Abstract
Background
We aimed to explore predictive measures for intravenous immunoglobulin (IVIG) resistance in children with Kawasaki disease (KD).
Methods
Patients diagnosed with KD were enrolled in this study. Univariate analysis and multiple logistic regression were utilized to analyze the clinical features and laboratory results prior to IVIG-treatment of the two groups. Independent predictors of IVIG resistance were analyzed, and a predictive model for KD children with IVIG resistance was constructed.
Results
A total of 277 children with KD, 180 boys and 97 girls, aged 2–128 (median 23) months, were enrolled in the study. Compared with the IVIG-responsive group, the IVIG-resistant group had higher levels of the peripheral neutrophil count, mean platelet volume, mean platelet volume-to-lymphocyte ratio and C-reactive protein, and total serum bilirubin, but lower levels of peripheral lymphocyte count, serum albumin and serum prealbumin. Age (in months), peripheral neutrophil count, lymphocyte count and mean platelet volume and serum albumin were independent indicators for IVIG resistance by multivariate logistic regression analysis. A logistic regression model and a scoring system were set up, where cut-off values of − 0.46 and 6.5 points yielded sensitivities of 83.9% and 77.4%, and specificities of 74.8% and 61.0%, respectively. The areas under the curve (AUC) were 0.808 in the logistic regression model, and 0.750 in the scoring system.
Conclusion
Our model for predicting IVIG-resistant children with KD, involving age (months), peripheral neutrophil count, lymphocyte count and mean platelet volume and serum albumin prior to IVIG-treatment, is helpful for clinical prediction of children with IVIG-resistant KD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Kawasaki disease (KD) with the highest incidence in the Northeast Asia especially in Japan, Korea and China, is the most common systemic vasculitis in children [1, 2]. In China, despite insufficient nationwide data, the latest epidemiological investigation showed that the incidence of KD was on the increase since 1998 and varied from 68.8 to 107.3 per 100,000 children under five in Shanghai City from 2013 to 2017 [3]. For children with KD, intravenous immunoglobulin (IVIG) is the first-line therapy; and patients who have repeated or persistent fever after IVIG therapy for 36–48 hours is defined as IVIG resistance [4]. The incidence of IVIG resistance in recent years has been reported as 4.9–20% [5,6,7,8,9]. The ratio of coronary ectasia is significantly higher in KD patients with IVIG resistance, who requiring a second-line treatment including the repeated dose of IVIG, corticosteroids, infliximab or cyclosporine. Long-term oral anticoagulant and antiplatelet drugs, and regular follow-up of coronary lesions are required in severe cases because of coronary complications with giant coronary aneurysm and myocardial infarction [5, 10].
Previous studies have indicated a variety of high-risk factors of IVIG resistance, including demographic features, clinical manifestations and laboratory findings [11,12,13,14,15,16,17,18,19,20,21,22,23]. A number of predictive models for KD patients with IVIG resistance were reported, such as Kobayashi, Egami and Sano scoring system from Japan, San Diego scoring system from American, and Formosa scoring system from Taiwan, China. However, the aforementioned models seemed relatively ineffective when used to predict children in the Mainland of China [24, 25], which might be related to heterogeneity of the populations. In recent years, investigators also reported useful predictive methods for IVIG resistance in China [11, 21, 26,27,28]. However, their sensitivity and/or specificity of the methods should be optimized.
The main pathology of KD is systemic vasculitis. In previous studies, the laboratory data used for the prediction of IVIG resistance mainly consisted of peripheral white blood cell count (WBC), C-reactive protein (CRP), serum liver enzymes, and platelet count. Actually, the indices reflecting the vascular inflammatory response in KD, including peripheral neutrophil count, lymphocyte count, monocyte count, monocyte-to-lymphocyte ratio (MLR), mean platelet volume (MPV) and mean platelet volume-to-lymphocyte ratio (MPVLR), have not been tested in the predictive tool for IVIG resistance. Therefore, the present research was designed to collect and analyze the clinical data in children with KD to identify the predictors of IVIG resistance and to establish valuable predictive models.
Methods
Study population
Patients diagnosed with KD for the first time in Peking University First Hospital from January 2014 to May 2019 were included in this retrospective research. The diagnosis of KD, including complete KD and incomplete KD, for each patient is consistent with the standards set by the American Heart Association [29]. Patients who received IVIG treatment before admission, or had other rheumatic diseases or infectious diseases were excluded. A total of 277 children (Chinese Han nationality) were enrolled, including 180 boys and 97 girls, aged 2–128 months. They were treated with a total dose of 2g/kg of IVIG and oral aspirin (30–50 mg/kg per day) after administration. The children were divided into two groups, including IVIG-responsive and IVIG-resistant groups. The definition of IVIG resistance was persistent or recrudescent fever (≥ 38 °C) for at least 48 hours after the end of the first IVIG infusion [22]. The Regional Ethics Committee of Peking University First Hospital approved this research.
Demographic data and clinical characteristics
Age (months), gender, weight, height, illness days of initial IVIG, skin rash, extremity changes, cervical lymphadenopathy, conjunctival congestion and changes in oral mucosal were recorded. The laboratory indices of peripheral blood test before IVIG-treatment included WBC, neutrophil count, lymphocyte count, monocyte count, hemoglobin, platelet count, MLR, red blood cell distribution width (RDW), platelet count-to-lymphocyte ratio (PLR), MPV, MPVLR and CRP. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, prealbumin, total bilirubin and serum sodium were also recorded.
Echocardiography
Coronary artery lesions were assessed by two-dimensional ultrasound. The frequency of the ultrasound probe was ≥ 5 MHz. By the short-axis view of the parasternal aorta in the supine position, the diameter of the left main coronary artery (LMCA) and the right coronary artery (RCA) were measured. The measurement is from one inner edge to the other inner edge. Z-value was the diameter of LMCA and RCA corrected by the body surface area [30]. The maximum Z value of coronary artery during hospitalization was determined. A Z value ≥ 2.5 was defined as the coronary artery injury [5].
Statistics analysis
Frequency and constituent ratio were used to describe categorical variables, and the difference between groups was compared by χ2 test. Continuous variables are showed as the mean ± standard deviation or median with quartile range, and independent sample T test or Mann–Whitney U test was used to compare the differences between the two groups depending on the data distribution. Variables with the p values less than 0.1 in the univariate analysis, together with the demographic characteristics (age, gender), were evaluated by logistic regression analysis with backward elimination to choose independent risk factors, and the logistic regression model was established.
To create an easy-to-perform scoring model, we performed the following analysis. To get the cut-off value of independent risk indicators for the prediction of IVIG-resistant patients, receiver operating characteristic (ROC) curve analysis was conducted. Two-categorical variables were converted from the continuous variables based on the optimal cut-off values derived by the abovementioned ROC curves. To verify the independent two-categorical variables, a second logistic regression analysis was performed and the odd ratios were estimated. Then, the score point of each variable was given to each patient according to the approximate odds ratio derived above. Total score points were then calculated for each patient.
Finally, ROC curves and the area under the curves (AUCs) were computed to evaluate the accuracy of the predictive model, and the cut-off values were identified by the Youden index derived from the calculated sensitivity and specificity. We also compared AUCs between the logistic regression model and the easy-to-perform scoring model. In the study, statistical analysis was carried out by SPSS V.25.0 and MedCalc V.15.2.2. P < 0.05 indicated significant difference.
Results
In this research, 277 children (180 boys and 97 girls) with KD meeting with the inclusion criteria were analyzed. The IVIG-responsive group consisted of 156 (63.4%) boys and 90 (36.6%) girls, and the IVIG-resistant group 24 boys (77.4%) and 7 girls (22.6%).
Univariate analysis
No significant differences in age, gender, symptoms and signs were observed between the two groups of children (Table 1). In the IVIG-resistant group, the rate of coronary artery lesion was much higher than that of the IVIG-responsive group (61.3%, 40.2%, P < 0.05).
Eighteen variables of laboratory examination were involved in the analysis of the research. In comparison to the IVIG-responsive group, serum total bilirubin, and peripheral neutrophil count, MPV, MPVLR and CRP were increased, and serum albumin and prealbumin were decreased in the IVIG-resistant group (Table 1, all P < 0.05).
Multivariate analysis and the logistic regression model
Eleven variables were evaluated by multivariate logistic regression, including age, gender, serum albumin, prealbumin, total bilirubin and sodium and peripheral neutrophil count, lymphocyte count, MPV, MPVLR and CRP. The results showed that age (months), peripheral neutrophil count, lymphocyte count and mean platelet volume, and serum albumin level were independent predictors of IVIG resistance (Table 2). And we obtained a logistic regression model by the following equation:
Establishment of predictive scoring system
The cut-off value of each independent predictor for IVIG resistance was obtained by ROC analysis. The results showed that the cut-off value to predict the IVIG resistance was as follows: age (months) ≤ 24 months, peripheral neutrophil count ≥ 10 × 109/L, peripheral lymphocyte count ≤ 3 × 109/L, peripheral mean platelet volume ≥ 10.5 fL and serum albumin ≤ 37 g/L (Table 3).
To create the scoring system, all independent predictors were converted to categorical variables, and multivariate analysis was performed. The odds ratio and score point of each variable were as follows: age (months) ≤ 24 months: odds ratio 2.820, 3.0 points; peripheral neutrophil count ≥ 10 × 109/L: odds ratio 2.903, 3.0 points; peripheral lymphocyte count ≤ 3 × 109/L: odds ratio 3.486, 3.5 points; peripheral mean platelet volume ≥ 10.5 fL: odds ratio 3.572, 3.5 points; and serum albumin ≤ 37 g/L: odds ratio 2.414, 2.5 points (Table 3). A total score points of a study subject were then calculated according to this scoring system.
ROC analysis
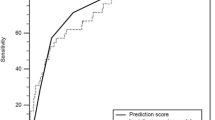
ROC curve was utilized to determine the accuracy of the model for predicting IVIG-resistant patients. A cut-off value of − 0.46 yielded a sensitivity of 83.9% and a specificity of 74.8%; and the AUC was 0.808 (95% confidence interval 0.741–0.876, P < 0.001) for the logistic regression model. A cut-off value of 6.5 points yielded a sensitivity of 77.4% and a specificity of 61.0% and the AUC was 0.750 (95% confidence interval 0.666–0.834, P < 0.001) for the scoring system. The comparison between the two models’ AUCs showed no statistical difference (P = 0.113) (Fig. 1).
Receiver operating characteristic (ROC) curve of the logistic regression model and the scoring system for predicting IVIG resistance in children diagnosed with KD. In the logistic regression model and the scoring system, cut-off values of − 0.46 and 6.5 points yielded sensitivities of 83.9% and 77.4%, and specificities of 74.8% and 61.0%, respectively. The area under the curve (AUC) was 0.808 in the logistic regression model and 0.750 in the scoring system, respectively
Discussion
Our research showed that age (months), peripheral neutrophil count, lymphocyte count and mean platelet volume, and serum albumin before IVIG-treatment were independent predictors for IVIG resistance. We used the five indicators to establish a logistic regression model for predicting IVIG-resistant children, and the cut-off of − 0.46 yielded a sensitivity of 83.9% and a specificity of 74.8%, respectively. A scoring system was set up for the convenient use in clinical practice, and the cut-off of 6.5 points yielded a sensitivity of 77.4% and a specificity of 61.0%, respectively. When the patient with KD is predicted to be at a high risk of IVIG resistance, we would consider using IVIG plus glucocorticoids in treatment.
In previous studies conducted on the Chinese population, younger age, particularly under 6-months and hypoalbuminemia were already found out as risk factors for IVIG-resistant patients [25]. However, peripheral neutrophil and lymphocyte count, and MPV were firstly involved in the predictive scoring system of IVIG resistance in KD.
Studies by Kobayashi et al. and Tan et al. showed that for IVIG-resistant patients with KD, younger age (months) was an independent risk factor [16, 21], and younger age especially in children under six months had a high risk of coronary artery lesion. Salgado et al. showed that children with KD younger than six months had high levels of peripheral WBC and CRP, but low levels of hemoglobin and serum albumin compared with those older than six months [31], which suggested that a severe inflammation occured in infants and young children with KD. When inflammatory reaction occurs, delayed apoptosis of neutrophils and stimulation of stem cells by growing factors lead to neutrophilia and redistribution in the lymphatic system, and increased apoptosis of lymphocyte leads to the lymphocytopenia [32]. In previous studies, the percentage of neutrophils was used to predict IVIG resistance, but the results of different studies were quite different. Kobayashi considered that the percentage of neutrophils ≥ 80% could predict IVIG resistance [16], Yang et al. showed the percentage of neutrophils ≥ 70% predicted IVIG resistance [27], while the Formosa predictive model assumed that neutrophil percentage ≥ 60% was the risk factor for IVIG resistance [18]. While several studies have focused on the association between the high level of peripheral neutrophil-to-lymphocyte ratio (NLR) and IVIG resistance. In Japan, Kawamura et al. found that NLR ≥ 3.83 and PLR ≥ 150 before IVIG-treatment could predict IVIG resistance [33]. In China, Hua et al. reported a model for the prediction of IVIG resistance including NLR [11], and Chen et al. found that NLR ≥ 2.51 was useful for predicting IVIG-resistant children under 12 months [34]. Considering that an elevated NLR ratio implies high neutrophil counts or low lymphocyte counts or both, this study underlines the association between these altered absolute counts and the higher risk of IVIG resistance. To further improve the capability of predicting IVIG resistance in KD, our study used the absolute count of neutrophil and lymphocyte as an entry point to predict IVIG resistance. Only univariate study confirmed that children with IVIG resistance had high peripheral neutrophil count and low lymphocyte count [17, 20, 21]. While we showed that the high level of neutrophil count and low level of lymphocyte count analyzed by multivariate regression could be used as independent predictors of IVIG resistance.
Hypoalbuminemia can predict IVIG resistance independently or in combination with other indicators [14, 27, 35]. Its possible mechanisms were not fully understood. Firstly, the increased vascular permeability during the systemic vasculitis largely leads to hypoalbuminemia. The severity of vascular leakage can be reflected by the level of vascular endothelial growth factor (VEGF) [36]. Serum VEGF concentration was inversely related to the serum albumin concentration in patients with KD [37]. Terai et al. reported that patients with IVIG resistance had higher VEGF levels and lower albumin levels compared with IVIG-responsive patients in the acute stage of the disease [38]. Secondly, previous studies indicated that indicators of liver function such as serum ALT, AST, GGT and bilirubin could be used as independent predictors of IVIG resistance [16, 17, 22]. Hepatic vasculitis resulting in the abnormal liver dysfunction might likely cause the poor ability of albumin synthesis.
MPV is a classic biomarker for inflammation. It is automatically calculated by blood analyzer according to the volume distribution of peripheral blood platelet morphology test. Peripheral MPV reflects platelet viability and function. The increased peripheral MPV has been found in some cardiovascular diseases, respiratory diseases, chronic kidney diseases, rheumatic diseases, diabetes and cancer. The increase in peripheral MPV is related to a growing number of large platelets during the inflammatory process. Large platelet facilitates the production of thromboxane A2 and β-platelet globulin [39,40,41]. But it has been unclear whether it plays a role in the prediction of IVIG-resistant KD. Our study firstly reported that MPV was an independent predictor of IVIG resistance. MPVLR represents mean platelet volume-lymphocyte ratio. Several studies have indicated that patients suffering from coronary artery disease with elevated MPVLR would have a poor prognosis [42,43,44]. In this study, patients from IVIG-resistant group had significantly increased levels of MPV and MPVLR compared with those in the IVIG-responsive group, which might be associated with the facilitated vascular inflammatory response.
In conclusion, however, there are some limitations in our study. This was a single-center research and the sample size of enrolled patients was not large enough. Further multi-center-based studies are needed to validate the predictive efficiency in the future.
References
Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100:1084–8.
Rowley AH, Shulman ST. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018;6:374.
Xie LP, Yan WL, Huang M, Huang MR, Chen S, Huang GY, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J Epidemiol. 2019. https://doi.org/10.2188/jea.JE20190065.
Phuong LK, Curtis N, Gowdie P, Akikusa J, Burgner D. Treatment options for resistant Kawasaki disease. Paediatr Drugs. 2018;20:59–80.
McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation. 2017;135:e927–e9.
Chen JJ, Ma XJ, Liu F, Yan WL, Huang MR, Huang M, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J. 2016;35:7–12.
Kim MK, Song MS, Kim GB. Factors predicting resistance to intravenous immunoglobulin treatment and coronary artery lesion in patients with Kawasaki disease: analysis of the Korean nationwide multicenter survey from 2012 to 2014. Korean Circ J. 2018;48:71–9.
Makino N, Nakamura Y, Yashiro M, Kosami K, Matsubara Y, Ae R, et al. Nationwide epidemiologic survey of Kawasaki disease in Japan, 2015–2016. Pediatr Int. 2019;61:397–403.
Skochko SM, Jain S, Sun X, Sivilay N, Kanegaye JT, Pancheri J, et al. Kawasaki disease outcomes and response to therapy in a multiethnic community: a 10-year experience. J Pediatr. 2018;203:e3.
Research Committee of the Japanese Society of Pediatric Cardiology, Cardiac Surgery Committee for Development of Guidelines for Medical Treatment of Acute Kawasaki D. Guidelines for medical treatment of acute Kawasaki disease: report of the Research Committee of the Japanese Society of Pediatric Cardiology and Cardiac Surgery (2012 revised version). Pediatr Int 2014;56:135–58.
Hua W, Sun Y, Wang Y, Fu S, Wang W, Xie C, et al. A new model to predict intravenous immunoglobin-resistant Kawasaki disease. Oncotarget. 2017;8:80722–9.
Hu P, Jiang GM, Wu Y, Huang BY, Liu SY, Zhang DD, et al. TNF-alpha is superior to conventional inflammatory mediators in forecasting IVIG nonresponse and coronary arteritis in Chinese children with Kawasaki disease. Clin Chim Acta. 2017;471:76–80.
Li X, Chen Y, Tang Y, Ding Y, Xu Q, Sun L, et al. Predictors of intravenous immunoglobulin-resistant Kawasaki disease in children: a meta-analysis of 4442 cases. Eur J Pediatr. 2018;177:1279–92.
Kuo HC, Liang CD, Wang CL, Yu HR, Hwang KP, Yang KD. Serum albumin level predicts initial intravenous immunoglobulin treatment failure in Kawasaki disease. Acta Paediatr. 2010;99:1578–83.
Takeshita S, Kanai T, Kawamura Y, Yoshida Y, Nonoyama S. A comparison of the predictive validity of the combination of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio and other risk scoring systems for intravenous immunoglobulin (ivig)-resistance in Kawasaki disease. PLoS ONE. 2017;12:e0176957.
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–12.
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166:131–7.
Lin MT, Chang CH, Sun LC, Liu HM, Chang HW, Chen CA, et al. Risk factors and derived formosa score for intravenous immunoglobulin unresponsiveness in Taiwanese children with Kawasaki disease. J Formos Med Assoc. 2016;115:350–5.
Masuzawa Y, Mori M, Hara T, Inaba A, Oba MS, Yokota S. Elevated D-dimer level is a risk factor for coronary artery lesions accompanying intravenous immunoglobulin-unresponsive Kawasaki disease. Ther Apher Dial. 2015;19:171–7.
Kong WX, Ma FY, Fu SL, Wang W, Xie CH, Zhang YY, et al. Biomarkers of intravenous immunoglobulin resistance and coronary artery lesions in Kawasaki disease. World J Pediatr. 2019;15:168–75.
Tan XH, Zhang XW, Wang XY, He XQ, Fan C, Lyu TW, et al. A new model for predicting intravenous immunoglobin-resistant Kawasaki disease in Chongqing: a retrospective study on 5277 patients. Sci Rep. 2019;9:1722.
Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–21.
Bar-Meir M, Kalisky I, Schwartz A, Somekh E, Tasher D, Israeli KG. Prediction of resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatric Infect Dis Soc. 2018;7:25–9.
Song R, Yao W, Li X. Efficacy of four scoring systems in predicting intravenous immunoglobulin resistance in children with Kawasaki disease in a children's hospital in Beijing. North China J Pediatr. 2017;184:120–4.
Qian W, Tang Y, Yan W, Sun L, Lv H. A comparison of efficacy of six prediction models for intravenous immunoglobulin resistance in Kawasaki disease. Ital J Pediatr. 2018;44:33.
Tang Y, Yan W, Sun L, Huang J, Qian W, Ding Y, et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in an east China population. Clin Rheumatol. 2016;35:2771–6.
Yang S, Song R, Zhang J, Li X, Li C. Predictive tool for intravenous immunoglobulin resistance of Kawasaki disease in Beijing. Arch Dis Child. 2019;104:262–7.
Fu PP, Du ZD, Pan YS. Novel predictors of intravenous immunoglobulin resistance in Chinese children with Kawasaki disease. Pediatr Infect Dis J. 2013;32:e319–e23.
Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the committee on rheumatic fever, endocarditis and Kawasaki Disease, council on Cardiovascular Disease in the Young. Am Heart Assoc Circ. 2004;110:2747–71.
Kobayashi T, Fuse S, Sakamoto N, Mikami M, Ogawa S, Hamaoka K, et al. A new Z score curve of the coronary arterial internal diameter using the Lambda-Mu-Sigma method in a pediatric population. J Am Soc Echocardiogr. 2016;29:794–801.e29.
Salgado AP, Ashouri N, Berry EK, Sun X, Jain S, Burns JC, et al. High risk of coronary artery aneurysms in infants younger than 6 months of age with Kawasaki disease. J Pediatr. 2017;185:e1.
Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102:5–14.
Kawamura Y, Takeshita S, Kanai T, Yoshida Y, Nonoyama S. The combined usefulness of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in predicting intravenous immunoglobulin resistance with Kawasaki disease. J Pediatr. 2016;178:e1.
Chen Y, Hua Y, Zhang C, Chen S, Zhang Q, Liao Y, et al. Neutrophil-to-lymphocyte ratio predicts intravenous immunoglobulin-resistance in infants under 12-months old with Kawasaki disease. Front Pediatr. 2019;7:81.
Ashouri N, Takahashi M, Dorey F, Mason W. Risk factors for nonresponse to therapy in Kawasaki disease. J Pediatr. 2008;153:365–8.
Soeters PB, Wolfe RR, Shenkin A. Hypoalbuminemia: pathogenesis and clinical significance. JPEN J Parenter Enteral Nutr. 2019;43:181–93.
Yasukawa K, Terai M, Shulman ST, Toyozaki T, Yajima S, Kohno Y, et al. Systemic production of vascular endothelial growth factor and fms-like tyrosine kinase-1 receptor in acute Kawasaki disease. Circulation. 2002;105:766–9.
Terai M, Honda T, Yasukawa K, Higashi K, Hamada H, Kohno Y. Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation. 2003;108:325–30.
Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074.
Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–56.
Sansanayudh N, Muntham D, Yamwong S, Sritara P, Akrawichien T, Thakkinstian A. The association between mean platelet volume and cardiovascular risk factors. Eur J Inter Med. 2016;30:37–42.
Kurtul A, Acikgoz SK. Usefulness of mean platelet volume-to-lymphocyte ratio for predicting angiographic no-reflow and short-term prognosis after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2017;120:534–41.
Hudzik B, Szkodziński J, Lekston A, Gierlotka M, Poloński L, Gąsior M. Mean platelet volume-to-lymphocyte ratio: a novel marker of poor short- and long-term prognosis in patients with diabetes mellitus and acute myocardial infarction. J Diabetes Complic. 2016;30:1097–102.
Wang Z, Ren L, Liu N, Peng J. Utility of hematological parameters in predicting no-reflow phenomenon after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2018;24:1177–83.
Funding
The work was supported by Peking University Clinical Scientist Program (BMU2019LCKXJ001), and Fundamental Research for the Central Universities.
Author information
Authors and Affiliations
Contributions
All authors contributed to all study data, revised and approved the final version of the manuscript. SW and JD designed the study, SW, YL, YS, CZ, HY, QZ, JQ, YC, XL, YW and JD acquired the data. SW, XL, HJ and JD analyzed the data, SW, HJ and JD drafted the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Regional Ethics Committee of Peking University, First Hospital.
Conflict of interest
No financial or non- financial benefits have been received or will be received from any party related directly or indirectly to the subject of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, S., Liao, Y., Sun, Y. et al. Prediction of intravenous immunoglobulin resistance in Kawasaki disease in children. World J Pediatr 16, 607–613 (2020). https://doi.org/10.1007/s12519-020-00348-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-020-00348-2