Abstract
Background
Idiopathic nephrotic syndrome is a common form of glomerular nephropathy in children, with an incidence rate of 1.15–16.9/100,000 depending on different nationalities and ethnicities. The etiological factors and mechanisms of childhood idiopathic nephrotic syndrome have not yet been fully elucidated. This review summarizes the progress of the immunopathogenesis of idiopathic nephrotic syndrome in children.
Data sources
We review the literature on the immunopathogenesis of idiopathic nephrotic syndrome in children. Databases including Medline, Scopus, and Web of Science were searched for studies published in any language with the terms “children”, “idiopathic nephrotic syndrome”, “immunopathogenesis”, “T cells”, “circulating permeability factors”, and “B cells”.
Results
Dysfunction in T lymphocytes and pathogenic circulatory factors were indicated to play key roles in the pathogenesis of idiopathic nephrotic syndrome. Recently, some studies have shown that cellular immune dysfunction may also be involved in the pathogenesis of idiopathic nephrotic syndrome.
Conclusions
Both T- and B-cell dysfunction may play significant roles in the pathogenesis of idiopathic nephrotic syndrome, like two sides of one coin, but the role of B cell seems more important than T cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edema, proteinuria, and hypoalbuminemia are characteristics of nephrotic syndrome (NS) [1]. As a common nephrosis in children, idiopathic NS (INS) has an incidence rate of 1.15–16.9/100,000 depending on different nationalities and ethnicities [2, 3]. Children with INS usually have podocyte injury, and most of them present with either of two major histologic variations: focal segmental glomerulosclerosis (FSGS) and minimal change disease (MCD) [4]. Whether MCD and FSGS are two different phases in the same context of INS or two separate nosological entities is still not totally clear. According to the response to corticosteroid therapy, INS can also be classified into steroid-resistant nephrotic syndrome (SRNS) and steroid-sensitive nephrotic syndrome (SSNS). Although steroid therapy can be effective in most affected patients, about 10–20% of children present with SRNS, and 8–35% of these children with SRNS will progress to end-stage renal disease within 5 years after diagnosis [5,6,7,8].
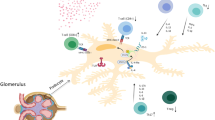
The pathogenesis of INS has not been fully clarified, so relapse of proteinuria, or steroid resistance challenges and conundrums remains common in clinical course. Previous studies have suggested that the main pathogenesis is T lymphocyte dysfunction and/or the abnormal secretion of certain glomerular permeability factors [9]. Recent studies have suggested that the pathogenesis may also be related to a dysfunction of B lymphocytes. Dysfunctions of T cells and B cells are similar to the two sides of a coin.
Dysfunction of T lymphocytes
T cell dysfunction is a classical theory for the pathogenesis of INS. Several studies have revealed that abnormal numbers and functions of T lymphocytes may be implicated in the pathogenesis of INS [10, 11]. The efficacy of calcineurin inhibitors, which include cyclosporine and tacrolimus, and the key role of steroids in the treatment of non-hereditary INS strongly suggest that T cell-mediated immune imbalance is involved in the etiopathogenesis of INS [12]; in some patients combined with measles, proteinuria was relieved simultaneously as the measles virus transiently inhibited cellular immunity [13]; injection of the cultural supernatant of T lymphocytes from MCD patients into rats can induce proteinuria [14]; 0.5–1% of patients with T cell-derived Hodgkin's disease; and thymoma are complicated with nephrotic syndrome [15, 16].
Recently, CD80, as a co-stimulatory factor of T cells, has become a hot spot in studies of podocyte injury. CD80, also named B7-1, is expressed on the surface of antigen-presenting cells (APCs), binds to homologous receptors on T cells, and regulates T cell immunity in both directions. Reiser et al. [17] revealed that the expression of CD80 in podocytes was significantly increased under certain conditions, bringing a breakthrough in revealing the pathogenesis of MCD in 2004. Subsequently, Garin et al. [18, 19] revealed that urine concentrations of soluble CD80 were increased in patients with relapsing MCD and implied that the increased CD80 entity originated from podocytes in urine. Immunohistochemical staining revealed that CD80 was overexpressed in the podocytes from MCD patients with relapse, while the down-regulation of CD80 expression was found in podocytes from patients with remission, suggesting that the level of CD80 is associated with the activity of MCD. In support of these findings, Ling et al. [20] reported that urinary CD80 levels in patients with recurrent MCD were significantly increased compared with those in patients with other nephropathies and healthy controls, with a specificity of 94.4% and a sensitivity of 81.8%. These results indicated that CD80 might be an early biological indicator for the diagnosis of MCD to distinguish it from FSGS. Researchers even discussed the possibility of using urine CD80 in the diagnosis of MCD instead of renal biopsy [21].
Abatacept (CTLA4–Ig) is a novel fusion protein designed to modulate the T cell co-stimulatory signal mediated through the CD28–CD80/86 pathway. Abatacept can compete with CD28 to bind CD80, thereby blocking the CD80–CD28 pathway and inducing the apoptosis and incompetence of T cells. Tsuji et al. [22] suggested that CTLA-4 was involved in the induction of remission in INS. Zhao et al. [23] found that the absent or minimal expression of CTLA-4 in glomeruli could distinguish steroid-sensitive from steroid-resistant MCD; MCD patients with strongly positive CD80 expression and simultaneous negative CTLA-4 expression or those with higher urinary CD80 levels and lower urinary CTLA-4 levels will achieve complete remission with glucocorticoid therapy. Yu et al. [24] demonstrated that CD80 was also positively expressed in patients with relapsed FSGS after kidney transplantation, and the proteinuria in patients with rituximab- and steroid-resistant nephrotic syndrome was alleviated after abatacept supplementation. These results indicated that CD80 may be involved in the pathogenesis of FSGS or MCD.
However, other researchers hold different opinions about this topic. Benign et al. [25] demonstrated that CD80 was not present in the renal biopsies of relapsed FSGS patients after kidney transplantation. Alachkar et al. [26] proposed that the remission of proteinuria in patients with recurrent FSGS after renal transplantation might not be due to CTLA-4 blocking CD80-induced T cell activation, because plasmapheresis, rituximab, and other immunosuppressants were adopted simultaneously in Yu’s study. Therefore, further large-scale multi-centre clinical studies will be necessary to verify the role of CD80 in FSGS and MCD.
Recently, several studies have suggested that the dysfunction of regulatory T cells (Tregs) plays an important role in the development of INS. Tsuji et al. performed a metagenomic analysis of gut microbiota in faeces from INS patients; the study showed that the proportion of butyric acid-producing bacteria was significantly lower in relapsing patients than that in controls. Their results suggested gut microbiota dysbiosis in children with relapsing, characterized by a decreased proportion of butyric acid-producing bacteria and lower faecal butyric acid quantities, concomitant with reduced circulatory Tregs, and dysfunctional Tregs due to gut dysbiosis played an important role in the development or exacerbation of INS [27]. As mentioned above, these studies have indicated that abnormalities in the quantity and functions of T lymphocytes are closely associated with the pathogenesis of INS. However, all these studies supplied with indirect evidences between T cells and INS, and abnormalities in T cells can not completely explain the pathogenesis of INS, as therapies targeting T cells are not always effective for all patients.
Pathogenic circulating factors
Recurrence in FSGS patients after renal transplantation was reported in 1972 [28], so the onset of FSGS is considered to be connected with circulating factors, which is also named as circulating permeability factors because the permeability of the glomerular filtration barrier increased by these factors. The podocyte damage of MCD and FSGS is considered to be the consequence of circulating factors. This view was not only derived from clinical observation but also supported by animal experiments [29].
According to the study by Ali et al. [30], two kidneys from one donor with biopsy-proven MCD were transplanted to patients with end-stage renal disease (ESRD); and the remission of proteinuria was observed in the recipients after transplantation, implying that the pathogenic factor of MCD is not the kidney itself, but may be due to the internal environment. Similarly, a Buffalo rat model study of FSGS also showed that proteinuria and renal lesions were significantly improved after transplanting the diseased kidneys into healthy rats [31].
Haffner et al. [32] found patients with FSGS had proteinuria in the early stage after kidney transplantation; and renal biopsy confirmed that it was due to FSGS recurrence rather than acute rejection. Complete and sustained remission can be achieved by plasmapheresis in combination with intensified immunosuppression in recurrent patients. Kemper et al. [33] found that the transmission of underlying osmotic factors to the fetus in women with primary FSGS may result in transient proteinuria in infants, suggesting that glomerular osmotic factors can be passed through the placenta and remain in the baby's circulation for several months.
According to the literature, FSGS recurs in the renal transplantation in 30–40% of patients, and idiopathic FSGS poses the highest risk of recurrence post-transplant [34]. These results imply the presence of a plasma factor or factors with unknown origin injure the integrity of glomerular filtration barrier.
Kashgary et al. [35] revealed that after a median of 12 plasma exchange treatments, 46.8% of 423 recurrent FSGS patients achieved a complete response after renal transplantation, and 28.1% achieved partial response. After a 19-month follow-up, 10.7% of the responders and 57.1% of the non-responders eventually progressed to ESRD, suggesting that plasma exchange can effectively alleviate recurrent FSGS after renal transplantation.
Several studies have reported the presence of different types of pathogenic circulating factors in INS, including hemopexin, cardiotrophin-like cytokine 1 (CLC-1), soluble urokinase receptor (suPAR), cathepsin L (CatL), angiopoietin-like-4 (Angptl4), apolipoprotein A-I (APOL1), sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b), and calcium/calmodulin-serine protein kinase (CASK).
Hemopexin
Hemopexin (Hx) is a 60-kD plasma glycoprotein in mammals and humans. In the late 1990s, it was used as the first potential cyclic permeability factor [36]. The treatment of human kidney tissue with Hx in vitro caused a series of classic glomerular changes, including a loss of anionic sites along the lamina internal of the glomerular basement membrane, decreased expression of extracellular ATPase, and a loss of glomerular sialoglycoproteins [37]. Plasma Hx activity was elevated during recurrence in 41 MCD patients. These reports indicated Hx as a potential effector in MCD [38]. Understanding the role of hemopexin in MCD may shed light on the pathogenesis of the disease.
Cardiotrophin-like cytokine 1
Cardiotrophin-like cytokine 1 (CLC-1) was first cloned from T cells, which is a member of the IL-6 cytokine family. Savin's group provided several lines of evidences to indicate that CLC-1 may be a pathogenic circulating factor in FSGS [29, 39]. They found that incubating murine podocytes with recombinant monomeric human CLC-1 increased permeability to albumin in isolated rat glomeruli. Clinically, CLC-1 is increased in patients with recurrent FSGS, and urinary protein is significantly reduced after the application of CLC-1 antibody. CLC-1 is a potential non-invasive biomarker for FSGS patients.
Soluble urokinase receptor (suPAR)
Wei et al. [40] found that the induction of urokinase receptor (uPAR) signaling in podocytes leads to foot process effacement and urinary protein loss via a mechanism that includes the lipid-dependent activation of alphavbeta3 integrin. Furthermore, they revealed that suPAR is elevated in geographically and ethnically diverse patients with FSGS. Our team also found a significant difference in plasma suPAR concentrations between SRNS and SSNS groups [41]. These clinical studies indicated that suPAR can be considered a specific diagnostic molecule to distinguish FSGS from MCD. In addition, suPAR has an important predictive value for recurrence after kidney transplantation; and the higher serum suPAR concentration is, the greater risk of FSGS relapse after kidney transplantation [42].
However, the pathogenic role of suPAR in FSGS remains controversial. Several studies have reported that suPAR levels can not be used to distinguish FSGS patients from those with other glomerular pathologies such as MCD, membranous nephropathy (MN), IgA nephropathy, lupus nephritis, or nonglomerular chronic kidney disease [43,44,45].
Cathepsin L
Cathepsin L (CatL) is involved in the breakdown of proteins in lysosomal chambers, which pertains to a subclass of cysteine proteases called lysosomal cathepsin. There is an intensity expression of CatL in rodent nephrotic podocytes. The increased expression and activity of CatL are associated with the onset of proteinuria [46]. Keisuke et al. [47] suggested that high levels of urinary CatL might be associated with the onset of INS and likely depend on the amount of protein leaking through the GBM.
Angiopoietin-like-4
Angiopoietin-like-4 (Angptl4) is a 45–65 kDa glycoprotein, which is strongly expressed in adipose and liver. Angptl4 has been suggested to play a part in the development of proteinuria in MCD [48,49,50]. Overexpression of Angptl4 in podocytes has been reported in MCD with relapse [49]; however, a large-sample study reported that Angptl4 was not expressed in glomeruli of MCD patients in relapse [51]. Serum or urine concentration of Angptl4 seems not to be a good biomarker for MCD.
Apolipoprotein A-I
Apolipoprotein A-I (APOLI) is found in numerous cell types, including monocytes, podocytes and platelets. Two coding variants, named G1 and G2 in the APOL1 gene, which are closely related to a risk of developing kidney disease in people of African ethnicity [52]. APOLI risk status is related to lower kidney function, more glomerulosclerosis, interstitial fibrosis, and a greater likelyhood to progress to ESRD [53]. The incidence of FSGS of African American is higher than that of European Americans, which is associated with variations in the gene encoding APOLI [52]. Clark et al. [54] reported urinary APOLI isoforms increased in many patients with FSGS; biopsies from FSGS patients showed increased APOLI staining at proximal tubule brush border, compared to diffuse cytoplasmic distribution in MCD.
Sphingomyelin phosphodiesterase acid-like 3b
Sphingomyelin phosphodiesterase acid-like 3b (SMPDL-3b) contributes to migration and actin remodeling in podocytes [55]. Ahmad et al. [56] revealed that radiation-induced smpdl-3b deletion changed the myelin homeostasis of the podocytes, leading to dysfunction of the podocytes. It was reported that Rituximab (RTX) pretreatment in kidney transplantation could prevent the recurrence of FSGS in an SMPDL-3b dependent manner by regulating the function of podocytosis [55].
Calcium/calmodulin-serine protein kinase
Calcium/calmodulin-serine protein kinase (CASK) is a membrane-associated kinase, which regulates a variety of protein–protein interactions among different cell types, including podocytes [57, 58] and neurons [59, 60]. It mediates the connection between actin and extracellular matrix. Thus, it participates in the organization of the cytoskeleton. Beaudreuil et al. found that the CASK was detected in the serum of patients with FSGS, but could not be detected in the serum of healthy controls, patients with significant proteinuria caused by diabetic nephropathy, MCD, idiopathic membranous nephropathy (IMN), and renal transplant patients without proteinuria. They suggest that the CASK is involved into the etiopathology of FSGS [61].
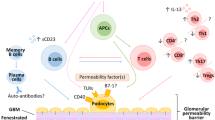
B lymphocyte dysfunction
Recent findings suggest that the dysfunction of B cells may play a more important role in the pathogenesis of INS than T cells. The number of activated B cells is increased in patients with INS, and the number is significantly reduced in patients after remission [62, 63]. A significant increase in serum immunoglobulin E (IgE) concentrations in patients with MCD is also an indirect evidence for the participation of B cells [64]. Clinical studies from two large samples (1700 cases) of B cell-derived Hodgkin's lymphoma reported that 0.4% of patients were complicated with MCD, which was often in remission after chemotherapy [65].
The most important evidence suggesting B cell participation in INS comes from the effect of rituximab (RTX) in INS. RTX is a human-mouse chimeric monoclonal antibody against the pre-B cell and mature B cell surface antigen CD20, recognizing and binding CD20 with high affinity. It mediates B cell apoptosis through signal transduction and a cascade of multiple kinases [66]. Recently, a multi-center, double-blind, randomized, placebo-controlled trial analyzed the therapeutic effects of RTX in 48 children with INS [67]. Notably, the median relapse-free period was shorter in the placebo group (101 days, 95% CI 70–155) than in the rituximab group (267 days, 95% CI 223–374). Furthermore, Basu et al. [68] investigated 176 consecutive children with steroid-dependent nephrotic syndrome (SDNS); and the results indicated that rituximab was more effective than tacrolimus in reducing corticosteroid exposure and maintaining remission. Lacking nephrotoxic effects and with good tolerance, rituximab can be considered a first-line corticosteroid-sparing treatment. Their findings suggest that RTX has a significant therapeutic effect on childhood-onset, steroid-sensitive but frequent relapse nephrotic syndrome and SDNS.
Another type of CD20 antibody, Ofatumumab, is a humanized CD20 monoclonal antibody which has increased affinity with CD20 and can prolong the dissociation time. Due to its stronger affinity for CD20, Ofatumumab is considered more effective than RTX [69]. To validate this hypothesis, Basu et al. [70] administered Ofatumumab in four children (2 MCD and 2 FSGS) with RTX-resistant idiopathic SRNS. After total dose administration, proteinuria decreased, the mean glomerular filtration rate improved, and remission was achieved. The results indicated that Ofatumumab may be better than RTX in the treatment of refractory SRNS. Nonetheless, further research is needed to verify these observations, and to determine the most effective dose and safety of ofatumumab in the treatment of SRNS or SDNS.
Idiopathic membranous nephropathy (IMN) is relatively rare in children but common in adults. There are strong evidences demonstrating that IMN is associated with abnormal B lymphocyte function. In 2009, M-type phospholipase A2 receptor (PLA2R), as a specific antigen of IMN [71], was identified in 70–80% of patients with IMN. Recently, additional autoantibodies have also been found IMN patients [72, 73], including thrombospondin type-1 domain-containing 7A (THSD7A, can account for 1–5%) [74], neural epidermal growth factor-like 1 protein (NELL-1, account for 5–10%) [75]. Currently, autoantibodies such as PLA2R can be applied as an important indicator for the diagnosis of MN and can even replace renal biopsy [76].
The target of chaos of the immune system in INS is also podocytes; however, unlike the knowledge for IMN, the target antigen of pathologic B cells in INS remains elucidated to the present time. Recently, lipoprotein apheresis (LA, the selective removal of lipoprotein particles from the blood with the return of the remaining components) or therapeutic plasma exchange (TPE, a therapeutic procedure in which blood of the patient is passed through a medical device which separates out plasma from other components of blood. The plasma is removed and replaced with a replacement solution such as colloid solution or a combination of crystalloid/colloid solution) is suggested in patients with steroid-resistant focal segmental glomerular sclerosis (FSGS) or recurrent FSGS after renal transplantation, and the recommendation grade is 2C [34]. The successful use of immunoabsorption techniques with various ligands demonstrates that putative circulating factors have immunoglobulin-like binding characteristics in INS.
Above all, there are direct and indirect evidences suggesting that B cell dysfunction is involved in the pathogenesis of INS. Future research should focus on novel biomarkers that can be used to make a precise diagnosis, guide therapy strategies, and forecast the prognosis of idiopathic nephrotic syndrome. Consequently, we have the rationale to speculate whether any autoantibody specific to podocytes exists in plasma of INS patients. Although no specific deposits of immune complexes in glomeruli have been confirmed in patients with INS, there may be certain antibodies that do exist yet shed from the podocytes. Capturing the presence of these antibodies in plasma is expected to be our further research strategy (Fig. 1).
Conclusions and future directions
The immunological mechanism of INS in children has not been fully elucidated yet, and there is no unitary theory that can fully explain the entire pathophysiological process of idiopathic nephropathy. For a long time, several studies suggest that the dysfunction of T cells is related to the pathogenesis of INS; however, with the discovery of the role of rituximab in idiopathic nephrotic syndrome, the dysfunction of B cells is also considered to be related to the pathogenesis of INS; and anti-podocyte antibodies may exist in the serum of children with INS, which will provide a novel way for precision diagnosis and potential therapeutic intervention in the future.
For a reasonable and practical point, it is necessary to identify new biomarkers in INS and validate their role in further large-sample, multi-center clinical studies. The continuous emergence of new biomarkers will lead to new paths for precise diagnosis and will have profound clinical significance in enhancing the efficacy of therapy and perfecting the prognosis of children with INS.
References
Radhakrishnan J, Cattran DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines–application to the individual patient. Kidney Int. 2012;82:840–56.
Noone DG, Iijima K, Parekh R. Idiopathic nephrotic syndrome in children. Lancet. 2018;392:61–74.
Roth KS, Amaker BH, Chan JC. Nephrotic syndrome: pathogenesis and management. Pediatr Rev. 2002;23:237–48.
Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis. 2013;62:403–41.
Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol. 2006;21:344–9.
Martinelli R, Okumura AS, Pereira LJ, Rocha H. Primary focal segmental glomerulosclerosis in children: prognostic factors. Pediatr Nephrol. 2001;16:658–61.
Paik KH, Lee BH, Cho HY, Kang HG, Ha IS, Cheong HI, et al. Primary focal segmental glomerular sclerosis in children: clinical course and prognosis. Pediatr Nephrol. 2007;22:389–95.
Trautmann A, Schnaidt S, Lipska-Zietkiewicz BS, Bodria M, Ozaltin F, Emma F, et al. Long-term outcome of steroid-resistant nephrotic syndrome in children. J Am Soc Nephrol. 2017;28:3055–65.
Davin JC. The glomerular permeability factors in idiopathic nephrotic syndrome. Pediatr Nephrol. 2016;31:207–15.
Daniel V, Trautmann Y, Konrad M, Nayir A, Scharer K. T-lymphocyte populations, cytokines and other growth factors in serum and urine of children with idiopathic nephrotic syndrome. Clin Nephrol. 1997;47:289–97.
Kaneko K, Tsuji S, Kimata T, Kitao T, Yamanouchi S, Kato S. Pathogenesis of childhood idiopathic nephrotic syndrome: a paradigm shift from T cells to podocytes. World J Pediatr. 2015;11:21–8.
Ishikura K, Ikeda M, Hattori S, Yoshikawa N, Sasaki S, Iijima K, et al. Effective and safe treatment with cyclosporine in nephrotic children: a prospective, randomized multicenter trial. Kidney Int. 2008;73:1167–73.
Lin CY, Hsu HC. Histopathological and immunological studies in spontaneous remission of nephrotic syndrome after intercurrent measles infection. Nephron. 1986;42:110–5.
Cunard R, Kelly CJ. T cells and minimal change disease. J Am Soc Nephrol. 2002;13:1409–11.
Federico A, Merletti MG, Lisi E, De Finis F, Trivelli G, Sopranzi F. Nephrotic syndrome as first presentation of malignant thymoma: description of a clinical case. G Ital Nefrol. 2010;27:674–9.
Geylis M, Rosen GB, Danino D, Schreiber R, Hassan D, Nalbandyan K, et al. Hodgkin’s lymphoma, nephrotic syndrome, and echinococcosis cysts: an unusual association and literature review. Pediatr Hematol Oncol. 2019;36:40–5.
Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, et al. Induction of B7–1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113:1390–7.
Cara-Fuentes G, Wasserfall CH, Wang H, Johnson RJ, Garin EH. Minimal change disease: a dysregulation of the podocyte CD80-CTLA-4 axis? Pediatr Nephrol. 2014;29:2333–40.
Garin EH, Diaz LN, Mu W, Wasserfall C, Araya C, Segal M, et al. Urinary CD80 excretion increases in idiopathic minimal-change disease. J Am Soc Nephrol. 2009;20:260–6.
Ling C, Liu X, Shen Y, Chen Z, Fan J, Jiang Y, et al. Urinary CD80 levels as a diagnostic biomarker of minimal change disease. Pediatr Nephrol. 2015;30:309–16.
Ahmed HM, Ezzat DA, Doudar NA, Adel M. Urinary CD80 as a replacement for renal biopsy for diagnosis of pediatric minimal change disease. Iran J Kidney Dis. 2018;12:107–11.
Tsuji S, Kimata T, Yamanouchi S, Kitao T, Kino J, Suruda C, et al. Regulatory T cells and CTLA-4 in idiopathic nephrotic syndrome. Pediatr Int. 2017;59:643–6.
Zhao B, Han H, Zhen J, Yang X, Shang J, Xu L, et al. CD80 and CTLA-4 as diagnostic and prognostic markers in adult-onset minimal change disease: a retrospective study. PeerJ. 2018;6:e5400.
Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, et al. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2013;369:2416–23.
Benigni A, Gagliardini E, Remuzzi G. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1261–3.
Alachkar N, Carter-Monroe N, Reiser J. Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med. 2014;370:1263–4.
Kaneko K, Tsuji S, Kimata T. Role of gut microbiota in idiopathic nephrotic syndrome in children. Med Hypotheses. 2017;108:35–7.
Hoyer JR, Vernier RL, Najarian JS, Raij L, Simmons RL, Michael AF. Recurrence of idiopathic nephrotic syndrome after renal transplantation. Lancet. 1972;2:343–8.
McCarthy ET, Sharma M, Savin VJ. Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2010;5:2115–21.
Ali AA, Wilson E, Moorhead JF, Amlot P, Abdulla A, Fernando ON, et al. Minimal-change glomerular nephritis. Normal kidneys in an abnormal environment? Transplantation. 1994;58:849–52.
Le Berre L, Godfrin Y, Gunther E, Buzelin F, Perretto S, Smit H, et al. Extrarenal effects on the pathogenesis and relapse of idiopathic nephrotic syndrome in Buffalo/Mna rats. J Clin Invest. 2002;109:491–8.
Haffner K, Zimmerhackl LB, von Schnakenburg C, Brandis M, Pohl M. Complete remission of post-transplant FSGS recurrence by long-term plasmapheresis. Pediatr Nephrol. 2005;20:994–7.
Kemper MJ, Wolf G, Muller-Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med. 2001;344:386–7.
Padmanabhan A, Connelly-Smith L, Aqui N, Balogun RA, Klingel R, Meyer E, et al. Guidelines on the use of therapeutic apheresis in clinical practice evidence-based approach from the writing committee of the American Society for Apheresis: the eighth special issue. J Clin Apher. 2019;34:171–354.
Kashgary A, Sontrop JM, Li L, Al-Jaishi AA, Habibullah ZN, Alsolaimani R, et al. The role of plasma exchange in treating post-transplant focal segmental glomerulosclerosis: A systematic review and meta-analysis of 77 case-reports and case-series. BMC Nephrol. 2016;17:104.
Cheung PK, Stulp B, Immenschuh S, Borghuis T, Baller JF, Bakker WW. Is 100KF an isoform of hemopexin? Immunochemical characterization of the vasoactive plasma factor 100KF. J Am Soc Nephrol. 1999;10:1700–8.
Lennon R, Singh A, Welsh GI, Coward RJ, Satchell S, Ni L, et al. Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol. 2008;19:2140–9.
Bakker WW, van Dael CM, Pierik LJ, van Wijk JA, Nauta J, Borghuis T, et al. Altered activity of plasma hemopexin in patients with minimal change disease in relapse. Pediatr Nephrol. 2005;20:1410–5.
Savin VJ, Sharma M, Zhou J, Gennochi D, Fields T, Sharma R, et al. Renal and hematological effects of CLCF-1, a B-cell-stimulating cytokine of the IL-6 family. J Immunol Res. 2015;2015:714964.
Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63.
Peng Z, Mao J, Chen X, Cai F, Gu W, Fu H, et al. Serum suPAR levels help differentiate steroid resistance from steroid-sensitive nephrotic syndrome in children. Pediatr Nephrol. 2015;30:301–7.
Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–60.
Kronbichler A, Saleem MA, Meijers B, Shin JI. Soluble urokinase receptors in focal segmental glomerulosclerosis: a review on the scientific point of view. J Immunol Res. 2016;2016:2068691.
Meijers B, Maas RJ, Sprangers B, Claes K, Poesen R, Bammens B, et al. The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int. 2014;85:636–40.
Sinha A, Bajpai J, Saini S, Bhatia D, Gupta A, Puraswani M, et al. Serum-soluble urokinase receptor levels do not distinguish focal segmental glomerulosclerosis from other causes of nephrotic syndrome in children. Kidney Int. 2014;85:649–58.
Reiser J, Oh J, Shirato I, Asanuma K, Hug A, Mundel TM, et al. Podocyte migration during nephrotic syndrome requires a coordinated interplay between cathepsin L and alpha3 integrin. J Biol Chem. 2004;279:34827–32.
Keisuke S, Kohei M, Takuji E, Tomoki M, Yuichi M, Rina O, et al. Role of cathepsin L in idiopathic nephrotic syndrome in children. Med Hypotheses. 2020;141:109718.
Chugh SS, Clement LC, Mace C. New insights into human minimal change disease: lessons from animal models. Am J Kidney Dis. 2012;59:284–92.
Clement LC, Avila-Casado C, Mace C, Soria E, Bakker WW, Kersten S, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–22.
Clement LC, Mace C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46.
Cara-Fuentes G, Segarra A, Silva-Sanchez C, Wang H, Lanaspa MA, Johnson RJ, et al. Angiopoietin-like-4 and minimal change disease. PLoS ONE. 2017;12:e0176198.
Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098–105.
Kopp JB, Winkler CA, Zhao X, Radeva MK, Gassman JJ, D’Agati VD, et al. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol. 2015;26:1443–8.
Clark AJ, Jabs K, Hunley TE, Jones DP, VanDeVoorde RG, Anderson C, et al. Urinary apolipoprotein AI in children with kidney disease. Pediatr Nephrol. 2019;34:2351–60.
Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46.
Ahmad A, Mitrofanova A, Bielawski J, Yang Y, Marples B, Fornoni A, et al. Sphingomyelinase-like phosphodiesterase 3b mediates radiation-induced damage of renal podocytes. FASEB J. 2017;31:771–80.
LaConte L, Mukherjee K. Structural constraints and functional divergences in CASK evolution. Biochem Soc Trans. 2013;41:1017–22.
Lehtonen S, Lehtonen E, Kudlicka K, Holthofer H, Farquhar MG. Nephrin forms a complex with adherens junction proteins and CASK in podocytes and in Madin-Darby canine kidney cells expressing nephrin. Am J Pathol. 2004;165:923–36.
Caruana G. Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int J Dev Biol. 2002;46:511–8.
Uhlen M, Bjorling E, Agaton C, Szigyarto CA, Amini B, Andersen E, et al. A human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteom. 2005;4:1920–32.
Beaudreuil S, Zhang X, Herr F, Harper F, Candelier JJ, Fan Y, et al. Circulating CASK is associated with recurrent focal segmental glomerulosclerosis after transplantation. PLoS ONE. 2019;14:e0219353.
Ling C, Wang X, Chen Z, Fan J, Meng Q, Zhou N, et al. Altered B-lymphocyte homeostasis in idiopathic nephrotic syndrome. Front Pediatr. 2019;7:377.
Printza N, Papachristou F, Tzimouli V, Taparkou A, Kanakoudi-Tsakalidou F. Peripheral CD19+ B cells are increased in children with active steroid-sensitive nephrotic syndrome. NDT Plus. 2009;2:435–6.
Hsiao CC, Tu KH, Hsieh CY, Lee CC, Chang CH, Fan PC, et al. Immunoglobulin E and G levels in predicting minimal change disease before renal biopsy. Biomed Res Int. 2018;2018:3480309.
Audard V, Larousserie F, Grimbert P, Abtahi M, Sotto JJ, Delmer A, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–60.
Maloney DG, Smith B, Rose A. Rituximab: mechanism of action and resistance. Semin Oncol. 2002;29:2–9.
Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384:1273–81.
Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, et al. Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: a randomized clinical trial. JAMA Pediatr. 2018;172:757–64.
Ravani P, Bonanni A, Ghiggeri GM. Randomised controlled trial comparing ofatumumab to rituximab in children with steroid-dependent and calcineurin inhibitor-dependent idiopathic nephrotic syndrome: study protocol. BMJ Open. 2017;7:e013319.
Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med. 2014;370:1268–70.
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21.
Ahmad SB, Appel GB. Antigens, antibodies, and membranous nephropathy: a decade of progress. Kidney Int. 2020;97:29–31.
De Vriese AS, Glassock RJ, Nath KA, Sethi S, Fervenza FC. A proposal for a serology-based approach to membranous nephropathy. J Am Soc Nephrol. 2017;28:421–30.
Godel M, Grahammer F, Huber TB. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2015;372:1073.
Sethi S, Debiec H, Madden B, Charlesworth MC, Morelle J, Gross L, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int. 2020;97:163–74.
van de Logt AE, Fresquet M, Wetzels JF, Brenchley P. The anti-PLA2R antibody in membranous nephropathy: what we know and what remains a decade after its discovery. Kidney Int. 2019;96:1292–302.
Funding
This study was supported by the National Natural Foundation of China (81770710), Key Research and Development Plan of Zhejiang Province (2019C03028), the Major projects jointly constructed by the Zhejiang Province, and National Health Commission (WKJ-ZJ-1908).
Author information
Authors and Affiliations
Contributions
All authors contributed to the preparation of this review and approved the text.
Corresponding author
Ethics declarations
Ethical approval
Not required for the review.
Conflict of interest
No financial or non-financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, J., Qiao, XH. & Mao, JH. Immunopathogenesis of idiopathic nephrotic syndrome in children: two sides of the coin. World J Pediatr 17, 115–122 (2021). https://doi.org/10.1007/s12519-020-00400-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-020-00400-1