Abstract
Background
During the coronavirus disease 2019 (COVID-19) pandemic, there is an urgent need for safe and effective COVID-19 vaccines to protect children and adolescents. This study aims to provide scientific evidence and recommendations for the application of COVID-19 vaccines in children and adolescents by analyzing the latest studies.
Methods
We systematically searched MEDLINE (accessed through PubMed), Embase, and Web of Science from January 1, 2020, to October 8, 2022. Eligible clinical trials, cohort studies, case‒control studies, and cross-sectional studies with extractable data were included in immunogenicity, effectiveness, and safety analyses. According to the heterogeneity, we chose a fixed-effect model (when I2 ≤ 50) or a random-effects model (when I2 > 50) to pool effect values.
Results
A total of 88 articles were included. The seroconversion rates after the first, second, and third doses of the vaccines were 86.10%, 96.52%, and 99.87%, respectively. After the first and second doses, vaccine effectiveness (VE) against severe acute respiratory syndrome coronavirus 2 infection was 42.87% [95% confidence interval (CI) = 27.09%–58.65%] and 63.33% (95% CI = 52.09%–74.56%), respectively. After the first and second doses, VE against COVID-19 was 60.65% (95% CI = 44.80%–76.50%) and 75.77% (95% CI = 63.99%–87.56%), respectively. VE against hospitalization due to COVID-19 after the first and second doses was 72.74% (95% CI = 51.48%–94.01%) and 82.78% (95% CI = 75.78%–89.78%), respectively. The most common adverse events were injection site pain, fatigue/asthenia/tiredness, headache, myalgia/muscle pain, and chills. The incidence rate of myocarditis or pericarditis was 2.42/100,000 people. In addition, the subgroup analysis showed that children aged ≤ 5 years had the lowest incidence of adverse events, and the incidence rate of adverse events was higher for mRNA vaccines than for inactivated vaccines.
Conclusions
COVID-19 vaccines have good immunogenicity, effectiveness, and safety among children and adolescents. We recommend that children and adolescents be vaccinated as soon as possible to protect them and slow the spread of COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19) is a respiratory infectious disease and is still in pandemic status [1]. According to the World Health Organization (WHO), there are 624,235,272 confirmed cases and 6,555,270 deaths worldwide as of October 24, 2022 [2]. Countries around the world have taken measures such as interventions and large-scale vaccinations in response to the COVID-19 pandemic. Previous meta-analyses and active surveillance studies have shown that the COVID-19 vaccine is a safe and effective way to prevent SARS-CoV-2 infection, symptomatic infection, severe cases, and death [3,4,5,6,7]. Currently, a total of 135 COVID-19 vaccines are in clinical trials worldwide, and 32 COVID-19 vaccines are in use [8]. By April 8, 2022, the WHO had assessed ten COVID-19 vaccines, including ChAdOx1-S/nCoV-19, Ad26.COV2.S, mRNA-1273, BNT162b2, BBIBP-CorV, CoronaVac, BBV152, NVX-CoV2373, and Ad5-nCoV to meet the necessary criteria for safety and efficacy [9]. A total of 5,392,424,039 people worldwide have received at least one dose of COVID-19 vaccines, and 4,951,178,365 people have been fully vaccinated as of October 18, 2022 [2].
Compared to adults, SARS-CoV-2 infection causes less severe illness and fewer deaths among children and adolescents [10]. However, there are still a considerable number of children and adolescents diagnosed with COVID-19. WHO surveillance data showed that from December 30, 2019, to September 13, 2021, the global numbers of COVID-19 cases < 5 years old and 5–14 years old were 1,695,265 and 6,020,084, respectively, accounting for 1.8% and 6.3% of the total number of cases [2, 10]. Children and adolescents infected with SARS-CoV-2 might also be underdiagnosed due to a less severe course of infection [10]. Furthermore, children and adolescents may develop severe COVID-19-related complications, such as multisystem inflammatory syndrome, which can lead to shock and multiple organ failure requiring intensive care [11]. The emergence of more transmissible Omicron variants has brought new challenges to the prevention and control of the pandemic. A study in 14 states in the United States found that the peak rate of COVID-19-related hospitalizations in children and adolescents aged 0–17 years during the Omicron-variant-dominant period was four times higher than that during the Delta-variant-dominant period [12].
The WHO recommends that countries consider vaccinating healthy children and adolescents over 5 years. The BNT162b2 vaccine is safe for children over 5 years, and the mRNA-1273 and BNT162b2 vaccines are approved for use in children over 12 years [13]. The effectiveness and safety of COVID-19 vaccines among children and adolescents are the focus of attention, with experimental studies [14,15,16] and observational studies [17,18,19,20,21,22,23,24] continuing to emerge. However, there is currently a lack of updated studies that systematically review the immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents. Therefore, this study collected published studies and systematically evaluated the immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years to provide scientific evidence and recommendations for the application of COVID-19 vaccines among children and adolescents.
Methods
This study was registered in the Prospective Register of Systematic Reviews (ID: CRD42022335219). The study process strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines [25].
Research strategies
We systematically searched MEDLINE (accessed through PubMed), Embase, and Web of Science from January 1, 2020, to October 8, 2022. The search terms consisted of following four parts: (1) SARS-CoV-2, COVID-19; (2) COVID-19 vaccine, SARS-CoV-2 vaccine; (3) infant, child, adolescent; and (4) immunogenicity, effectiveness, safety. The above four parts are logically connected using "AND". The detailed research strategy is shown in Supplementary Table 1.
Inclusion and exclusion process
Inclusion criteria were as follows: (1) research topic: studies that evaluated the immunogenicity, effectiveness or safety among children and adolescents (≤ 18 years old) after vaccination with COVID-19 vaccines; (2) study design: clinical trial, cohort study, case‒control study, and cross-sectional study were included; and (3) vaccine type: unlimited. Exclusion criteria are as follows: (1) topic was irrelevant: such as vaccination among adults, using a model to evaluate, or population does not be vaccinated; (2) study design: review, case series, case report, letter, conference abstract, or comment; (3) data were not sufficient for analysis, such as unable to extract or unable to pool effect values; and (4) duplicate articles.
The records downloaded from databases were managed using EndNote 20 (Thomson Research Soft, Stanford, CA, USA). First, we used EndNote to exclude duplicates. Then, two researchers independently screened the titles and abstracts of the records one by one. To obtain as much data as possible, only articles that clearly met the exclusion criteria were excluded when reading the title and abstract. The researchers read the full texts of the remaining records, and those who met the inclusion criteria were finally included. Disagreements between the two researchers in the above process were resolved through discussion or seeking the opinion of a third researcher.
Data extraction
The following data of included studies were extracted: (1) basic information: title, publication year, first author, and study design; (2) characteristics of population: age, nationality, sample size, and follow-up time; (3) information on COVID-19 vaccine: type and the number of vaccine doses; (4) information on immunogenicity: antibody detection methods and the number of seroconverted people; (5) information on effectiveness: the number of people infected with SARS-CoV-2, suffering from COVID-19, and hospitalized due to COVID-19, or any other data that can be used to determine vaccine effectiveness (VE); and (6) information on safety: the number of adverse events after each dose of vaccine. Data extraction was performed independently by two researchers. Disagreements were resolved through discussion or seeking the opinion of a third researcher.
Assessment of article quality
The following measurement tools were used for evaluation: (1) the revised Cochrane risk-of-bias tool [26] was used for randomized trials, and the results were divided into low risk of bias, some concerns, and high risk of bias; (2) the risk of bias in non-randomized studies of interventions assessment tool [27] was used for non-randomized studies, and the results were divided into low risk of bias, moderate risk of bias, serious risk of bias, critical risk of bias, and no information; (3) the Newcastle‒Ottawa scale [28] was used for cohort studies and case‒control studies, and the results were divided into low risk of bias (7–9 scores), moderate risk (5–6 scores), and high risk of bias (0–4 scores); and (4) the checklist recommended by the Agency for Healthcare Research and Quality [29] was used for cross-sectional studies, and the results were divided into low risk of bias (8–11 scores), moderate risk of bias (4–7 scores), and high risk of bias (0–3 scores). Assessment was performed independently by two researchers. Disagreements were resolved through discussion or seeking the opinion of a third researcher.
Outcomes and statistical analysis
For immunogenicity, the outcome was seroconversion rate. For effectiveness, the three outcomes we focused on were the VE against SARS-CoV-2 infection, COVID-19, and hospitalization. VE was defined as (1-RR) × 100% for clinical trials and cohort studies and (1-OR) × 100% for case‒control studies. RR was the risk of the three outcomes in the vaccinated group compared with the unvaccinated group. OR compared the odds of vaccination between cases and controls and can be used as an approximation of RR. VE indicates the reduction in risk of the three outcomes in the vaccinated group compared to the unvaccinated group and is expressed as a percentage (%). In the effectiveness analysis, vaccination status was divided into "fully vaccinated" and "partially vaccinated". "Fully vaccinated" was defined as being vaccinated with one dose of vaccines that only needed one dose (e.g., Ad26.COV2.S), or being vaccinated with two doses of vaccines that needed two doses (e.g., BNT162b2). “Partially vaccinated” was defined as being vaccinated with one dose of vaccine that needed two doses. For safety, the outcome was the incidence rate of adverse events after each dose of vaccines. Myocarditis, pericarditis, hypersensitivity, acute allergic reaction, Bell's palsy, convulsions, seizures, and thrombosis were called "special adverse events" in this study, occurring at a low rate with a denominator set to "per 100,000 people".
Effect values were pooled at the "cohort" level. Populations that differed in terms of age, vaccine type, the number of vaccine doses, or study time were considered different cohorts in our study. Heterogeneity was measured by the I2 statistic [30]. When I2 ≤ 50%, it can be considered that the heterogeneity between studies is low or moderate, and we use a fixed effect model to pool the effect value; when I2 > 50%, it can be considered that the heterogeneity between studies is high, and we use a random effects model to pool the effect value. In addition, we performed subgroup analyses of the above indicators with vaccine type and age. Data analysis was conducted by R (version 4.1.0).
Results
Characteristics of the included studies
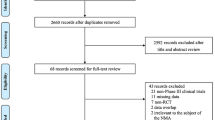
The study selection process is shown in Fig. 1. We obtained 8721 records from MEDLINE (accessed through PubMed), Embase and Web of Science. In addition, 5892 records remained for screening after excluding duplicates by EndNote. After reading titles and abstracts, 501 records remained. After reading the full texts, 88 articles were eligible to be included. Of the 88 articles included, there were 12 RCTs, two non-randomized clinical trials, 40 cohort studies, 19 cross-sectional studies, and 15 case‒control studies. In the studies included, 16 articles were available for immunogenicity analysis (Supplementary Table 2) [14, 15, 18, 31,32,33,34,35,36,37,38,39,40,41,42,43]; 38 articles were available for effectiveness analysis (Supplementary Table 3) [14, 17, 19, 31, 44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]; and 49 articles were available for safety analysis (Supplementary Table 4) [14,15,16, 18, 20,21,22,23,24, 31, 34, 35, 37, 41, 43, 45, 47, 50, 55, 78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107]. Children and adolescents are involved in 27 countries, including the United States, China, Australia, Argentina, Italy, Israel, France, Denmark, and South Korea, which have larger cohorts (> 100,000 people). Vaccine types included mRNA vaccines (BNT162b2, mRNA-1273), inactivated vaccines (CoronaVac, BBIBP-CorV, BBV152, PastoCoVac), recombinant adenovirus-vectored vaccines (Ad5-nCoV-S, ChAdOx1-S/nCoV-19, Ad26.COV2.S), and DNA vaccine (ZyCoV-D). Among them, BNT162b2 was vaccinated most wildly among children and adolescents. The results of the article quality assessment showed that only six articles had a high risk of bias. Other articles had a low risk or moderate risk of bias. Overall, the quality of the included studies was good (Supplementary Tables 5, 6, 7).
Immunogenicity
Antibodies were generally measured on day 28 post-vaccination for most studies. The immunogenicity results are shown in Table 1. The seroconversion rate increased sequentially after the first (86.10%), second (96.52%), and third (99.87%) doses of COVID-19 vaccines. Those who were vaccinated with mRNA vaccines had a higher seroconversion rate than those who were vaccinated with inactivated vaccines (98.78% vs. 92.77%). The seroconversion rate was higher in children aged 5–11 years than in children and adolescents aged 12–18 years (97.59% vs. 91.31%).
Effectiveness
The effectiveness results are shown in Table 2. Compared with the partially vaccinated group, the fully vaccinated group showed higher effectiveness in the pooled VEs against SARS-CoV-2 infection (63.33% vs. 42.87%), COVID-19 (75.77% vs. 60.65%), and hospitalization due to COVID-19 (82.78% vs. 72.74%). In addition, in the fully vaccinated group, the pooled VE against hospitalization due to COVID-19 (82.78%) was higher than the VE against SARS-CoV-2 infection (63.33%) and the VE against COVID-19 (75.77%). All of the pooled VEs against SARS-CoV-2 infection (66.82% vs. 38.66%), COVID-19 (74.94% vs. 59.63%), and hospitalization due to COVID-19 (90.07% vs. 65.96%) in children and adolescents aged 12–18 were higher than those in children under 11 years. The pooled VEs against SARS-CoV-2 infection (33.41% vs. 63.00%), COVID-19 (55.98% vs. 79.81%), and hospitalization due to COVID-19 (72.10% vs. 91.47%) in the period of Omicron variant predominance were lower than those in the period of Delta variant predominance.
Safety
The occurrence of adverse events after each dose of vaccines is shown in Fig. 2, and the detailed data are shown in Supplementary Table 8. After the first dose of injection, the five adverse events with the highest incidence rates were tenderness (52.77%), injection site pain (50.98%), fatigue/asthenia/tiredness (24.04%), headache (20.30%), and myalgia/muscle pain (15.43%). However, the number of cohorts reporting tenderness was small (five cohorts). Considering the stability of the results, when the number of cohorts was limited to at least 10, the most common adverse events were injection site pain, fatigue/asthenia/tiredness, headache, myalgia/muscle pain, and chills (12.19%). Incidence rates of any adverse events (42.28%), any local adverse events (41.17%), and any systemic adverse events (32.57%) after the first dose were all over 30%.
After the second dose of injection, when the number of cohorts was still limited to at least 10, the five most common adverse events were injection site pain (46.70%), fatigue/asthenia/tiredness (30.66%), headache (28.90%), myalgia/muscle pain (19.65%), and chills (16.58%). These five adverse reactions were consistent with those after the first dose. Except for injection site pain, the incidence rates of the other four adverse events after the second dose increased compared to those after the first dose. The incidence rates of any adverse events (38.04%), any local adverse events (38.49%), and any systemic adverse events (38.11%) were similar to those after the second dose. The incidence rates of adverse events after the third dose were very different from those after the first and second doses. However, the number of cohorts was less (all ≤ 6), and the stability was lower.
As shown in Table 3, the incidence of special adverse events was low. The most concerning adverse event was myocarditis or pericarditis, with an incidence rate of 2.42/100,000 people. The incidence rates of hypersensitivity/acute allergic reaction (3.86/100,000 people) and convulsions/seizures (2.15/100,000 people) were similar to those of myocarditis or pericarditis, but the number of cohorts was much smaller. The results of the subgroup analysis are shown in Table 4. We found that in age subgroups, the incidence rate of adverse events in children aged ≤ 5 years was lower than that in children aged 6–11 years and 12–18 years. In the vaccine type subgroup, the incidence rates of adverse events with mRNA vaccines, whether after the first, second, or third dose, were obviously higher than those with inactivated vaccines.
Discussion
This article is an update of a previous study we conducted, which was the first meta-analysis to evaluate the effectiveness and safety of COVID-19 vaccines among children and adolescents. In this study, a total of 88 relevant articles were included, of which 16 articles were used for immunogenicity analysis, 38 articles were used for effectiveness analysis, and 49 articles were used for safety analysis. The present study showed that the seroconversion rates after the first, second, and third doses of the vaccines were 86.10%, 96.52%, and 99.87%, respectively. VEs against SARS-CoV-2 infection in the partially vaccinated group and fully vaccinated group were 42.87% and 63.33%, respectively. VEs against COVID-19 in the partially vaccinated group and fully vaccinated group were 60.65% and 75.77%, respectively. VEs against hospitalization due to COVID-19 in the partially vaccinated group and fully vaccinated group were 72.74% and 82.78%, respectively. The incidence rates of any adverse events (42.28% vs. 38.04%) and local adverse events (41.17% vs. 38.49%) after the first dose were slightly higher than those after the second dose, while the incidence rate of systemic adverse events after the first dose (32.57% vs. 38.11%) was slightly lower than that after the second dose. Common adverse events included injection site pain, fatigue/asthenia/tiredness, headache, myalgia/muscle pain, and chills. The incidence of myocarditis or pericarditis was 2.42/100,000 people. In addition, the subgroup analysis showed that the incidence rates of adverse events of mRNA vaccines were higher than those of inactivated vaccines, whether after the first or second dose. The incidence rates of adverse events in children aged ≤ 5 years were the lowest, which may be related to the fact that they were all vaccinated with inactivated vaccines.
This study found that COVID-19 vaccines have good immunogenicity among children and adolescents. In particular, the seroconversion rate increased sequentially after the first (86.10%), second (96.52%), and third (99.87%) doses of COVID-19 vaccines. Du et al. [108] conducted a meta-analysis including three RCT studies by November 9, 2021 and found that the seroconversion rates of children and adolescents aged 3–17 after the first and second doses were 69.81%–94.63% and 98%–100%, respectively. They also found that the seroconversion rate in the vaccinated group was significantly higher than that in the unvaccinated group, especially after the second dose. Previous studies among healthy adults also suggested that two doses of vaccines can induce a stronger humoral immune response than a single dose [109, 110]. Therefore, there is a need for a two-dose vaccination strategy among children and adolescents. Furthermore, as a result of waning immunity and reduced protection after two doses of vaccines, offering third or booster doses was taken into consideration [111]. There is evidence that a third dose can boost antibody and neutralizing responses among adults [111, 112]. However, the WHO does not currently recommend that children and adolescents under 18 years receive a booster dose [13]. More studies are expected to explore the safety, immunogenicity, and effectiveness of booster vaccination among children and adolescents.
As a special population, children and adolescents present many influencing factors to consider when getting vaccinated. Vaccine safety and effectiveness are the most important considerations for children, adolescents, and their parents [113]. A meta-analysis including 44 articles by December 12, 2021, showed that the overall proportion of parents who intended to vaccinate their children against COVID-19 was 60.1%, and concerns about adverse events and effectiveness were important factors affecting parents’ willingness to vaccinate their children [114]. Vaccine hesitancy is also one of the main obstacles to the prevention and control of COVID-19 [115]. Previous studies showed that the major reasons for parents’ hesitancy to vaccinate their children included insufficient safety information and concerns about adverse effects and effectiveness [116, 117]. Our study suggested that COVID-19 vaccines have good safety and effectiveness among children and adolescents. This finding can help improve parents’ willingness to vaccinate their children, reduce vaccine hesitancy, and promote vaccination in children and adolescents.
For effectiveness, our results indicated that full vaccination with COVID-19 vaccines showed high VE against SARS-CoV-2 infection, COVID-19, and hospitalization due to COVID-19. However, the effectiveness was slightly lower than that in a previous meta-analysis without the Omicron variant [118]. Since the first report of the Omicron variant in South Africa on November 24, 2021, this variant has quickly become the predominant variant worldwide, and new and more contagious subtypes BA.4 and BA.5 have emerged [119]. The effectiveness of COVID-19 vaccines against the Omicron variant has become the focus. A meta-analysis involving 57 studies by March 4, 2022 showed that the VE against the Omicron variant (55.9%) was lower than that against the α variant (88.0%), β variant (73.0%), γ variant (63.0%), and Delta variant (77.8%) in the general population with complete vaccination, while the VE against the Omicron variant reached 80.8% after boost vaccination [120]. A prospective cohort study among 136,127 children aged 5–11 years found that the VE against symptomatic COVID-19 was 48% during the Omicron variant pandemic, lower than 90.7% during the Delta variant pandemic [17]. The lower VE might be related to the ability of Omicron variants to escape most neutralizing antibodies of SARS-CoV-2 [121, 122]. We also found that the VEs among children under 11 years were lower than those among adolescents aged 12–18 years. The potential reason is the later time of vaccination for children under 11 years. They were vaccinated when the Omicron variant was prevalent; therefore, the lower VEs among them might just reflect the lower VEs against this variant. In the future, more studies are needed to explore the effectiveness of COVID-19 vaccination and booster doses against Omicron variants among children and adolescents.
For safety, this study showed that the incidence rates of any adverse events, local adverse events, and systemic adverse events after the first and second doses of COVID-19 vaccines among children and adolescents were slightly higher than 30%, and the incidence rates of adverse events after vaccination with mRNA vaccines were higher than those after vaccination with inactivated vaccines. A meta-analysis involving 73,633 subjects in 14 RCT studies showed that the incidence rate of adverse events after COVID-19 vaccine vaccination was 36% [123]. A meta-analysis of six RCTs among participants aged 3–17 years found that the risk of total, local, and systemic adverse events in the mRNA vaccine group and adenovirus-vectored vaccine group significantly increased, while only the risk of local adverse events in the inactivated vaccine group was higher than that in the control group [108]. We also found that injection site pain, fatigue, and headache were the most common adverse events, similar to previous studies [118]. Additionally, myocarditis or pericarditis is a serious adverse event in children and adolescents, especially in male adolescents [124]. Between December 14, 2020, and July 16, 2021, 397 cases of myocarditis occurred among 8.9 million 12- to 17-year-old adolescents vaccinated with BNT162b2 in the United States [89]. Between December 2020 and August 2021, the incidence rates of myocarditis after the second dose of the BNT162b2 vaccine among adolescent males aged 12–15 and 16–17 years in the United States were 70.73/million doses and 105.86/million doses, respectively [124]. Currently, there is a lack of research on the long-term effects of COVID-19 vaccine-related myocarditis. In the future, more efforts are needed to strengthen the monitoring and follow-up of serious adverse events such as myocarditis or pericarditis and explore the treatment and management strategies of these adverse events.
However, there are some limitations in our study. First, heterogeneity between the included studies was somewhat high, making the results in need of future verification. Second, for immunogenicity, we only focused on the antibody seroconversion rate, not the antibody titer. Moreover, these studies did not use the same detection methods and reagents for SARS-CoV-2 antibodies, which may have an impact on the results. Third, for effectiveness, the vaccination status of most studies included in this meta-analysis was partially or fully vaccinated. More research is needed in the future to explore the effectiveness of booster doses of COVID-19 vaccines. Finally, for safety, the follow-up time of adverse events in most studies was within 30 days after vaccination, and there are only a few long-term follow-up studies.
In conclusion, as far as the current studies are concerned, COVID-19 vaccines have good immunogenicity, effectiveness, and safety among children and adolescents aged 2–18 years. COVID-19 vaccines can effectively prevent children and adolescents from being infected with SARS-CoV-2 and suffering from COVID-19. During the COVID-19 pandemic, we suggest that children and adolescents should be vaccinated as soon as possible to protect them and slow the spread of COVID-19. However, studies on the effectiveness of booster doses of COVID-19 vaccines among children and adolescents are currently insufficient. More basic research, clinical trials, and real-world studies are needed in the future to explore the immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents.
Data availability
The data analyzed in this study is available from the corresponding author on reasonable request.
References
WHO. Coronavirus disease 2019 Q&As: coronavirus disease (COVID-19), 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19. Accessed 18 Jul 2022.
WHO. Coronavirus (COVID-19) dashboard, 2022. https://covid19.who.int/. Accessed 25 Oct 2022.
Liu Q, Qin C, Liu M, Liu J. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty. 2021;10:132.
Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, immunogenicity and safety of COVID-19 vaccines: a systematic review and meta-analysis. Front Immunol. 2021;12:714170.
Riad A, Pokorná A, Klugarová J, Antalová N, Kantorová L, Koščík M, et al. Side effects of mRNA-based COVID-19 vaccines among young adults (18–30 years old): an independent post-marketing study. Pharmaceuticals (Basel). 2021;14:1049.
Riad A, Hocková B, Kantorová L, Slávik R, Spurná L, Stebel A, et al. Side effects of mRNA-based COVID-19 vaccine: nationwide phase IV study among healthcare workers in Slovakia. Pharmaceuticals (Basel). 2021;14:873.
Lounis M, Rais MA, Bencherit D, Aouissi HA, Oudjedi A, Klugarová J, et al. Side effects of COVID-19 inactivated virus vs. adenoviral vector vaccines: experience of Algerian healthcare workers. Front Public Health. 2022;10:896343.
Vaccine Centre at the London School of Hygiene & Tropical Medicine. COVID-19 vaccine tracker, 2022. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/. Accessed 18 Jul 2022.
WHO. COVID-19 advice for the public: getting vaccinated, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines/advice. Accessed 18 Jul 2022.
WHO. COVID-19 disease in children and adolescents: scientific brief, 29 September, 2021. https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1. Accessed 18 Jul 2022.
Jiang L, Tang K, Levin M, Irfan O, Morris SK, Wilson K, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20:e276–88.
Marks KJ, Whitaker M, Anglin O, Milucky J, Patel K, Pham H, et al. Hospitalizations of children and adolescents with laboratory-confirmed COVID-19-COVID-NET, 14 states, July 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:271–8.
WHO. Coronavirus disease 2019 Q&As-coronavirus disease (COVID-19): vaccines, 2022. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines. Accessed 18 Jul 2022.
Creech CB, Anderson E, Berthaud V, Yildirim I, Atz AM, Baez IM, et al. Evaluation of mRNA-1273 covid-19 vaccine in children 6 to 11 years of age. N Engl J Med. 2022;386:2011–23.
Khobragade A, Bhate S, Ramaiah V, Deshpande S, Giri K, Phophle H, et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): the interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet. 2022;399:1313–21.
Li G, Cappuccini F, Marchevsky NG, Aley PK, Aley R, Anslow R, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in children aged 6–17 years: a preliminary report of COV006, a phase 2 single-blind, randomised, controlled trial. Lancet. 2022;399:2212–25.
Cohen-Stavi CJ, Magen O, Barda N, Yaron S, Peretz A, Netzer D, et al. BNT162b2 vaccine effectiveness against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:227–36.
Greish K, Alawadhi A, Jaradat A, Almarabheh A, AlMadhi M, Jawad J, et al. Safety and immunogenicity of COVID-19 BBIBP-CorV vaccine in children 3–12 years old. Vaccines. 2022;10:586.
Kildegaard H, Lund LC, Hojlund M, Stensballe LG, Pottegard A. Risk of adverse events after covid-19 in Danish children and adolescents and effectiveness of BNT162b2 in adolescents: cohort study. BMJ. 2022;377:e068898.
Nygaard U, Holm M, Dungu KHS, Matthesen AT, Stensballe LG, Espenhain L, et al. Risk of myopericarditis after COVID-19 vaccination in Danish children aged 5–11 years. Pediatrics. 2022;150:e2022057508.
Ouldali N, Bagheri H, Salvo F, Antona D, Pariente A, Leblanc C, et al. Hyper inflammatory syndrome following COVID-19 mRNA vaccine in children: a national post-authorization pharmacovigilance study. Lancet Reg Health Eur. 2022;17:100393.
Alami A, Krewski D, Mattison D, Wilson K, Gravel CA, Villeneuve PJ, et al. Risk of myocarditis and pericarditis among young adults following mRNA COVID-19 vaccinations. Vaccines. 2022;10:722.
Lee CW, Sa S, Hong M, Kim J, Shim SR, Han HW. Adverse events and safety profile of the COVID-19 vaccines in adolescents: safety monitoring for adverse events using real-world data. Vaccines (Basel). 2022;10:744.
Tavakoli N, Nafissi N, Shokri S, Fallahpour M, Soleimani S, Riahi T, et al. Pediatric and adolescent COVID-19 vaccination side effects: a retrospective cohort study of the Iranian teenage group in 2021. J Med Virol. 2022;94:4890–900.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca//programs/clinical_epidemiology/oxford.asp. Accessed 30 Jun 2022.
Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Evidence reports/technology assessments, No. 104. Appendix D. Quality assessment forms, 2004. Celiac disease. Rockville (MD): Agency for Healthcare Research and Quality (US); 2004. https://www.ncbi.nlm.nih.gov/books/NBK35156/. Accessed 24 May 2022.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Ali K, Berman G, Zhou H, Deng W, Faughnan V, Coronado-Voges M, et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N Engl J Med. 2021;385:2241–51.
Bratic JS, Gans HA, Chen SF, Banaei N, Johnston EM, Sear K, et al. Pediatric solid organ transplant recipients demonstrate robust cell-mediated and humoral responses to three doses of mRNA SARS-CoV-2 vaccine. Am J Transplant. 2022;22:3047–52.
Ella R, Reddy S, Jogdand H, Sarangi V, Ganneru B, Prasad S, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect Dis. 2021;21:950–61.
Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–53.
Kastl A, Weaver KN, Zhang X, Strople JA, Adler J, Dubinsky MC, et al. Humoral immune response and safety of SARS-CoV-2 vaccination in pediatric inflammatory bowel disease. Am J Gastroenterol. 2022. https://doi.org/10.14309/ajg.0000000000002016.
Poparn H, Srichumpuang C, Sosothikul D, Jantarabenjakul W, Lauhasurayotin S, Techavichit P, et al. Immune response after 2 doses of BNT162b2 mRNA COVID-19 vaccinations in children and adolescents with cancer and hematologic diseases. Asian Pac J Cancer Prev. 2022;23:2049–55.
Rosa Duque JS, Wang X, Leung D, Cheng SMS, Cohen CA, Mu X, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines BNT162b2 and CoronaVac in healthy adolescents. Nat Commun. 2022;13:3700.
Sattler A, Thumfart J, Tóth L, Schrezenmeier E, Proß V, Stahl C, et al. SARS-CoV2 mRNA vaccine-specific B-, T- and humoral responses in adolescents after kidney transplantation. Transpl Int. 2022;35:10677.
Torres JP, Saure D, Basso LJ, Zuniga M, Cazor A, O’Ryan M. SARS-COV-2 IgG positivity in vaccinated and non-vaccinated Chilean children: a national cross-sectional study in schools. Int J Infect Dis. 2022;121:89–91.
Udaondo C, Cámara C, Miguel Berenguel L, Alcobendas Rueda R, Muñoz Gómez C, Millán Longo C, et al. Humoral and cellular immune response to mRNA SARS-CoV-2 BNT162b2 vaccine in adolescents with rheumatic diseases. Pediatr Rheumatol Online J. 2022;20:64.
Vadrevu KM, Reddy S, Jogdand H, Ganneru B, Mirza N, Tripathy VN, et al. Immunogenicity and reactogenicity of an inactivated SARS-CoV-2 vaccine (BBV152) in children aged 2–18 years: interim data from an open-label, non-randomised, age de-escalation phase 2/3 study. Lancet Infect Dis. 2022;22:1303–12.
Xia S, Duan K, Zhang Y, Zeng X, Zhao D, Zhang H, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, WIBP-CorV, in healthy children: interim analysis of a randomized, double-blind, controlled, phase 1/2 trial. Front Immunol. 2022;13:898151.
Zhu F, Jin P, Zhu T, Wang W, Ye H, Pan H, et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored coronavirus disease 2019 (COVID-19) vaccine with a homologous prime-boost regimen in healthy participants aged ≥ 6 years: a randomized, double-blind, placebo-controlled, phase 2b trial. Clin Infect Dis. 2021;75:e783–91.
Amodio E, Genovese D, Mazzeo L, Martino L, Restivo V, Vella G, et al. Effectiveness of mRNA COVID-19 vaccines in adolescents over 6 months. Pediatrics. 2022;150:e2022057394.
Choe YJ, Yi S, Hwang I, Kim J, Park YJ, Cho E, et al. Safety and effectiveness of BNT162b2 mRNA Covid-19 vaccine in adolescents. Vaccine. 2022;40:691–4.
Fowlkes AL, Yoon SK, Lutrick K, Gwynn L, Burns J, Grant L, et al. Effectiveness of 2-dose BNT162b2 (Pfizer BioNTech) mRNA vaccine in preventing SARS-CoV-2 infection among children aged 5–11 years and adolescents aged 12–15 years-PROTECT cohort, July 2021-February 2022. MMWR Morb Mortal Wkly Rep. 2022;71:422–8.
Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 covid-19 vaccine in adolescents. N Engl J Med. 2021;385:239–50.
González S, Olszevicki S, Gaiano A, Baino ANV, Regairaz L, Salazar M, et al. Effectiveness of BBIBP-CorV, BNT162b2 and mRNA-1273 vaccines against hospitalisations among children and adolescents during the Omicron outbreak in Argentina: a retrospective cohort study. Lancet Reg Health Am. 2022;13:100316.
Lutrick K, Rivers P, Yoo YM, Grant L, Hollister J, Jovel K, et al. Interim estimate of vaccine effectiveness of BNT162b2 (Pfizer-BioNTech) vaccine in preventing SARS-CoV-2 infection among adolescents aged 12–17 years-Arizona, July-December 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1761–5.
Molteni E, Canas LS, Kläser K, Deng J, Bhopal SS, Hughes RC, et al. Post-vaccination infection rates and modification of COVID-19 symptoms in vaccinated UK school-aged children and adolescents: a prospective longitudinal cohort study. Lancet Reg Health Eur. 2022;19:100429.
Naleway AL, Groom HC, Crawford PM, Salas SB, Henninger ML, Donald JL, et al. Incidence of SARS-CoV-2 infection, emergency department visits, and hospitalizations because of COVID-19 among persons aged ≥12 years, by COVID-19 vaccination status-Oregon and Washington, July 4-September 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1608–12.
Sacco C, Del Manso M, Mateo-Urdiales A, Rota MC, Petrone D, Riccardo F, et al. Effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection and severe COVID-19 in children aged 5–11 years in Italy: a retrospective analysis of January–April, 2022. Lancet. 2022;400:97–103.
Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–16.
Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021;385:1761–73.
Walter EB, Talaat KR, Sabhar C, Gurtman A, Lockhart S, Paulsen GC, et al. Evaluation of the BNT162b2 covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46.
Yigit M, Ince YE, Kalayci F, Santaflioglu B, Kurt F, Ozkaya-Parlakay A, et al. The impact of childhood and parental vaccination on SARS-CoV-2 infection rates in children. Pediatr Infect Dis J. 2022;41:841–5.
Ziv A, Heshin-Bekenstein M, Haviv R, Kivity S, Netzer D, Yaron S, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine among adolescents with juvenile-onset inflammatory rheumatic diseases. Rheumatology (Oxford). 2022. https://doi.org/10.1093/rheumatology/keac408.
Chiew CJ, Premikha M, Chong CY, Wei WE, Ong B, Lye DC, et al. Effectiveness of primary series and booster vaccination against SARS-CoV-2 infection and hospitalisation among adolescents aged 12–17 years in Singapore: a national cohort study. Lancet Infect Dis. 2022. https://doi.org/10.1016/S1473-3099(22)00573-4.
Cocchio S, Zabeo F, Tremolada G, Facchin G, Venturato G, Marcon T, et al. COVID-19 vaccine effectiveness against Omicron variant among underage subjects: the Veneto region’s experience. Vaccines (Basel). 2022;10:1362.
Glatman-Freedman A, Hershkovitz Y, Kaufman Z, Dichtiar R, Keinan-Boker L, Bromberg M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg Infect Dis. 2021;27:2919–22.
Husin M, Tok PSK, Suah JL, Thevananthan T, Tng BH, Peariasamy KM, et al. Real-world effectiveness of BNT162b2 vaccine against SARS-CoV-2 infection among adolescents (12 to 17-year-olds) in Malaysia. Int J Infect Dis. 2022;121:55–7.
Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387:525–32.
Jara A, Undurraga EA, Zubizarreta JR, Gonzalez C, Acevedo J, Pizarro A, et al. Effectiveness of CoronaVac in children 3 to 5 years during the SARS-CoV-2 Omicron outbreak in Chile. Nat Med. 2022;28:1377–80.
Chadeau-Hyam M, Eales O, Bodinier B, Wang H, Haw D, Whitaker M, et al. Breakthrough SARS-CoV-2 infections in double and triple vaccinated adults and single dose vaccine effectiveness among children in Autumn 2021 in England: REACT-1 study. EClinicalMedicine. 2022;48:101419.
Andeweg SP, de Gier B, Eggink D, van den Ende C, van Maarseveen N, Ali L, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. 2022;13:4738.
Mallah N, Pardo-Seco J, López-Pérez LR, González-Pérez JM, Rosón B, Otero-Barrós MT, et al. Effectiveness of COVID-19 vaccine booster in the general population and in subjects with comorbidities. A population-based study in Spain. Environ Res. 2022;215:114252.
Oliveira CR, Niccolai LM, Sheikha H, Elmansy L, Kalinich CC, Grubaugh ND, et al. Assessment of clinical effectiveness of BNT162b2 COVID-19 vaccine in US adolescents. JAMA Netw Open. 2022;5:e220935.
Oliveira EA, Oliveira MCL, Colosimo EA, Simões ESAC, Mak RH, Vasconcelos MA, et al. Vaccine effectiveness against SARS-CoV-2 variants in adolescents from 15 to 90 days after second dose: a population-based test-negative case-control study. J Pediatr. 2022. https://doi.org/10.1016/j.jpeds.2022.09.039.
Florentino PTV, Alves FJO, Cerqueira-Silva T, Oliveira VA, Júnior JBS, Jantsch AG, et al. Vaccine effectiveness of CoronaVac against COVID-19 among children in Brazil during the Omicron period. Nat Commun. 2022;13:4756.
Britton A, Fleming-Dutra KE, Shang N, Smith ZR, Dorji T, Derado G, et al. Association of COVID-19 vaccination with symptomatic SARS-CoV-2 infection by time since vaccination and Delta variant predominance. JAMA. 2022;327:1032–41.
Fleming-Dutra KE, Britton A, Shang N, Derado G, Link-Gelles R, Accorsi EK, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during Omicron predominance. JAMA. 2022;327:2210–9.
Florentino PTV, Millington T, Cerqueira-Silva T, Robertson C, de Araújo OV, Júnior JBS, et al. Vaccine effectiveness of two-dose BNT162b2 against symptomatic and severe COVID-19 among adolescents in Brazil and Scotland over time: a test-negative case-control study. Lancet Infect Dis. 2022;22:1577–86.
Rudan I, Millington T, Antal K, Grange Z, Fenton L, Sullivan C, et al. BNT162b2 COVID-19 vaccination uptake, safety, effectiveness and waning in children and young people aged 12–17 years in Scotland. Lancet Reg Health Eur. 2022;23:100513.
Klein NP, Stockwell MS, Demarco M, Gaglani M, Kharbanda AB, Irving SA, et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 mRNA vaccination in preventing COVID-19-associated emergency department and urgent care encounters and hospitalizations among nonimmunocompromised children and adolescents aged 5–17 years-VISION network, 10 states, April 2021-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:352–8.
Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of Pfizer-BioNTech mRNA vaccination against COVID-19 hospitalization among persons aged 12–18 years-United States, June-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1483–8.
Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, et al. Effectiveness of BNT162b2 vaccine against critical covid-19 in adolescents. N Engl J Med. 2022;386:713–23.
Price AM, Olson SM, Newhams MM, Halasa NB, Boom JA, Sahni LC, et al. BNT162b2 protection against the Omicron variant in children and adolescents. N Engl J Med. 2022;386:1899–909.
Akgün O, Çakmak F, Guliyeva V, Demirkan FG, Tanatar A, Hançerli Torun S, et al. Humoral response and safety of BNT162b2 mRNA vaccine in children with rheumatic diseases. Rheumatology (Oxford). 2022;61:4482–90.
Alamer E, Alhazmi A, Qasir NA, Alamer R, Areeshi H, Gohal G, et al. Side effects of COVID-19 Pfizer-BioNTech mRNA vaccine in children aged 12–18 years in Saudi Arabia. Vaccines (Basel). 2021;9:1297.
Arslanoglu Aydin E, Baglan E, Bagrul I, Tuncez S, Ozdel S, Bulbul M. Safety of COVID-19 vaccines and disease flares after vaccines in children with rheumatic disease. Postgrad Med. 2022;13:616–21.
Bartsch YC, St Denis KJ, Kaplonek P, Kang J, Lam EC, Burns MD, et al. SARS-CoV-2 mRNA vaccination elicits robust antibody responses in children. Sci Transl Med. 2022;14:9237.
Bloise S, Marcellino A, Frasacco B, Gizzone P, Proietti Ciolli C, Martucci V, et al. Cross-sectional survey on BNT162b2 mRNA COVID-19 vaccine serious adverse events in children 5 to 11 years of age: a monocentric experience. Vaccines (Basel). 2022;10:1224.
Capponi M, Pulvirenti F, Cinicola BL, Brindisi G, Conti MG, Colaiocco G, et al. Short-term side effects and SARS-CoV-2 infection after COVID-19 Pfizer–BioNTech vaccine in children aged 5–11 years: an Italian real-world study. Vaccines. 2022;10:1056.
Chan EWW, Leung MTY, Lau LKW, Leung J, Lum D, Wong RS, et al. Comparing self-reported reactogenicity between adolescents and adults following the use of BNT162b2 (Pfizer-BioNTech) messenger RNA COVID-19 vaccine: a prospective cohort study. Int J Infect Dis. 2022;116:47–50.
Chantasrisawad N, Puthanakit T, Tangsathapornpong A, Techasaensiri C, Phongsamart W, Suwanpakdee D, et al. Immunogenicity and reactogenicity of mRNA BNT162b2 COVID-19 vaccine among Thai adolescents with chronic diseases. Vaccines. 2022;10:871.
Cheng DR, Clothier HJ, Morgan HJ, Roney E, Shenton P, Cox N, et al. Myocarditis and myopericarditis cases following COVID-19 mRNA vaccines administered to 12–17-year olds in Victoria. Australia BMJ Paediatrics Open. 2022;6:e001472.
Hause AM, Baggs J, Marquez P, Myers TR, Gee J, Su JR, et al. COVID-19 vaccine safety in children aged 5–11 years-United States, November 3-December 19, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1755–60.
Hause AM, Baggs J, Marquez P, Myers TR, Su JR, Hugueley B, et al. Safety monitoring of Pfizer-BioNTech COVID-19 vaccine booster doses among children aged 5–11 years-United States, May 17-July 31, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1047–51.
Hause AM, Gee J, Baggs J, Abara WE, Marquez P, Thompson D, et al. COVID-19 vaccine safety in adolescents aged 12–17 years-United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1053–8.
Hause AM, Marquez P, Zhang B, Myers TR, Gee J, Su JR, et al. COVID-19 mRNA vaccine safety among children aged 6 months-5 years-United States, June 18, 2022-August 21, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1115–20.
Hause AM, Shay DK, Klein NP, Abara WE, Baggs J, Cortese MM, et al. Safety of COVID-19 vaccination in US children ages 5–11 years. Pediatrics. 2022;150:e2022057313.
Heshin-Bekenstein M, Ziv A, Toplak N, Hagin D, Kadishevich D, Butbul YA, et al. Safety and immunogenicity of BNT162b2 mRNA COVID-19 vaccine in adolescents with rheumatic diseases treated with immunomodulatory medications. Rheumatology (Oxford). 2022;61:4263–72.
Kaur U, K LA, Chauhan M, Joshi A, Das A, Kansal S, et al. A prospective observational study on BBV152 coronavirus vaccine use in adolescents and comparison with adults: interim results of the first real-world safety analysis. Drug Saf. 2022;45:1099–109.
Kim S, Hwang I, Ko M, Kwon Y, Lee YK. Safety monitoring of COVID-19 vaccination among adolescents aged 12 to 17 years old in the Republic of Korea. Osong Public Health Res Perspect. 2022;13:230–7.
Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326:1390–9.
Krug A, Stevenson J, Hoeg TB. BNT162b2 vaccine-associated myo/pericarditis in adolescents: a stratified risk-benefit analysis. Eur J Clin Invest. 2022;52:e13759.
Lai FTT, Chua GT, Chan EWW, Huang L, Kwan MYW, Ma T, et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population-based retrospective cohort study. Emerg Microbes Infect. 2022;11:885–93.
Lee DS, Kim JW, Lee KL, Jung YJ, Kang HW. Adverse events following COVID-19 vaccination in South Korea between February 28 and August 21, 2021: a nationwide observational study. Int J Infect Dis. 2022;118:173–82.
Lee KJ, Choi SY, Lee YM, Kim HW. Neutralizing antibody response, safety, and efficacy of mRNA COVID-19 vaccines in pediatric patients with inflammatory bowel disease: a prospective multicenter case-control study. Vaccines (Basel). 2022;10:1265.
Li T, Qi R, Chen B, Luo Y, Zhang W, Zhou YH, et al. COVID-19 vaccination coverage among adolescents aged 12–17 years in three provinces of eastern China: a cross-sectional survey, 2021. Front Public Health. 2022;10:919190.
Lu Q, Wang YY, Wang QH, Tang LN, Yang XY, Dun S, et al. Safety of inactivated COVID-19 vaccine in tuberous sclerosis complex patients with epilepsy treated with rapamycin. Seizure. 2022;99:71–4.
Marglani OAR, Qashqari MB, Alnashri MT, Alharbi HA, Namenkani MI, Alnashri NT, et al. Adverse effects of Pfizer-BioNTech vaccine among adolescents aged 12–18 in Saudi Arabia. Med Sci. 2021;25:3421–30.
Myers V, Saban M, Wilf-Miron R. Covid-19 in children aged 5–11: examining the issues surrounding vaccination and public health policy. Paediatr Respir Rev. 2022;43:85–90.
Nygaard U, Holm M, Bohnstedt C, Chai Q, Schmidt LS, Hartling UB, et al. Population-based incidence of myopericarditis after covid-19 vaccination in danish adolescents. Pediatr Infect Dis J. 2022;41:e25–8.
Sutardi AQI, Ramatillah DL. Evaluation comparison between sinovac and pfizer vaccine among indonesian children and teenager under 18 years old. Int J App Pharm. 2022;14:22–30.
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22:196–208.
Yang X, Wu L, Zheng D, Yang B, Wu D. COVID-19 vaccination for patients with benign childhood epilepsy with centrotemporal spikes. Epilepsy Behav. 2022;134:108744.
Du Y, Chen L, Shi Y. Safety, immunogenicity, and efficacy of COVID-19 vaccines in adolescents, children, and infants: a systematic review and meta-analysis. Front Public Health. 2022;10:829176.
Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–91.
Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51.
Costa Clemens SA, Weckx L, Clemens R, Almeida Mendes AV, Ramos Souza A, Silveira MBV, et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet. 2022;399:521–9.
Munro APS, Janani L, Cornelius V, Aley PK, Babbage G, Baxter D, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–76.
Smith LE, Amlôt R, Weinman J, Yiend J, Rubin GJ. A systematic review of factors affecting vaccine uptake in young children. Vaccine. 2017;35:6059–69.
Galanis P, Vraka I, Siskou O, Konstantakopoulou O, Katsiroumpa A, Kaitelidou D. Willingness, refusal and influential factors of parents to vaccinate their children against the COVID-19: a systematic review and meta-analysis. Prev Med. 2022;157:106994.
Wiysonge CS, Ndwandwe D, Ryan J, Jaca A, Batouré O, Anya BM, et al. Vaccine hesitancy in the era of COVID-19: could lessons from the past help in divining the future? Hum Vaccin Immunother. 2022;18:1–3.
Temsah MH, Alhuzaimi AN, Aljamaan F, Bahkali F, Al-Eyadhy A, Alrabiaah A, et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: a national survey. Front Public Health. 2021;9:752323.
Middleman AB, Klein J, Quinn J. Vaccine hesitancy in the time of COVID-19: attitudes and intentions of teens and parents regarding the COVID-19 vaccine. Vaccines (Basel). 2021;10:4.
Gao P, Cai S, Liu Q, Du M, Liu J, Liu M. Effectiveness and safety of SARS-CoV-2 vaccines among children and adolescents: a systematic review and meta-analysis. Vaccines (Basel). 2022;10:421.
Callaway E. What Omicron’s BA.4 and BA.5 variants mean for the pandemic. Nature. 2022;606:848–9.
Zeng B, Gao L, Zhou Q, Yu K, Sun F. Effectiveness of COVID-19 vaccines against SARS-CoV-2 variants of concern: a systematic review and meta-analysis. BMC Med. 2022;20:200.
Cao Y, Wang J, Jian F, Xiao T, Song W, Yisimayi A, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–63.
Cele S, Jackson L, Khoury DS, Khan K, Moyo-Gwete T, Tegally H, et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature. 2022;602:654–6.
Chen M, Yuan Y, Zhou Y, Deng Z, Zhao J, Feng F, et al. Safety of SARS-CoV-2 vaccines: a systematic review and meta-analysis of randomized controlled trials. Infect Dis Poverty. 2021;10:94.
Oster ME, Shay DK, Su JR, Gee J, Creech CB, Broder KR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327:331–40.
Acknowledgements
Thanks to all authors for their contributions to this article.
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers: 71934002, 72122001), the National Key Research and Development Project of China (grant numbers: 2021ZD0114104, 2021ZD0114101, and 2021ZD0114105).
Author information
Authors and Affiliations
Contributions
GP and KLY contributed equally as first authors. GP developed the search searches, extracted the data, assessed the study quality, performed the statistical analysis, and wrote the manuscript. KLY developed the search searches, extracted the data, assessed the study quality, and wrote the manuscript. LJ and LM conceived and designed the study. All the authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, P., Kang, LY., Liu, J. et al. Immunogenicity, effectiveness, and safety of COVID-19 vaccines among children and adolescents aged 2–18 years: an updated systematic review and meta-analysis. World J Pediatr 19, 1041–1054 (2023). https://doi.org/10.1007/s12519-022-00680-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-022-00680-9