformation of the Maremma coast should be noted. The plain did not exist, the seaside slowly went into interruption, and the Ombrone and Bruna rivers flowed into the Prile coastal lake (Fig. 9; Ceccarelli and Niccolucci 2003).
The standard linear mixing model (Fig. 4) points out very high values that are unlikely to be considered because they entail vast quantities of edible meat and dairy products, and even though several faunal remains have been recovered, they cannot account for the sole animal product expenditure. In spite of the scanty information available, a general overview of plant gathering and cultivation could be roughly ascertained from the published list of findings and indirect evidence (Bellini et al. 2008) suggesting how cereals and legumes were widely recovered in central Italian archeological surveys. Indeed, Triticum sp. (both glumen and naked wheats) and barley (Hordeum sp.) represented the most frequent occurrences, even though some Panicum miliaceum seeds are described in Tuscany in the Eneolithic/Bronze Age time edge (Bellini et al. 2008; Mariotti-Lippi et al. 2017). This latter evidence seems to be consistent with other research (Tafuri et al. 2009; Varalli et al. 2016) into how millet could be an important crop during the Middle Bronze Age in Italy. The research shows that it could be cultivated from at least the Early Bronze Age onward, and perhaps before then, though no isotopic evidence suggests its edible usage in presented eneolithic communities.
As stated above, if only wheat (Fig. 5) was the origin of the plant protein intake, every site would be characterized by a vegetarian eating program. This is unlikely, given the ecological and structural biological needs, as grains contain less protein than animal flesh, legumes, and seeds, at an average of about 10–12% of their dry weight (Shewry and Halford 2002). Moreover, wheat and other cereal proteins do not contain as many nutritionally essential amino acids as animal protein. Further, if consumed as a sole protein source, they are not utilized with the same efficiency as animal protein compounds to meet the physiological requirements for the people living there as a total protein, or to provide specific indispensable amino acids such as lysine, threonine, and tryptophan. However, when combined with other food such as legumes, oil seeds, and animal products, the proteins of wheat exhibit excellent nutritional complementarity (Young and Pellett 1985). The δ15N values of wheat reported by Vaiglova et al. (2014) seem too high for the Italian herbivore samples that sit at lower values (4.3‰) than their putative forages (5.6 ‰) and it should be noted that these high values could be ascribed to manuring practices that were employed as early as the Neolithic period in Europe to intensify arable production leading to increases in populations and to more opportunities for trading (Guttmann et al. 2005). The manuring effect is well documented as a modifying factor for nitrogen values (Fraser et al. 2011; Kanstrup et al. 2011, 2014), which could affect proper nitrogen identification in the recorded wheat. The result obtained through the modern crops appears to be more conceivable (Fig. 8): despite the known differences in δ15N values due to differential degree of manuring and irrigation between modern and ancient horticultural practices, the proposed model fits the economic sustainability of ancient communities, whose subsistence should be based on a mixed animal and plant resource consumption.
The barley-based model (Fig. 6) seems to be even comparable: this cereal is usually cultivated in warm climates and complements wheat, which thrives in cooler climates. Their nutritional features are quite similar: it is considered high in carbohydrates, fat, dietary fiber, vitamin B, vitamin C, calcium, iron, magnesium, phosphorus, potassium, zinc, and folate but is deficient in the essential mineral selenium (Biel and Jacyno 2013).
A similar scenario would be outlined if we considered the pulses (Fig. 7) the main plant resources. Pulses typically contain about twice the amount of protein found in whole grain cereals such as wheat and barley and can constitute a major source of protein in a diet. Moreover, when other foods are combined with pulses, their nutritional value is further enhanced, because other foods enable the body to better absorb the nutrients found in pulses (Trichopoulou et al. 2009). Thus, they could be a primary foodstuff for ancient people, an idea that is supported by evidence related to agronomy information regarding the legume cultivation attested in the Tuscany area (Oliva 1939), and in general these plants gained greater edible magnitude during the Eneolithic (Mariotti-Lippi et al. 2006). All the proposed scenarios consider communities with mixed diets of plants, cereals, pulses, and animal proteins, and undoubtedly people from Le Lellere preferred a more mixed diet than the other peoples, mainly based on their arable resources. No differences could be outlined between the two sides of the Apennines, which makes sense, given the shared open trade net between Tuscany and Marche (Sarti 2005). Cultural contacts between the two sides of the Apennines have been extensively documented, as well as cultural swaps in material culture and funerary architectures (Cazzella and Silvestrini 2005; Leonini and Sarti 2006), especially between the eastern Marche region and the southern area of Tuscany. The northeastern area, meanwhile, seems to preserve a more autarchic trait: in this frame, the difference between Le Lellere and the other evaluated Tuscanian samples could mirror specific features in dietary habits. Of course, this scenario was sporadically intermixed, as suggested by the condition of Podere Cucule, which falls in the quite homogenous scenario proposed for southern Tuscany and Marche as to the exploited animal protein fraction highlighted by the δ15N values. Although the sample sizes for some funerary contexts—such as Le Lellere, Podere Cucule, and Buca di Spaccasasso—are quite low and render any statistical evaluation unacceptable, the Kolmogorov-Smirnov test fails to identify significant differences between the Tuscan sites and the Fontenoce di Recanati isotopes values (δ13C, D = 0.10; p = 0.98; δ15N, D = 0.14; p = 0.75), confirming the overall dietary similarities between the two sides of the Apennines.
This homogeneity notwithstanding several peculiar elements could indicate some differences between these subsistence economies and could be ascribed to local environmental exploitation. Indeed, if the extremely small sample size could lead to misinterpretation, Podere Cucule shows very different δ13C values. These values could be ascribed to several factors despite the lack of carpological elements, making C4 plant consumption less plausible despite their presence in Tuscany in other surveys (Bellini et al. 2008). Trade from the Adriatic regions suggests at least a sporadic and not continuous exploitation that could move the carbon values toward less negative results. In spite of this evidence, the changes in plant management strategies could address this heterogeneity. Indeed, many studies have shown that plants grown under water stress produce higher δ13C values (Saugier et al. 1993; Ferrio et al. 2005), allowing for the determination of a significant expected relationship between δ13C and the environmental parameters related to water availability. This proposed approach has been pioneeringly used (Araus and Bux 1993) in grains from archeological sites to gain insight into the environmental conditions of early agriculture, allowing for the relative quantification of water inputs and crop yields (Araus et al. 2002). It could also be a proxy for obtaining further information about climate-derived and anthropogenic effects on plants’ water status, as well as for distinguishing different strategies of water management. Thus, the more positive δ13C values could mask different strategies to obtain plant resources in Podere Cucule, even though the limited sample size makes it hard to properly understand the subsistence economy in such a community. Of course, this bias underlines a need to magnify the human sample size related to this community even though the current one represents all the human specimens recovered to date. Moreover, local seeds or plant macroremains and faunal individuals should be classified in order to confirm this subsistence role for the community and clarify inferences about the putative inter-site differences.
A local environmental constraint also seems to be responsible for the isotopic results at the Marche site of Fontenoce di Recanati. This area (Silvestrini and Pignocchi 1997) is located close to the Potenza and Menocchia rivers, which could have represented valuable hunting and fishing grounds for the people living in their vicinity. Despite substantial introgression evidence from the eastern shores of the Adriatic sea (Cazzella and Silvestrini 2005), there is no nutritional awareness of foods that could be attributed to Aegean/Balkan populations, such as C4 metabolism plants, that were later introduced to Italy from the northeast (Tafuri et al. 2009, 2018).
Conclusions
This paper presents the first data about subsistence strategies for Copper Age communities in Central Italy, filling an existing gap in the bio-molecular analysis of the prehistoric Italian inhabitants. The dissection of the diet of people buried in the Tuscan and Marche areas during the Eneolithic seems to indicate a dietary pattern mainly based on terrestrial resources, with the greatest role played by animal protein. If the backbone dietary component for all the communities came from C3 plants, it cannot be denied that there was a remarkable recourse to protein foodstuffs in every community, with consumption of animal sources between 60 and 100%, except in Le Lellere, where the animal protein input dropped to low extent (30–50%). C3 plant sources such as wheat, barley, and pulses do not seem exclusively important to the diets of those samples, while the animal protein component would mainly come from the flesh of terrestrial herbivores (the livestock of the community), even though the use of milk and dairy products cannot be ruled out.
The archeological findings and the topographical locations of Buca di Spaccasasso, Grotta del Fontino, and Fontenoce di Recanati could represent evidence of the consumption of marine and freshwater resources, and their burning should not be ignored as merely occasional. These marine and freshwater protein resources could also be highlighted in Neolithic coastal sites in Italy (Lelli et al. 2012) and in the Mediterranean region (Lightfoot et al. 2011), suggesting that consumption of this food clearly supported the rise of refined farming societies. These results support the hypothesis that the land exploitation maximization that occurred during the Eneolithic period had to contend with environmental constraints that, in such cases, provided beneficial habitats for spontaneous foraging. This might be corroborated by the evidence of putative eating support in the form of spontaneous C3 plants in Podere Cucule, where the watering strategies could not be so finely tuned as to guarantee steady opportunities for cereal reaping. Despite a lack of carpological evidence, occasional C4 plant consumption could be conceivable and a negligible amount of marine resource exploitation cannot be totally ruled out.
The existing fully developed trade routes between the two sides of the Apennines could address the highlighted overall dietary homogeneity of the studied communities, especially between Fontenoce di Recanati and the southern Tuscan human groups such as those at Grotta del Fontino and Buca di Spaccasasso, with lesser influence for Le Lellere and Podere Cucule, which seem to suggest a more locally based subsistence strategy, even though the funerary affinities do not match this overall diet homogeneity.
References
Ambrose SH (1990) Preparation and characterization of bone and tooth collagen for isotopic analysis. J Archaeol Sci 17:431–451
Araus JL, Bux R (1993) Changes in carbon isotope discrimination in grain cereals from the north-western Mediterranean Basin during the past seven millennia. Aust J Plant Physiol 20:117–128
Araus JL, Slafer GA, Reynolds MP, Royo C (2002) Plant breeding and water stress in C3 cereals: what to breed for? Ann Bot 89:925–940
Barfield LH, Sturt WM, Valzolgher E, Higham TFG (2010) A wiggle-matched date for the copper age cemetery at Manerba del Garda Northern Italy. Radiocarbon 52:984–1001
Bellini C, Mariotti-Lippi M, Mori Secci M, Aranguren B, Perazzi P (2008) Plant gathering and cultivation in prehistoric Tuscany (Italy). Veg Hist Archaeobotany 17:103–112
Biel W, Jacyno E (2013) Chemical composition and nutritive value of spring hulled barley varieties. Bulg J Agric Sci 19:721–727
Bocherens H, Drucker D (2003) Trophic level isotopic enrichments for carbon and nitrogen in collagen: case studies from recent and ancient terrestrial ecosystems. Int J Osteoarchaeol 13:46–53
Borgognini Tarli S (1992) Aspetti antropologici e paleodemografici dal Paleolitico Superiore alla prima eta’del ferro In: Guidi A, Piperno M (eds) Italia Preistorica. Laterza, pp 238–273
Braun A, Auerswald K, Vikari A, Schnyder H (2013) Dietary protein content affects isotopic carbon and nitrogen turnover. Rapid Commun Mass Spectrom 27:2676–2684
Brescia MA, Di Martino G, Fares C, Di Fonzo N, Platani C, Ghelli S, Reniero F, Sacco A (2002) Characterization of Italian durum wheat semolina by means of chemical analytical and spectroscopic determinations. Cereal Chem 79:238–242
Brown TA, Nelson DE, Vogel JS, Southon JR (1988) Improved collagen extraction by modified Longin method. Radiocarbon 30:171–177
Cavanna C (2007) La preistoria nelle grotte del parco naturale della Maremma. Museo Di Storia Naturale Della Maremma, Grosseto
Cazzella A, Guidi A (2011) Il concetto di Eneolitico in Italia. In: Atti XLIII Riunione Scientifica IIPP. Ist Italiano di Preistoria, Florence, pp 25–32
Cazzella A, Silvestrini M (2005) L’Eneolitico delle Marche nel contesto degli sviluppi culturali dell’Italia centrale. In Atti IIPP XXXVIII Ist Italiano di Preistoria, Florence, pp 371–386
Cazzella A, Pignocchi G, Silvestrini M (2013) Cronologia eneolitica delle Marche. In: Cocchi Genick D (ed) Cronologia assoluta e relativa dell’età del rame in Italia. Atti dell’Incontro di Studi Università di Verona, Verona, pp 119–136
Ceccarelli L, Niccolucci F (2003) Modelling time through GIS technology: the Ancient Prile Lake (Tuscany Italy). In: Doerr M, Apostolis S (eds) The Digital Heritage of Archaeology Proceedings of the 30th CAA Conference Heraklion, pp 133–138
Cencetti S, Pacciani E (1994) Resti umani di età eneolitica a Colle Val d’Elsa (Siena). Bulletino di Paletnologia Italiana 85:287–306
Champion T, Gamble C, Shennan S, Whittle AW (2009) Prehistoric Europe. Left Cost Press, Walnut Creek
Chapman R (1990) Emerging complexity: the later prehistory of South-East Spain Iberia and the West Mediterranean. Cambridge University Press, Cambridge
Chilleri F, Pacciani E (2002) Note antropologiche sui resti umani della necropoli eneolitica di Poggialti Vallelunga (Pitigliano - GR) In: Atti del V incontro di studi “Preistoria e Protostoria in Etruria” vol II. Centro studi di Preistoria e Archeologia, Milano, pp 513–520
Cocchi Genick D (2014) Cronologia assoluta e relativa dell'età del rame in Italia. QuiEdit, Verona
Crittenden AN, Schnorr SL (2017) Current views on hunter-gatherer nutrition and the evolution of the human diet. Am J Phys Anthropol 162:84–109
Dahnke K, Thamdrup B (2013) Nitrogen isotope dynamics and fractionation during sedimentary denitrification in Boknis Eck Baltic sea. Biogeosci Discuss 10:681–709
De Angelis F, Di Giannantonio S, Scorrano G, Catalano P, Rickards O (2016) An integrated approach to subsistence of the Eneolithic communities of Via Casetta Mistici and Osteria del Curato – via Cinquefrondi. In: Rickards O, Sarti L (eds) Biological and cultural heritage of the central-southern Italian population through 30 thousand years. Universitalia, pp 107–123
DeNiro MJ (1985) Post-mortem preservation and alteration of in vivo bone collagen isotope ratios in ralation to paleodietary reconstruction. Nature 317:806–809
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Dolfini A (2010) The origins of metallurgy in central Italy: new radiometric evidence. Antiquity 84:707–723
Doppler S, Vohberger M, Carnap-Bornheim C, Heinrich D, Peters J, Grupe G (2011) Biodiversity of archaeological fauna in the estuarine palaeoecosystem of the Schleifjord Northern Germany: isotopic evidence. In: Grupe G, McGlynn G, Peters J (eds) Archaeobiodiversity, a european perspective. Verlag Marie Leidorf, Rahden-Westfalen, pp 21–70
Drucker D, Bocherens H (2004) Carbon and nitrogen stable isotopes as tracers of change in diet breadth during Middle and Upper Palaeolithic in Europe. Int J Osteoarchaeol 14:162–177
Dufour E, Bocherens H, Mariotti A (1999) Palaeodietary implications of isotopic variability in Eurasian lacustrine fish. J Archaeol Sci 26:617–627
Fernandes R, Nadeau MJ, Grootes PM (2012) Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol Anthropol Sci 4:291–301
Ferrio JP, Mateo MA, Bort J, Abdalla O, Voltas J, Araus JL (2005) Relationships of grain delta C-13 and delta O-18 with wheat phenology and yield under water-limited conditions. Ann Appl Biol 150:207–215
Fontanals-Coll M, Eulàlia Subirà M, Díaz-Zorita Bonilla M, Gibaja JF (2016) First insight into the Neolithic subsistence economy in the north-east Iberian Peninsula: paleodietary reconstruction through stable isotopes. Am J Phys Anthropol 62:36–50
Fraser RA, Bogaard A, Heaton T, Charles M, Jones G, Christensen BT, Halsteadc P, Merbache I, Poultonf PR, Sparkesg D, Styring AK (2011) Manuring and stable nitrogen isotope ratios in cereals and pulses: towards a new archaeobotanical approach to the inferences of land use and dietary practices. J Archaeol Sci 38:2790–2804
Fraser RA, Bogaard A, Schäfer M, Arbogast R, Heaton THE (2013) Integrating botanical faunal and human stable carbon and nitrogen isotope values to reconstruct land use and palaeodiet at LBK Vaihingen an der Enz Baden-Wurttemberg. World Archaeol 45:492–517
Gannes LZ, Martínez del Rio C, Koch P (1998) Natural abundance variations in stable isotopes and their potential uses in animal physiological ecology. Comp Biochem Physiol A Mol Integr Physiol 119:725–737
García Sanjuán L, Lucianez Trivino M, Schuhmacher TX, Wheatley D, Banerjee A (2013) Ivory craftsmanship trade and social significance in the southern Iberian Copper Age: the evidence from the PP4-Montelirio sector of Valencina de laConcepción (Seville Spain). Eur J Archaeol 16:610–635
Greenfield HJ (2010) The secondary products revolution: the past the present and the future. World Archaeol 42:29–54
Greenfield HJ (2013) Animal secondary products: archaeological perspectives on domestic animal exploitation in the Neolithic and Bronze Age. Oxbow, Oxford
Guttmann EB, Simpson I, Davidson D (2005) Manuring practices in antiquity: a review of the evidence. In: Smith DN, Brickley MB, Smith W (eds) Fertile ground: papers in honour of Susan Limbrey. Oxbow, Oxford, pp 68–76
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:9
Hedges REM, Reynard LM (2007) Nitrogen isotopes and the trophic level of humans in archaeology. J Archaeol Sci 34:1240–1251
Hedges RE, Clement JG, Thomas CD, O'Connell TC (2007) Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements American. J Phys Anthropol 133:808–816
Herrscher E, Le Bras-Goude G (2010) Southern French Neolithic populations: isotopic evidence for regional specificities in environment and diet. Am J Phys Anthropol 141:259–271
Jim S, Jones V, Ambrose SH, Evershed RP (2006) Quantifying dietary macronutrient sources of carbon for bone collagen biosynthesis using natural abundance stable carbon isotope analysis. Br J Nutr 95:1055–1062
Kanstrup M, Thomsen IK, Andersen AJ, Bogaard A, Christensen BT (2011) Abundance of 13C and 15N in emmer spelt and naked barley grown on differently manured soils: towards a method for identifying past manuring practice. Rapid Commun Mass Spectrom 25:2879–2887
Kanstrup M, Holst M, Jensen P, Thomsen IK, Christensen BT (2014) Searching for long-term trends in prehistoric manuring practice δ15N analyses of charred cereal grains from the 4th to the 1st millennium BC. J Archaeol Sci 51:115–125
Katzenberg M (2008) Stable isotope analysis: a tool for studying past diet demography and life history. In: Katzenberg M, Saunders S (eds) Biological anthropology of the human skeleton. Wiley-Liss, New York, pp 413–442
Kostov RI (2005) Precious and decorative minerals from the Eneolithic necropolis in Northeastern Bulgaria and their significance in the history of gemology. In: Society YBG (ed) 80 Years Bulgarian Geological Society Society. YBG, Sofia, pp 205–208
Lee-Thorp JA (2008) On isotopes and old bones. Archaeometry 50:925–950
Lelli R, Allen R, Biondi G, Calattini M, Conati Barbaro C, Gorgoglione MA, Manfredini A, Martínez-Labarga C, Radina F, Silvestrini M, Tozzi C, Rickards O, Craig OE (2012) Examining dietary variability of the earliest farmers of south-eastern Italy. Am J Phys Anthropol 149:380–390
Leonini V, Sarti L (2006) Sepolture e rituali funerari nell’Eneolitico e al passaggio all’età del Bronzo in Italia. In: Martini F (ed) La cultura del morire nelle società preistoriche e protostoriche italiane. Ist Italiano di Preistoria, Florence, pp 129–160
Leonini V, Sarti L, Volante N (2013) La cronologia dell’età del Rame in area fiorentina nel quadro dell’Italia centrale tirrenica Stato dell’arte e nuove datazioni. In: Cocchi Genick D (ed) Cronologia assoluta e relativa dell’età del Rame in Italia. Atti Incontro di studi Verona, University of Verona, Verona, pp 67–80
Lightfoot E, Boneva B, Miracle P, Šlaus M, O'Connell T (2011) Exploring the Mesolithic and Neolithic transition in Croatia through isotopic investigations. Antiquity 85:73–86
Longin R (1971) New method of collagen extraction for radiocarbon dating. Nature 230:241–242
Manfredini A, Fugazzola-Delpino MA, Sarti L, Silvestrini M, Martini F, Conati Barbaro C, Muntoni I, Pizziolo G, Volante N (2013) Adriatico e Tirreno a confronto: analisi dell’occupazione territoriale tra il Neolitico fonale e l’età del Rame in alcune aree campione dell’Italia centrale. Riv Sc Preist 59:115–180
Mannino MA, Talamo S, Tagliacozzo A, Fiore I, Nehlich O, Piperno M, Tusa S, Collina C, Di Salvo R, Schimmenti V, Richards MP (2015) Climate-driven environmental changes around 8200 years ago favoured increases in cetacean strandings and Mediterranean hunter-gatherers exploited them. Sci Rep 5:16288
Mariotti A (1983) Atmospheric nitrogen is a realiable standard for natural 15N abundance measurements. Nature 303:685–687
Mariotti-Lippi M, Mori Secci M, Bellini C, Gonnelli T (2006) Plants in the diet in Prehistoric Tuscany. Atti Soc Nat Mat Modena 343–353
Mariotti-Lippi M, Pisaneschi L, Sarti L, Lari M, Moggi-Cecchi J (2017) Insights into the Copper-Bronze Age diet in Central Italy: plant microremains in dental calculus from Grotta dello Scoglietto (Southern Tuscany Italy). J Archaeol Sci Rep 15:30–39
Martini F (2006) La cultura del morire nelle società preistoriche e protostoriche italiane. Ist Italiano di Preistoria, Florence
Micheli R (2004) Gli ornamenti in conchiglia del Neolitico dell’Italia settentrionale. Preistoria Alpina 40:53–70
Milner N, Craig OE, Bailey GN, Pedersen K, Andersen SH (2004) Something fishy in the Neolithic? A re-evaluation of stable isotope analysis of Mesolithic and Neolithic coastal populations. Antiquity 78:9–22
O'Brien DM (2015) Stable isotope ratios as biomarkers of diet for Health Research. Annu Rev Nutr 35:565–594
Oliva A (1939) I frumenti le leguminose da granella e gli altri semi repertati a Belverde. Studi Etruschi 13:343–351
Pacciani E (1993) Resti ossei umani da una sepoltura di tipo Rinaldoniano in località Podere Cucule (Pogibonsi – SI). In: Negroni-Catacchio N (ed) Atti del Primo Incontro di Studi “La cultura di Rinaldone – Ricerche e Scavi”. Eureka, Milano
Pickard C (2016) Prehistoric molluscan remains from tell Aqab Northeast Syria. In: Yeshuran R, Weissbrod L, Marom N, Bar-Oz G (eds) Bones and identity: zooarchaeological approaches to reconstructing social and cultural landscapes in Southwest Asia. Oxbow, Oxford, pp 157–169
Recchia G, Baroni I (2011) Aspetti demografici nell’analisi delle comunità eneolitiche dell'Italia centro-meridionale. In: Atti della XLIII Riunione Scientifica dell'IIPP. Ist Italiano di Preistoria, Florence, pp 49–56
Richards MP, Hedges REM (1999) Stable isotope evidence for similarities in the types of marine foods used by late Mesolithic humans at sites along the Atlantic coast of Europe. J Archaeol Sci 26:717–722
Rubinetto V, Appolonia L, De Leo S, Serra M, Borghi A (2013) A petrographic study of the anthropomorphic Stelae from the megalithic area of Saint-Martin-De-Corléans (Aosta Northern Italy). Archaeometry 56:927–950
Salesse K, Fernandes R, de Rochefort X, Brůžek J, Castex D, Dufour É (2018) IsoArcH.eu: an open-access and collaborative isotope database for bioarcheological samples from Graeco-Roman World and its margins. J Archaeol Sci Rep 19:1050–1055
Sarti L (2005) Rapporti tra Marche e Toscana nell’Eneolitico sulla base dell’indicatore ceramico. In: Atti IPP. Ist Italiano di Preistoria, Florence, pp 387–398
Saugier B, Ehleringer JR, Hall AE, Graham D (1993) Stable isotopes and plant carbon-water relations. Academic Press, Cambridge
Schwarcz HP, Schoeninger MJ (1991) Stable isotope analyses in human nutritional ecology. Yearb Phys Anthropol 34:283–321
Schwarcz HP, Schoeninger MJ (2012) Stable isotopes of carbon and nitrogen as tracers for Paleo-diet reconstruction. In: Baskaran M (ed) Handbook of environmental isotope geochemistry advances in isotope geochemistry. Springer, Berlin
Scorrano G, Baldoni M, Brilli M, Rolfo MF, Fornaciari G, Rickards O, Martínez-Labarga C (2018) Effect of Neolithic transition on an Italian community: Mora Cavorso (Jenne Rome). Archaeol Anthropol Sci. https://doi.org/10.1007/s12520-018-0615-9
Sherratt A (1993) What would a Bronze-Age world system look like? Relations between temperate Europe and the Mediterranean in later prehistory. J Eur Archaeol 1:1–58
Shewry PR, Halford NG (2002) Cereal seed storage proteins structures properties and role in grain utilization. J Exp Bot 53:947–958
Silvestrini M, Pignocchi G (1997) La necropoli eneolitica di Fontenoce di Recanati: lo scavo 1992. Rivista di Scienze Preistoriche 48:309–366
Silvestrini M, Cilla G, Pignocchi G (1993) La necropoli eneolitica di Fontenoce (Recanati) Picus 12-13: 127–185
Tafuri MA, Craig OE, Canci A (2009) Stable isotope evidence for the consumption of millet and other plants in Bronze Age Italy. Am J Phys Anthropol 139:146–153
Tafuri MA, Rottoli M, Cupitò M, Pulcini ML, Tasca G, Carrara N, Bonfanti F, Salzani L, Canci A (2018) Estimating C4 plant consumption in Bronze Age Northeastern Italy through stable carbon and nitrogen isotopes in bone collagen. Int J Osteoarchaeol 28:131–142
Trichopoulou A, Bamia C, Trichopoulos D (2009) Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 338:b2337
Tykot RH (2004) Stable isotopes and diet: you are what you eat. In: Martini F, Milazzo M, Piacentini M (eds) Proceedings of the International School of Physics “Enrico Fermi”. Course CLIV IOS Press, Amsterdam, pp 433–444
Vaiglova P, Bogaard A, Collins M, Cavanagh W, Mee C, Renard J, Lamb A, Gardeisen A, Fraser R (2014) An integrated stable isotope study of plants and animals from Kouphovouno southern Greece: a new look at Neolithic farming. J Archaeol Sci 42:210–215
van Klinken GJ (1999) Bone collagen quality indicators for Palaeodietary and radiocarbon measurements. J Archaeol Sci 26:687–695
Varalli A, Moggi-Cecchi J, Moroni A, Goude G (2016) Dietary variability during Bronze Age in Central Italy: first results. Int J Osteoarchaeol 26:431–446
Vargiu R, Cucina A, Coppa A (2009) Italian populations during the Copper Age: assessment of biological affinities through morphological dental traits. Hum Biol 8:479–493
Vigliardi A (2002) La grotta del Fontino – Una cavità funeraria eneolitica del grossetano. EDIFIR, Florence
Vogel JC (1980) Fractionation of the carbon isotopes during photosynthesis. Springer Verlag, Berlin
Volante N (2014) La Collina di Spaccasasso: evidenze funerarie e minerarie nel Parco regionale della Maremma Nuovi dati. In: Negroni Catacchio N (ed) Paesaggi cerimoniali – ricerche e scavi. Arbor Sapientae, Rome, pp 625–636
Walker PL, DeNiro MJ (1986) Stable nitrogen and carbon isotope ratios in bone collagen as indices of prehistoric dietary dependence on marine and terrestrial resources in southern California. Am J Phys Anthropol 71:51–61
Young VR, Pellett PL (1985) Wheat proteins in relation to protein requirements and availability of amino acids. Am J Clin Nutr 41:1077–1090
Acknowledgements
We are grateful to every Institution involved in this project for granting access to the skeletal remains that have been analyzed. This work was supported by the Italian Ministry of Education, Universities and Research (MIUR) through PRIN 2010–2011 action (EPIC Project: Biological and cultural heritage of the central-southern Italian population through 30 thousand years. Grant ID: 2010EL8TXP) allotted to OR. We are grateful to the anonymous reviewers for the critical reading of the paper and for the useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Angelis, F., Scorrano, G., Martínez-Labarga, C. et al. Eneolithic subsistence economy in Central Italy: first dietary reconstructions through stable isotopes. Archaeol Anthropol Sci 11, 4171–4186 (2019). https://doi.org/10.1007/s12520-019-00789-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12520-019-00789-5