Abstract
Optimum water supply along with appropriate planting date plays the main role with respect to the yield and quality in okra, which is usually cultivated for its pods. This study was carried out at the research farm of Azad Islamic University, Tehran, Iran, to examine the effect of irrigation regimes and sowing dates on physiological traits, the quality and quantity of seed, and pod water use efficiency of okra (Abelmoschus esculentus L.). The results showed a reduction in the growth period from sowing to pod harvesting as planting time was delayed. Total chlorophyll content, plant height, and the number of pods/plants were decreased by drought stress and late planting. However, ash and mucilage content increased under mild drought conditions. Also, protein content and pod water use efficiency were significantly increased in late planting. Leaf area (LA) and carbohydrate content were considerably affected by the interaction of irrigation regimes and sowing at the flowering stage. Based on the results of cluster analysis, it was found that mild drought + June 4, mild drought + June 18, and mild drought + July 2 treatments were more closely related to all studied traits. Based on the biplot results, it was observed that the protein content, yield, mucilage, and LA had the highest correlation with mild drought + June 4 and mild drought + June 18 treatments. Also, well watered + June 4, well watered + June 18, and well watered + July 2 treatments showed a strong relationship with plant height, total chlorophyll, and fat content of the seed. These findings suggest that delay in planting improves water use efficiency, but yield and pod protein content were reduced under this condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Okra (Abelmoschus esculentus L.) originated in Ethiopia then propagated in the Mediterranean and North Africa. Now, it is cultivated in tropical and subtropical parts of the world, Southeast Asia, Arabia, Thailand, and India (Das et al. 2018; Keshavarz and Sadegh-Ghol-Moghadam 2017). The different useful fractions of this plant include stem fiber, leaves, seed, seed oil, and pods, which have been used as vegetables. Also, the okra pod (fruit) contains proteins, carbohydrates, mucilage, vitamin C, anthocyanin, and other phenolic compounds, which play a vital role in the human diet and also have medical importance like plasma replacement or blood volume expander (Da-Costa-Rocha et al. 2014). Okra needs a temperature above 25 °C for normal growth and development, but the flowering stage is postponed with an increase in air temperature. It is a short-day plant, and the critical day length reported is 12.30 h (Abd El-Fattah et al. 2020).
Many environmental agronomic factors influence the growth, yield, and quality of okra plants (Xu et al. 2019). Drought stress is the most important obstacle to achieving a high crop yield around the world. Furthermore, saving irrigation water and improving crop yields are two related and important global issues. Proper planting dates and practices of irrigation management are some effective techniques for raising utilization of limited water resources in these regions (Keshavarz Mirzamohammadi et al. 2021a). It has been reported that increasing intervals between irrigation and drought conditions, gave a significant decrease in yield and yield components but an increase in active constituents of okra (Abelmoschus esculentus) such as vitamin C and total phenols (Adejumo et al. 2019; Keshavarz et al. 2021). However, another researcher reported an increase in flowering and dry yield of okra in mild drought conditions (Singh et al. 2014). In another study on basil (Ocimum basilicum L), mild drought stress subsequent to the increase in irrigation intervals led to an increment in mucilage due to carbohydrate accumulation that reshape into secondary metabolites (Ghanbari and Ariafar 2013). Fallahi et al. (2017) found that drought conditions under light soil did not affect stem diameter and plant height of roselle, but instead, calyx yield and the number of flowers increased in mild drought stress. Keshavarz et al. (2018) defined that drought stress led to a reduction in biological and essential oil yield of two mint species.
Maximum production potential due to agronomic practices such as time of sowing has been related to high yield. An appropriate sowing date promotes plant growth and development resulting in higher biological yield and economic use of the land. The optimum sowing date ensures that the susceptible growth stage of the plant does not coincide with harmful environmental conditions. Okra is a short-day plant, and long days at the wrong developmental stage lead to yield losses (Ghayour et al. 2020). Morphological traits and yield components like stem diameter, plant height, and branch numbers are influenced by planting due to the longer growth period of the first planting date (Singh et al. 2013). The results of Ghannad et al. (2014) for okra highlighted a significant correlation between plant dry matter and sowing date and concluded that a wrong sowing date brings the loss of economic yield by affecting yield components. Asadpour et al. (2020) showed that a delayed sowing date decreases the grain yield of maize (Zea mays L.). In a study on the effect of five sowing dates on rice, Basyouni Abou-Khalifa (2010) concluded that the greatest grain yield was achieved at sowing dates of May 10 and April 30 (stage of milky and maturity, respectively). It was reported that a delay in the sowing date from mid-May to mid-July led to a 60% flower yield loss and a 58% reduction in the yield of the calyx (Ghayour et al. 2020). Ghannad et al. (2014) stated that the delay in sowing from March 30 to May 22, resulted in lower plant height, harvest index, and fruit yield per unit. Also, Morwal and Patel (2017) showed that a delay in the sowing time leads to loss of flower yield and biological yield but increased water use efficiency of the pod and biological yield.
Studies on the effect of irrigation and planting date on the quality of seed and water use efficiency are still rare. Changes in irrigation scheduling, water deficit techniques, and planting date have been widely used to improve the quality and quantity of yield and water use efficiency of plants. However, the present study aimed to examine the influence of different irrigation regimes and panting date on some physiological and morphological traits, pod yield, seed quality, and water use efficiency of okra (Abelmoschus esculentus L.) and to determine the exact quantity of irrigation water to be applied on okra plant grown.
Materials and methods
Site study
This study was carried out at the farm station of Azad Islamic University, Tehran, Iran to study the effect of water deficit stress and sowing date on the quantitative and qualitative yield of okra (Abelmoschus esculentus L.) during two consecutive growing seasons of 2017 and 2018. This experiment includes nine treatments, which were interaction between three irrigation regimes (well-watered, mild, and drought stress) and three planting dates (June 4, June 18, and July 2). The climate in this province is semidry, and annual rainfall is about 250 mm, which mostly occurs in autumn and winter between November and April. The average minimum and maximum temperatures and precipitation of both years of the present study are shown in Table 1. The soil type of the field was sandy loam, which contains total N (0.031%), available P (35 mg kg−1), and available K (225 mg kg−1) with pH and EC 6.96 and 0.82 dS m−1, respectively. Phosphorus (P) at 90 kg P2O5 ha−1 in the form of single superphosphate, potassium (K) at 60 85 kg ha−1 phosphorous, and kg K2O ha−1 in the form of potassium sulfate and 350 kg ha−1 urea were applied and incorporated into the soil before plant sowing.
Experiment design
The nine treatments were randomly employed in a split plot with three replicates. Irrigation regimes were distributed in the main plots, which included three irrigation levels (irrigation after 30%, 45%, and 60% (severe stress) depletion of available water were known as control, mild, and drought stress, respectively) (Keshavarz Mirzamohammadi et al. 2021b), while planting date treatments were arranged in subplots. Seeds of okra (Abelmoschus esculentus L.) were directly sowed in prepared plots (divided into four rows with 60 apart and 30 cm between the plants on the row) on June 4, June 18, and July 2 in both years. After germination, the seedlings were thinned to reach optimum density. The seedlings were irrigated in 80% field capacity when needed until they were completely established. The amounts of water to plots were controlled by contour. All plots were fertilized uniformly during soil preparation in the spring of each year.
Data collection
For the total chlorophyll assay and LA, the samples of leaves were harvested within 4 weeks after flowering. The total chlorophyll content was determined by the method of Arnon (1949) based on Eq. 1, while leaf area was determined by using the model [LA = 0.34(LW)1.12] modified by Omolaiye et al. (2015), where LA = leaf area, L = leaf length, and W = leaf width.
The harvest was performed at the physiological maturity stage from the first of October to the first of November (in both years) by harvesting the four middle rows. The traits included plant height, number of pods (fruit) per plant, and pod yield. Edible pods were harvested, counted (per plant), and weighed. Samples of okra fruits from each plot were analyzed for the carbohydrate, crude protein, crude fat, and ash of the okra fruits, which were determined using standard chemical methods described by the Association of Official Analytical Chemists (AOAC 2003). For this purpose, the pods of okra were blended. After that, they were diluted with ten times their weight with water (1:10). The viscous solution was separated from the debris using a fine cloth. Mucilage of the extracted viscous liquid was measured using a viscometer (Thanatcha and Pranee 2011). The pod yield per plot was converted to pod yield as kg ha−1. Pod water use efficiency (WUEpod) was calculated by dividing the dry pod yield (marketable yields) by the volume of applied water.
Statistical analysis
Analyses of variance (ANOVA) were performed using the SAS software (SAS Institute Inc. ver. 9.2). Two-year data was analyzed according to combined years because Bartlett’s test was not significant for all traits measured. Differences between the mean values of okra plant responses with the level of irrigation regimes and planting date were analyzed with the least significant difference (LSD) test at a significance of α ≤ 0.05. General correlations between parameters were examined with Pearson’s correlation coefficients. Principal component analysis (PCA) based on biplot (SAS 9.1) was applied to consider the visualization of similarities or differences and interrelationships by acute and obtuse angles among all parameters. Clustering analysis (S-PLUS ver. 6.1 software, Insightful Corporation, USA) aims at classifying objects based on the minimum variance linking method and similarity of input data for the existing parameters using Ward’s hierarchical approach and Euclidean distance to organize data into groups.
Results and discussion
The results showed that the different irrigation regimes differed in all studied traits except WUE (Table 2). Sowing date treatments differed significantly in terms of LA, plant height, the number of pods/plants, yield, protein, and WUE. The two-way interaction of irrigation regimes × sowing date treatments was significant for LA and carbohydrates (Table 2).
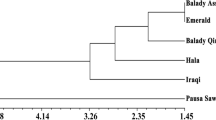
The results of cluster analysis showed that all the treatments were divided into three separate groups so that the interaction of severe drought + June 4, severe drought + June 18, and severe drought + July 2 (T7, T8, and T9, respectively) treatments were placed in one group and well watered + June 4, well-watered + June 18, and well-watered + July 2 (T1, T2, and T3, respectively) treatments were placed in the other group. Also, based on the results of cluster analysis, it was found that mild drought + June 4, mild drought + June 18, and mild drought + July 2 (T4, T5, and T6) treatments were more closely related to all studied traits and were placed in a group (Fig. 1). The results of the principal component analysis showed that the first and second components had the highest relative variance with 58% and 23%, respectively, and accounted for a total of 82% of the total variance. Based on the biplot obtained from the first and second components, it was observed that the protein content, yield, mucilage, and LA had the highest correlation with T5 and T4 treatments. Also, T1, T2, and T3 treatments showed a strong relationship with plant height, total chlorophyll, and fat content of the seed (Fig. 1).
The results of dendrogram based on cluster analysis (A) and biplot of first and second components based on principal component analysis. T1: well watered + June 4, T2: well watered + June 18, T3: well watered + July 2, T4: mild drought + June 4, T5: mild drought + June 18, T6: mild drought + July 2, T7: severe drought + June 4, T8: severe drought + June 18, T9: severe drought + July 2. Y1: plant height, Y2: total chlorophyll, Y3: leaf area, Y4: yield, Y5: pod water use efficiency, Y6: number of pods/plants, Y7: protein content, Y8: carbohydrate content, Y9: mucilage, Y10: ash, and Y11: fat
Total chlorophyll, leaf area (LA), and plant height
The data relating to the total chlorophyll content (Table 3), the well-watered irrigation (3.6 mg g−1 FW), showed maximum value for total chlorophyll content, which was higher than mild (22%) and severe (30%) drought stress. However, there was a significant difference between mild and severe drought in terms of total chlorophyll content. When compared with the control irrigation (well-watered), mild and severe drought stress decreased LA by 29% and 39%, respectively (averaged over the sowing date treatments) (Table 4). The maximum LA belonged to the well-watered treatment and on-time planting (July 4) with an average of 0.74 m2, which was 9.4% and 14.8% higher than the mild and severe drought stress, respectively (Table 4). Well-watered took a significantly greater plant height (60.2 cm) than mild and severe drought stress by an average of 24% and 35%, respectively (Table 3). The generation of reactive oxygen species (ROS) and free radicals such as O2−, H2O2, and OH in the plant cell is another result of stomatal closure, which yielded in peroxidation of chlorophyll, mitochondria, peroxisomes, and chloroplasts (Keshavarz 2020). Also, to avoid consequent damage, the chlorophyll needs to be degraded quickly by the chlorophyllase enzyme (Aghdasi et al. 2018b). In fact, water shortage leads to increase ROS in chloroplast and caused destruction to chlorophyll molecules. Our results are in harmony with those found by Karami et al. (2016) on soybean cultivars [Glycine max (L.) Merr.], which indicated that drought stress reduced plant growth, leaf area index (LAI), and plant height due to shortage in the vegetative growth stage and also disturbance in photosynthesis and low carbohydrate production. In addition, a prolonged growth period allowed the crops to use growth resources like light, water, and nutrient, which finally increased the growth of the crops. Similar findings in respect to drought stress and sowing date have been reported by Asadipour and Madani (2017) and Rah Khosravani et al. (2017) on okra (Abelmoschus esculentus L.) and maize (Zea mays L.) hybrids, respectively.
Number of pods/plants
A decrease from 10.5 pod/plant to 7.2 pod/plant of okra was recorded with decreasing the irrigation supply from control to severe drought stress, and this reduction was about 31.4%. However, there was no significant difference between mild and severe drought stress treatments (Table 3). El-Dissoky et al. (2020) and Bake et al. (2014) studied the effect of irrigation frequency on calyx yield of roselle (Hibiscus sabdariffa L.) and pod/plant in okra, respectively, and found that mild drought stress improved the calyx production and the number of pods, but the severe drought condition reduced these traits. Stress conditions have been shown to decrease the number of flowers and fruits (depending on the time of the stress severity) because the flowering phase involves several processes that are vulnerable to stress conditions (Jasim et al. 2020). From Table 5, it is clear that the first sowing date (June 4) registered a maximum number of pod/plant (9.7), while the lowest one (8.1) was recorded in the third sowing date (July 2) with 16.4% reduction in pod number. Given the favorable growth conditions such as temperature and sunlight on June 4, plants produced more assimilates and yielded higher flowers. Due to a shortened vegetative phase, flowering occurred when summer temperatures exceed and flowers are aborted. Previous research has reported that optimal planting time leads to better-developed plants with higher LAI and carbohydrates than those sown later (Rah Khosravani et al. 2017). Accordingly, flowering abortion would be expected to increase during drought stress as it decreases the flux of photosynthate supply from source leaves to the vegetative tissues. Also, drought stress may change the concentration of abscisic acid (ABA) in the plants, and thereby induce flower abortion in drought-stressed crops (Kumar et al. 2016).
Yield
A decrease from 2632 to 1882 kg ha−1 in okra yield was recorded with increasing the drought severity from well-watered to severe drought stress, and this condition significantly decreased okra economic yield to 1882 kg ha−1 (Table 3), which was 28.4% lower than the well-watered condition. In the well-watered condition, there was a strong association between leaf area index and plant height and the number of pods (Table 6), which means that plants with higher high have a much greater number of leaves and pods in plants. Stress condition reduces the yield production of crops by limitations in the water and essential fertilizer uptake (Keshavarz Mirzamohammadi et al. 2021b). It had been reported that reductions in grain yield of rice (Oryza sativa L.) in drought conditions seems attributed to the reduction in water availability, which reduces cell division, lower LAI, and plant height, finally resulting in lower dry matter and grain yield (Aghdasi et al. 2018a). They stated that the increase in allocation of photosynthates to the plant root compared to the shoots is the other reason for the reduction in shoot biological yield. In respect of sowing dates, a significant maximum yield (2377 kg ha−1) was achieved when the plant was sown on 4 June with a minimum yield (2027 kg ha−1) in the case of July 2 sown crop (Table 5), which was lower than 4 June by 14.7%. However, there was no significant difference between June 18 and July 2. These results are in accordance with those of Keshavarz and Khodabin (2019) and Rah Khosravani et al. (2017). The higher yield in early sowing was mainly due to more number of effective branch m2, more plant height, and greater stem diameter (some data not show). Also, the other reason for this result is probably due to the fact that the first sowing date resulted in a longer growing period; therefore, crops have time to extend their canopy. Also, an adequate irrigation supply would eventually yield a higher growth rate.
Carbohydrate
Carbohydrate content was higher in the well-watered condition + July 18 (with an average of 37.1%) (Table 4). The minimum carbohydrate content (29.6% and 29.6%) was recorded in severe drought stress + June 4 and June 18, which was statistically in the same group (Table 4). In mild drought conditions, there was a positive correlation between leaf area and fat content but a negative correlation was observed between the leaf area carbohydrate content of fruits (Table 6). Water availability limiting significantly is the main factor for carbon assimilation and carbohydrate production for plant growth (Keshavarz et al. 2016). Therefore, water shortage and drought stress result in considerable yield losses. Under drought conditions, the limited diffusion of CO2 by a reduction in the enzyme activity involved in the catalytic reactions in the Calvin–Benson cycle and along the mesophyll pathway resulted in the lower carboxylation rate efficiency of RuBisCO (Bake et al. 2017).
Crude fat and protein content
On average, the fat content under mild and severe drought treatments was significantly lower compared to the plants treated by well-watered (22.1% and 22.2%, respectively) (Table 3), while, mild and severe drought stress were at the same statistical level. The highest pod crude protein content (16.9% and 16.4%) was recorded in plants treated with well-watered and mild drought stress, respectively, while the lowest protein (14.08%) was recorded in severe drought stress treatment (Table 3). The effect of the sowing date was significant for the pod protein content of okra (Table 2). In other words, the mild drought condition decreases fat content but increases protein fraction. On average, crude protein content increased by 9.8% compared to on-time planting (June 4) when the seed was planted on July 2 (Table 5). Crude fat comes from carbon assimilation of leaves and green pod walls (Karami et al. 2016). Then, the carbohydrates are converted into triacylglycerol in the cellular organs (cytosol, plastid, and endoplasmic reticula). Drought stress, by affecting the lipid biosynthesis pathways and their enzymatic panel, has been reported to decrease the fat content. Stress conditions would affect the number of pods or pod length (some data not shown), and consequently, reduce available carbon assimilation for fat synthesis in the plants. Also, low oxygen content in the high air temperature caused a reduction in adenosine triphosphate (ATP) value and fat accumulation (Marika et al. 2018). Mariani and Ferrante (2017) reported a negative correlation between air temperature and oxygen level, which affects the fat content. In well-watered treatments, the plant uptakes optimal nitrogen, but in drought conditions, the water and nitrogen absorption were disturbed, which leads to a lower plant protein content. In late sowing dates, due to reduction in plant canopy (by high air temperature and changes in water balance), the nitrogen availability could have been superior to the demand, thus boosting the plant protein. Tamagno et al. (2018) reported that the foliar application of nitrogen increased the total amino acid content of phloem and was directly correlated with the plant protein and nitrogen content of soybean [Glycine max (L.) Merr].
Ash and mucilage content
Ash content was affected significantly by irrigation levels (Table 2), and the amount of ash under severe drought stress (6.1%) was higher than well-watered irrigation and mild drought stress (by an average of 21.3% and 19.6% lower than severe stress, respectively). In terms of mucilage content, severe drought stress considerably increased the mucilage content by 5.1% and 4.1% higher than well-watered and mild stress, respectively (Table 3). Changes in ash and mucilage under drought stress have been shown to depend on stress severity, drought duration, and cultivar (Alam et al. 2020; Ghanbari and Ariafar 2013). The increase in ash, protein, and mucilage contents of plants treated by drought stress compared to those of well-watered plants is in agreement with the findings of Yang et al. (2010) who stated that plant production special high molecular protein during drought condition assist them in resisting the effect of water shortage. Similarly, Keshavarz Mirzamohammadi et al. (2021b) reported that drought stress caused increased synthesis of ash, protein, and mucilage in isabgol (Plantago ovata Forsk).
Pod water use efficiency (WUEpod)
The results showed that (Table 5) the pod water use efficiency increased when the sowing date was delayed after June 4. Based on our results (Table 5), the highest pod water use efficiency was observed on July 2, and it was higher by 14.7% and 9.3% than the first and second planting dates, respectively. However, there was no significant difference between the second and third planting dates. Based on harvested pod yield and the amount of irrigation, the severe drought stress was detected as the best irrigation regime in terms of the WUEpod. As previously mentioned, the yield of okra was decreased by delay planting. In our study, since the average amount of irrigation water was reduced among the delay in planting treatments, the higher pod yield compared to water consumption led to a greater WUEpod. In fact, with the delay in planting conditions, reduction in water consumption was more than pod yield loss.
Conclusions
In brief, the drought stress improved the ash and mucilage content of pod yield, but the pod yield and protein content of pod reduced drought conditions. These results suggest that the delay of on-time planting date from June 4 to early July (July 2) is possible in improved pod WUE in arid and semiarid regions like Tehran (probably due to reducing irrigation supply) but not a good strategy to increase the quality and quantity of pod production.
References
Abd El-Fattah BES, Haridy AG, Abbas HS (2020) Response to planting date, stress tolerance and genetic diversity analysis among okra [Abelmoschus esculentus (L.) Moench.] varieties. Genet. Resour. Crop Evol. 67:831–851
Adejumo SA, Ezeh OS, Mur LAJ (2019) Okra growth and drought tolerance when exposed to water regimes at different growth stages. Int J Veg Sci 25(3):226–258. https://doi.org/10.1080/19315260.2018.1501788
Aghdasi S, ModaresSanavy SAM, Aghaalikhani M, Keshavarz H (2018a) Effect of foliar application of iron and manganese on yield and yield components of mungbean under water deficit stress. Water Soil Sci 28(3):13–25
Aghdasi S, ModaresSanavy SAM, Aghaalikhani M, Keshavarz H (2018b) Impact of water deficit stress and foliar application of iron and manganese on some morphological and physiological of mungbean (Vigna radiata L.). J Plant Proc Fun 7(26):101–116. http://jispp.iut.ac.ir/article-1-670-en.html
Alam M, Hayat Kh, Ullah I, Sajid M, Ahmad M, Basit A, Ahmad I, Muhammad A, Akbar S, Hussain Z (2020) Improving okra (Abelmoschus esculentus L.) growth and yield by mitigating drought through exogenous application of salicylic acid. Fresenius Environ. Bull. 29(01):529–535
AOAC (2003) Official methods of analysis of AOAC International. AOAC, Arlington
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenoxidase in beta vulgaris. Plant Physiol 24:1–15. https://doi.org/10.1104/pp.24.1.1
Asadipour A, Madani V (2017) The effects of irrigation and sowing date on the quantitative traits of okra (Abelmoschus esculentus L.). Int J Farm and Alli Sci 3(5):497–501
Asadpour S, Madani H, NourMohammadi G, MajidiHeravan I, Heidari Sharif Abad H (2020) Improving maize yield with advancing planting time and nano-silicon foliar spray alone or combined with zinc. Silicon. https://doi.org/10.1007/s12633-20-00815-5 (Online Available (2020))
Bake ID, Singh BK, Singh AK, Moharana DP, Maurya AK (2014) Impact of planting distances and sowing dates on yield attributing traits of okra [Abelmoschus esculentus (L.) Moench] cv. Kashi Pragati. Int J Curr Microbiol App Sci 6(7):4112–4125
Bake ID, Singh BK, Singh AK, Moharana DP, Maurya AK (2017) Effect of sowing dates and planting distances on quantitative attributes of okra [Abelmoschus esculentus (L.) Moench] cv. Kashi Pragati. Pharma Innovation 6(12):142–148
Basyouni Abou-Khalifa AA (2010) Response of some rice varieties to irrigation withholding under different sowing dates. Agric Biol J N Am 1(1):56–64
Da-Costa-Rocha I, Bonnlaender B, Sievers H, Pischel I, Heinrich M (2014) Hibiscus sabdariffa L. A phytochemical and pharmacological review. Food Chem 165:424–443. https://doi.org/10.1016/j.foodchem.2014.05.002
Das S, Pandey V, Mishra S (2018) Growth and fruit yield of okra as influenced by different growing environment. Int Agric Inn Res 6(5):232–234
El-Dissoky R, Attia AM, Awad AM (2020) Managing roselle plant (Hibiscus sabdariffa L.) requirements of fertilizers and irrigation grown under upper Egypt conditions. J Soil Sci Agric Eng 11(12):693–700
Fallahi HR, Ghorbany M, Aghhavani-Shajari M, Samadzadeh AR, Asadian AH (2017) Qualitative response of roselle to planting methods, humic acid application, mycorrhizal inoculation and irrigation management. J Crop Improv 31(2):192–208
Ghanbari M, Ariafar S (2013) The study of different levels of zeolite application on quantitative and qualitative parameters in basil (Ocimum basilicum L) under drought conditions. Intl J Agric: Res & Rev 3(4):844–853
Ghannad M, Madani H, Darvishi HH (2014) Responses of okra crop to sowing time, irrigation interval and sowing methods in Shahrood region. Intl J Agric Crop Sci 7(10):676–682. https://doi.org/10.15835/nsb346171
Ghayour M, Taherian M, Baghban S, Khavari S (2020) Effect of early planting dates and different treatments of seed priming on germination and seedling establishment of roselle (Hibiscus sabdariffa). Iranian J Seed Res 6(2):95–109
Jasim AA, Jabr AK, Rawdhan SA, Abdullatif ZA (2020) Effect of subsurface irrigation and drip irrigation on growth and yield of roselle under different irrigation periods. Indian J. Economic. Business 47(12):98–100
Karami S, Modarres-Sanavy SAM, Ghanehpoor S, Keshavarz H (2016) Effect of foliar zinc application on yield, physiological traits and seed vigor of two soybean cultivars under water deficit. Not Sci Biol 8(2):181–191
Keshavarz H (2020) Study of water deficit conditions and beneficial microbes on the oil quality and agronomic traits of canola (Brassica napus L.). Grasas Y Aceites 71(3):e373
Keshavarz H, Khodabin G (2019) The role of uniconazole in improving physiological and biochemical attributes of bean (Phaseolus vulgaris L.) subjected to drought stress. J Crop Sci Biotech 22(2):161–168
Keshavarz H, Sadegh-Ghol-Moghadam R (2017) Seed priming with cobalamin (vitamin B12) provides significant protection against salinity stress in the common bean. Rhizosphere 3:143–149. https://doi.org/10.1016/j.rhisph.2017.04.010
Keshavarz H, Modarres-Sanavy SAM, SadeghGholMoghadam R (2016) Impact of foliar application with salicylic acid on biochemical characters of canola plants under cold stress condition. Not Sci Biol 8(1):98–105
Keshavarz H, Modarres-Sanavy SAM, Mahdipour Afra M (2018) Organic and chemical fertilizer affected yield and essential oil of two mint species. J Essential Oil Bear Plant 21(6):1674–1681. https://doi.org/10.1080/0972060X.2018.1497545
Keshavarz H, Hosseini SJ, Sedibe MM, Achilonu MC (2021) Arbuscular mycorrhizal fungi used to support Iranian barley (Hordeum vulgare L.) cultivated on cadmium contaminated soils. Appl Ecol Environ Res 20(1):43–53. https://doi.org/10.15666/aeer/2001_043053
Keshavarz Mirzamohammadi H, Modarres-Sanavy SAM, Sefidkon F, Mokhtassi-Bidgoli A, Mirjalili MH (2021a) Irrigation and fertilizer treatments affecting rosmarinic acid accumulation, total phenolic content, antioxidant potential and correlation between them in peppermint (Mentha piperita L.). Irrigation Sci. https://doi.org/10.1007/s00271-021-00729-z (Online Available)
Keshavarz Mirzamohammadi H, Tohidi-Moghadam HR, Hosseini SJ (2021b) Is there any relationship between agronomic traits, soil properties and essential oil profile of peppermint (Mentha piperita L.) treated by fertiliser treatments and irrigation regimes?. Ann Appl Biol 179(3):331-344: https://doi.org/10.1111/aab.12707
Kumar V, Dhankar SK, Chandrashive AV, Yadav N (2016) Effect of spacing on growth and yield parameters of two varieties of okra (Abelmoschus esculentus). Int Farm Sci 6(1):163–168
Mariani L, Ferrante A (2017) Agronomic management for enhancing plant tolerance to abiotic stresses-drought, salinity, hypoxia, and lodging. Horticulturae 3(4):52
Bocchini M, D'Amato R, Ciancaleoni S, Fontanella MC, Palmerini CA, Beone GM, Onofri A, Negri V, Marconi G, Albertini E, Businelli D (2018) Soil selenium (Se) biofortification changes the physiological, biochemical and epigenetic responses to water stress in Zea mays L. by inducing a higher drought tolerance. Front Plant Sci 9:389. https://doi.org/10.3389/fpls.2018.00389
Morwal BR, Patel MC (2017) Growth and yield of okra (Abelmoschus esculentus L.) as affected by date of sowing and spacing under north Gujarat condition. J Krishi Vigyan 6(1):93–96
Omolaiye JA, Jayeoba OJ, Akoun J, Ogunbanjo OR, Adams BA, Ashidi JS (2015) Development of Leaf Area Prediction Model of Okra (Abelmoschus spp). PAT. 11(1):130–136
Rah Khosravani AT, Mansourifar C, Modarres-Sanavy SAM, Asilan KS, Keshavarz H (2017) Effects of sowing date on physiological characteristics, yield and yield components for different maize (Zea mays L) hybrids. Not Sci Biol 9(1):143–147. https://doi.org/10.15835/nsb919913
Singh S, Nathiram JP, Kaushik RH (2013) Seed quality of okra cultivars as affected by sowing dates and plant geometry. Asian J Hort 8(2):683–685
Singh P, Chauhan V, Tiwari BK, Chauhan SS, Simon S, Bilal S, Abidi AB (2014) An overview on okra (Abelmoschus esculentus) and it’s importance as a nutritive vegetable in the world. Int J Pharma Bio Sci 4(2):227–233
Tamagno S, Sadras VO, Haegele JW, Armstrong PR, Ciampitti IA (2018) Interplay between nitrogen fertilizer and biological nitrogen fixation in soybean: implications on seed yield and biomass allocation. Sci Rep 8:17502
Thanatcha R, Pranee A (2011) Extraction and characterization of mucilage in Ziziphus mauritiana Lam. Int Food Res J 18:201–212
Xu HJ, Wang XP, Zhao CY, Zhang XX (2019) Responses of ecosystem water use efficiency to meteorological drought under different biomes and drought magnitudes in northern China. Agric. For. Meteorol 278:107660
Yang X, Dong M, Huang Z (2010) Role of mucilage in the germination of Artemisia sphaerocephala (Asteraceae) achenes exposed to osmotic stress and salinity. Plant Physiol Biochem 48:131–135
Acknowledgements
The authors thank Dr. Olumide Samuel Daramola (Department of Plant Physiology and Crop Production, Federal University of Agriculture, Abeokuta, Nigeria) for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Haroun Chenchouni
Rights and permissions
About this article
Cite this article
Keyvan Rad, S., Madani, H., Heidari Sharifabadi, H. et al. Effects of different irrigation intervals and sowing time on yield attributing traits of okra (Abelmoschus esculentus L.). Arab J Geosci 15, 740 (2022). https://doi.org/10.1007/s12517-022-09663-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-09663-6