Abstract
Groundwater is the most important source for domestic and agricultural usage. Surface water supplies are meager in Saudi Arabia; therefore, it depends on the available groundwater reserves. Twenty-two groundwater samples were collected from different locations in Najran area according to the standard protocol recommended by the American Public Health Association (APHA, 1995). The study physio-chemical parameters of groundwater including pH, electrical conductivity, total dissolved solids, turbidity, calcium, magnesium, sulfate, and chloride parameters were measured and compared with the WHO standards. The results illustrate the concentration ranges of cations and anions as follows: pH (7.03–7.48), electrical conductivity (240–877 μS cm−1), total hardness (560–808 mg L−1), total dissolved solids (1287–1848 mg L−1), total alkalinity (107–183 mg L−1), Fe2+ (0.02–1.08 mg L−1), Cl− (341–601 mg L−1), Ca+2 (323–508 mg L−1), Mg+2 (132–316 mg L−1), and SO4 (250–680 mg L−1). Results indicate that the quality of groundwater in the study area is not suitable for drinking but can be safely used for agricultural purposes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater usage is dependent on its quality and its assessment forms an important aspect of groundwater studies where variations in groundwater quality from region to region depend on, to great extent, geogenic conditions (geology, amount of rainfall, hydraulic gradient) or due to anthropogenic activities (agricultural, domestic, or industrial pollution) (Belkhiri et al. 2010). The groundwater quality assessment issue has been studied worldwide throughout different researchers for various areas (Aghazadeh and Mogaddam 2010; Essumang et al. 2011; Spanos et al. 2015; Annapoorna and Janardhana 2015; Selvakumar et al. 2017; Khan and Jhariya 2017; Abbasnia et al. 2018; Celestino et al. 2018; Ibrahim 2019). While in Saudi Arabia, a limited research work has been carried out in this theme (Zaidi et al. 2015; Al Suhaimi et al. 2019).
Accordingly, the interpretation of the constituents presents in groundwater helps in understanding its suitability for different types of practice and also helps in understanding the governing mechanism (geogenic or anthropogenic) which influences its chemistry (Kelley 1940; Wilcox 1955). The groundwater quality to be used for a given purpose rests on the concentration of the dissolved ions and it is ideal to use the collective chemistry of all the ions rather than seeing the ions in pairs or segregation (Handa 1964, 1965; Hem 1985). Results of hydrochemical analysis are frequently utilized for interpretation of groundwater facies, determination of ionic relationships, and for geostatistical assessment.
The selected study area lies in Najran region at southern Saudi Arabia between longitudes from 45° 1′ 15″ to 45° 09′ E and latitudes from 19° 01″ to 19° 5′ 30″ N (Fig. 1). Najran city, situated along Wadi Najran, is the main administrative and commercial capital of the region. Wind-borne deposits from Ar Rub Alkhali cover the eastern part of Najran region. There are two major drainage systems of Wadi Habawnah and Wadi Najran gutter eastward into the Ar Rub Al Khali. The altitudes of Najran region range from about 950 to 2432 m above mean sea level. Moreover, the region has a rugged topography including a deeply dissected plateau.
On 2011, the Ministry of Environment, Water and Agriculture has been decided to drill 22 of deep groundwater wells to supply Najran urban area with water demand due to the scarcity of water resources Najran region besides the continuous expansion of the urban extension of Najran city. Furthermore, several environmental problems have arisen in near-surface water resources as a result of the dumping of sewage products into surface junkyards, which has become a threat to citizens and the surrounding environment throughout causing serious diseases.
Based on aforementioned facts, this study aims to assess the groundwater quality in the study area though physio-chemical analysis of 22 groundwater samples by evaluating the spatial distribution of dissolved ions in groundwater and defining its aptness for drinking and agricultural usage.
Geologic setting
The rocks in the region include the Proterozoic basement rocks along the southeastern margin of the Arabian Shield, the Cambro-Ordovician Wajid sandstone, and Quaternary surficial deposits (Fig. 2). The Proterozoic basement includes the mafic to intermediate volcanic rocks and sedimentary rocks that are regionally correlated with the Halaban group. The lithology of the oldest units and the inferred depositional environments of the layered rocks have been interpreted by Greenwood et al. (1982), to constitute the record of a Late-Proterozoic volcanic are. The andesitic and basaltic volcanic rocks probably formed from melting subducted, dominantly ensimatic crust along a zone of plate convergence that dipped southwest (Schmidt et al. 1979; Greenwood 1981; Greenwood et al. 1982).
Except for various Late-Proterozoic dikes, the granite-Syenite suite represents the final Proterozoic rock-forming event in the Najran quadrangle. Uplift and panation were followed by deposition of epiclastic sediment to from the Paleozoic Wajid Sandstone, which was probably overlain by younger, now-eroded sedimentary rocks. The eastern part of the Najran region includes the west margin of the Ar Rub’ al Khali, the vast basin that covers most of the southeastern part of the Arabian Peninsula.
Hydrogeology
Hydrogeologically, the study area is occupied by the upper Wajid aquifer. The thickness of the Upper Wajid aquifer varies from 100 m in the western part of Wadi Ad Dawasir to approximately 1500 m in the central part of the Rub’ Al Khali basin. The average thickness within the study area is about 200 m. The Upper Wajid aquifer is characterized as an unconfined aquifer. Except in outcrop areas, the Upper and the Lower Wajid aquifer are combined as one aquifer. Transmissivity value for the Upper Wajid aquifer is in the range of 7.1 × 10−4 m2 s−1 and 4.0 × 10−2 m2 s−1.
The average storage coefficient is 3.8 × 10−4. Specific yield is estimated to be 3–10%. Within the study area, the groundwater level in the Upper Wajid aquifer ranges between 118.72 and 132 m (Fig. 3). There is a gradual increase in depth to groundwater levels from the north to the south in the study area. The watershed for the study area was delineated using ArcGIS (Fig. 4). All the wells except well number 18 and 22 (extreme south) lie outside the watershed. The watershed has an area of 144.6 km2. It is a 4th order watershed with the main channel flowing from the south to the north. The highest elevations are seen in the eastern margin of the watershed. The elevation ranges from 827 to 1027 m above mean sea level.
Materials and methods
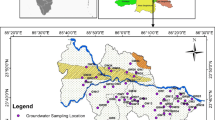
Twenty-two groundwater samples were collected from bore wells in the study area (Fig. 1). The total depth of these wells ranges from 650 to 849 m below the ground surface. These samples were analyzed for various physio-chemical parameter which includes pH, EC, TDS, total alkalinity (TA), total hardness (TH), and major ions. Samples were collected from unconfined aquifer in duplicate in new pre-cleaned polypropylene bottles (450-mL capacity). The wells were intensively pumped for about 30 min before collecting the water samples to avoid collection from well bore storage and any local contamination as well (Subyani 2005). The physical parameters were measured in situ and included temperature, EC (using conductivity meter), and pH (using pH meter). For the chemical constituents, the samples were sealed properly and sent to the laboratory for analysis. Figure 5 represents the adopted methodology flowchart for the present study.
Results and discussion
The results of the groundwater sample analysis from Najran area have been given in Table 1. The results are also compared with WHO (2004, 2011) guideline value for drinking water (Table 2).
Water quality assessment
Human health to a great extent depends on the quality of water used for drinking. If the dissolved constituents in the water including inorganic and organic constituents exceed a certain limit, it becomes unfit for consumption and can have adverse impact on human health. The World Health Organization has fixed a desirable limit and maximum permissible limit for different dissolved matter in water based on the effect of that particular matter when taken in excess or lesser amount (WHO 2004, 2011) (Table 2).
The concentration of dissolved constituent in water depends on geogenic as well as anthropogenic factors. The quality of water depends upon the assessment of the dissolved constituents and their comparison with a given water quality standard. The type of water quality required depends on its intended use. The same quality of water is not required for domestic, industrial, and agricultural purposes.
Total dissolved solids (TDS) are the sum of all dissolved chemical constituents and can be measured directly or can be estimated from the electrical conductivity (EC) of the water. Conductivity which has units of microsiemens is an indirect measure of the level of dissolved ionic constituents (impurities) in the water (Freez and Cherry 1979). Spatial distribution maps of EC, TDS, and other major ions were prepared to understand their distribution in the study area.
pH
Water having pH less than 7 is termed as acidic whereas more than 7 is termed as alkaline. This property of water is used to evaluate its interaction with rock or other materials (Hem 1985). The permissible range for pH as per global standards is 6.5–8.5 (WHO 2004) for drinking water. The pH range for the collected groundwater samples ranges from 7.03 to 7.51 (Table 1; Fig. 6).
Total dissolved solids
Total dissolved solids refer to the inorganic chemical constituents dissolved in water. It is generally equal to the sum of the concentration of major ions dissolved in water. Water with high TDS can affect its taste and hardness. WHO does not prescribe any health-based limit for TDS concentration in water; however, the taste of water changes significantly beyond 1000 mg L−1. Water with very low TDS has a bland taste (Khan and Jhariya 2017). TDS of water is influenced by the geology as different minerals have different solubility in water (WHO 2011). TDS concentration is also influenced by rock water interaction and rainfall recharge. In general, TDS determines the overall quality of groundwater to be used for a given purpose.
Sewage waste disposal or saline water intrusion (Sarath Prasanth et al. 2012; Subba Rao 2001; Ramesh et al. 2012) may also lead to high TDS concentration in groundwater. The groundwater suitability for various purposes based on TDS values has been shown in Table 3. TDS values for the collected groundwater sample vary from 1287 to 1880 mg L−1 (Table 1; Fig. 7). As per Table 3, all groundwater samples exceed the permissible limits for drinking.
The high salinity of groundwater in the study wells is due to the presence of thicker and massive salt layers to the east of the study area. This salt column is rather homogeneous, uniform density with depth and usually encountered at different depths (Mogren et al. 2014).
Electrical conductivity
EC is frequently used as a criterion to classify drinking and irrigation waters. EC value in the present study ranges from 1791 to 2622 μS cm−1 (Fig. 8). The maximum permissible limit of EC is 1500 μS cm−1, as per WHO 2004 guidelines. Thirty-three percent of the samples shows higher values in this study. According to Fig. 7, EC values decrease towards the south.
Higher EC values can have negative impact on human health (Annapoorna and Janardhana 2015). Geochemical processes such as ion exchange, reverse exchange, evaporation, silicate weathering, rock water interaction, sulfate reduction, and oxidation processes lead to high EC values in groundwater. Arid climate and high evaporation rates may be responsible for higher EC values in the present study.
Generally, the electrical conductivity (μs cm−1) can be categorized into four classes as follows: C1: low (0–249); C2: medium (250–749); C3: high (750–2249); C4: very High (2250–5000).
A total of 27.27% of groundwater samples fall under type-3 indicating high salinity, while 72.73% of samples falls under type 4 indicating very high salinity. Samples falling under type 3 and type 4 show that the high EC values may be a result of agricultural practices in the area of study
Major ion distribution
The distribution of major ions in groundwater gives an estimate about the recharge and discharge areas, determination of hydrochemical facies, and identification of main hydrochemical processes and the relative groundwater flowrates. Spatial distribution maps were prepared in the present study to show the distribution of major ions and hydrochemical facies.
Sulfate
According to WHO (2004), 250 mg L−1 is the desirable limit of sulfate in groundwater whereas 400 mg L−1 is the maximum permissible limit. The sulfate concentration in the present study ranges from 250 to 618 mg L−1 (Table 1; Fig. 9). The occurrence of sulfate in groundwater can be attributed to the presence of sulfide-bearing minerals, gypsum dissolution, application of sulfate-bearing fertilizers, and industrial wastes. Higher values of sulfate are observed in the eastern side of the study area.
Ammonia
Ammonia is a toxic substance and it becomes a health hazard when present in water above the ppm level. Ammonia can be present in water either as unionized ammonia (NH3) or the ammonium ion (NH4+). The value generally reported is the sum of the two forms and referred to as total ammonia or simply ammonia.
Natural level of ammonia in groundwater is generally below 0.2 mg L−1. The ammonia concentration ranges from 0.01 to 0.12 mg L−1 in the study area. Low values are observed in the northeast and high values are observed in the southwest (Fig. 10).
Total hardness
The presence of CO2 in the atmosphere and the plant roots makes the percolating rainwater acidic. The percolating acidic water has the greater capacity to dissolve the insoluble carbonates and other anions like sulfates, chlorides, and silicates increasing the hardness of groundwater and thus making it unsuitable for domestic and industrial use (Sarath Prasanth et al. 2012; Gopinath et al. 2015; Nag and Lahiri 2012).
The carbonate hardness is attributed to the presence of bicarbonate salts of calcium and magnesium. Salts of calcium and magnesium other than carbonate and bicarbonate lead to the non-carbonate hardness. The non-carbonate salts include CaSO4, CaCl2 salts. Total hardness of groundwater, however, includes both carbonate and non-carbonate. In general, most of the hardness in groundwater is related to the anions affiliated with calcium rather than magnesium, sodium, and potassium.
Dissolution of carbonate minerals such as calcite and dolomite leads to carbonate hardness. Hardness refers to the presence of magnesium and calcium in water and is expressed as equivalent concentration of dissolved calcite (CaCO3). The total hardness of water is expressed as (Todd 1980)
Total hardness = 2.5 Ca + 4.1 Mgwhere the concentration of calcium and magnesium is expressed in mg L−1.
The total hardness of the water samples in the present study ranges from 556 to 808 mg L−1 (Table 1 and Fig. 11). Using Sawyer and McCarty (1967) classification, the water samples have been classified as soft, moderately hard, hard, and very hard (Table 4). All the collected water samples in this study fall in the very hard category.
Calcium and magnesium
As per WHO (2004), the maximum permissible level for calcium in groundwater is 200 mg L−1. The Ca concentration in the collected groundwater sample ranges from 323 to 508 mg L−1 in Table 1 and Fig. 12. Limestone and the carbonate cement in sandstone are important sources of Ca (Hem 1985). In the study area, the groundwater shows high values in the north and low values in the south east.
Mg2+ concentrations are generally less as compared to Ca concentration in groundwater and are true in the present study as well. The Mg values range from 132 to 316 mg L−1 (Table 1; Fig. 13), and some samples exceed the maximum permissible limits in drinking water quality. Magnesium is mainly derived from ferromagnesian minerals in igneous rocks. The maximum permissible limit for magnesium in groundwater as suggested by WHO (2004) is 150 mg L−1. High values of Mg in groundwater give an unpleasant taste to groundwater (Ramesh and Elango 2011). Figure 12 shows that Mg values show an increase in the northern part of study area.
Chloride
The taste threshold of the chloride ion in water depends on the associated cation. Taste thresholds for sodium chloride and calcium chloride in water are ranges from 200 to 300 mg L−1. Consumers get accustomed to concentrations of chloride in excess of 250 mg L−1.
Concentration of Cl in groundwater in excess of 200 mg L−1 can give it an unpleasant taste. Whereas at concentrations in excess of 250 mg L−1, it can have negative impact on human health. Elevated Cl concentration in groundwater may be due to geogenic as well as anthropogenic reasons. Reverse ion exchange, halite dissolution, or saline water intrusion may lead to high values of Cl in water. Anthropogenic sources may include the use of inorganic fertilizes, leachates from landfill, septic tanks, and industrial waste disposal sites. Irrigation return flows are also a major source of elevated Cl concentration in water. Chloride concentration in uncontaminated waters seldom exceeds 10 mg L−1 and in some cases is also below 1 mg L−1.
The desirable level for chloride in drinking water is 250 mg L−1 and maximum permissible level is 1000 mg L−1. Cl− concentration in this study ranges from 341 to 601 mg L−1 (Table 1, Fig. 14). These chloride values are decreasing in the southeast and increasing in north east.
Iron
In anaerobic groundwater where iron is in the form of iron ions (Fe2+ and Fe3+), the concentration ranges from 0.5 to 10 mg L−1 and can reach up to 50 mg L−1 (National Research Council 1979). Naturally dissolved iron seldom exceeds 0.3 mg L−1 in water; however, it can increase significantly if iron salts are used as coagulating agents in water treatment plants. Use of cast iron, steel, and galvanized iron pipes in water distribution are other sources of elevated iron concentration in water. In the study area, the iron concentration ranges from 0.02 to 0.97 mg L−1. Low values are towards the southeast (Fig. 15).
Nitrate and nitrite
Concentrations of nitrate in rainwater can vary up to 5 mg L−1 in industrial areas (van Duijvenboden and Matthijsen 1989). In rural areas, concentrations are somewhat lower. The nitrate concentration in uncontaminated surface water is normally low (0–18 mg L−1) but agricultural runoff and irrigation return flows can greatly increase the nitrate concentration in surface water and groundwater, respectively. Unplanned discharge of sewage effluent and certain other industrial wastes can also lead to nitrate contamination in water. Nitrate values seldom increase 10 mg L−1 in uncontaminated water.
Nitrite concentration in drinking water is usually below 0.1 mg L−1. Nitrate value ranges from 0.14 to 25.08 mg L−1 in the study area (Fig. 16). The values are low in southwestern part and increase in the northern and eastern parts. Nitrite value ranges from 0.015 to 0.136. High values are seen in the southern part of the study area (Fig. 17).
Alkalinity
The carbonate, bicarbonate, and hydroxide content in surface water and groundwater contribute to its alkalinity. Higher alkalinity leads to the greater capacity of water to neutralize acids and vice versa. In neutral water, total alkalinity is associated with bicarbonate (Shov et al. 2014). The alkalinity ranges from 48.1 to 183 mg L−1 in the analyzed samples. All groundwater samples fall within the prescribed limits of 200 mg L−1 (WHO 2011). Recent rainfall recharge, silicate, and carbonate weathering are the main source of bicarbonate ions in groundwater (Srinivasamoorthy 2014). The values are low in the center of the study area and increase in the northeast (Fig. 18, Table 2).
Turbidity
Turbidity, expressed as nephelometric turbidity units (NTU), refers to the cloudiness of water due to suspended particles (e.g., clay and silts), chemical precipitates (e.g., manganese and iron), organic particles (e.g., plant debris), and microorganisms. Turbid water reduces its esthetic acceptability for drinking. Turbidity can range in appearance and color from milky-white clay-based particles to muddiness from sediments and soils, red-brown iron-based particles, and black manganese-based particles. Clear water has a turbidity below 1 NTU and the water becomes cloudy at 4 NTU and above.
Turbidity value in the study area ranges from 0.25 to 50 NTU. The values are high in the south eastern part (Fig. 19). This study shows that groundwater turbidity of 6 wells (27.27%) is less than 1 NTU, 10 wells (45.45%) between 1 and 4 NTU while 6 wells (27.27%) greater than 4 NTU.
Discussion and conclusions
The population in Najran district relies primarily on groundwater supplies for domestic and agricultural usage. In this study, the groundwater samples drawn from 22 wells were analyzed for their chemical contents and physical attributes: pH (7.03–7.48), electrical conductivity (240–877 μS cm−1), total hardness (560–808 mg L−1), total dissolved solids (1287–1848 mg L−1), total alkalinity (107–183 mg L−1), Fe2+ (0.02–1.08 mg L−1), Cl− (341–601 mg L−1), Ca+2 (323–508 mg L−1), Mg+2 (132–316 mg L−1), and SO4 (250–680 mg L−1). The results were compared with the WHO 2004 and 2011 values for drinking water. The groundwater is characterized by higher concentrations of TDS, EC, Ca+2, Cl−, Mg2+, and alkalinity in addition to hardness. The groundwater belongs to the hard to very hard type and is generally unfit for drinking purposes but suitable for irrigation and agricultural activities. The raised concentrations of some of the anions in groundwater are mainly due to rock water interaction and agricultural events where there are thicker and massive layers of salt are recorded at different depths in the eastern and southeastern provinces of Saudi Arabia.
Periodic monitoring of groundwater quality is important in the region over exploitation of the aquifer may lead to the deterioration of groundwater quality. This will be disastrous for the entire livestock in Najran region. Spatial distribution maps of selected parameters prepared from the hydrochemical data in ArcGIS were helpful in determining the regional variability of groundwater quality.
References
Abbasnia A, Radfard M, Mahvi AH, Nabizadeh R, Yousefi M, Soleimani H, Alimohammadi M (2018) Groundwater quality assessment for irrigation purposes based on irrigation water quality index and its zoning with GIS in the villages of Chabahar, Sistan and Baluchistan, Iran. Data Brief 19:623–631
Aghazadeh N, Mogaddam AA (2010) Assessment of groundwater quality and its suitability for drinking and agricultural uses in the Oshnavieh area. Northw Iran J Environ Protect 2010(1):30–40
AlSuhaimi AO, AlMohaimidi KM, Momani KA (2019) Preliminary assessment for physicochemical quality parameters of groundwater in Oqdus area, Saudi Arabia. J Saudi Soc Agric Sci 18:22–31
Annapoorna H, Janardhana MR (2015) Assessment of groundwater quality for drinking purpose in rural areas surrounding a defunct copper mine. Aquat Procedia 4:685–692
Belkhiri L, Boudoukha A, Mouni L, Baouz T (2010) Application of multivariate statistical methods and inverse geochemical modeling for characterization of groundwater - a case study: Ain Azel plain (Algeria). Geoderma 159:390–398
Celestino AEM, Cruz DAM, Sánchez EMO, Reyes FG, Sot DV (2018) Groundwater quality assessment: an improved approach to K-means clustering. Prin Comp Analy Spat Analys 2018(10):437. https://doi.org/10.3390/w10040437
Davis DV (1966) Hydrogeology. Wiley, New York, p 453
Essumang DK, Senu J, Fianko JR, Nyarko BK, Adokoh CK, Boamponsem L (2011) Groundwater quality assessment: a physicochemical properties of drinking water in a rural setting of developing countries. Can J Sci Indust Res 2(3):102–126
Freez RA, Cherry JA (1979) Groundwater. Prentice Hall Inc., Englewood Cliffs
Gopinath S, Srinivasamoorthy K, Saravanan K, Prakash R, Suma CS, Khan F, Senthilnathan D, Sarma VS, Devi P (2015) Hydrogeochemical characteristics of coastal groundwater in Nagapattinam and Karaikal aquifers: implications for saline intrusion and agricultural suitability. Coast Sci J, figshare. https://doi.org/10.6084/m9.figshare.1512790.v1
Greenwood (1981) Geology of the Bir Idimah quadrangle, sheet 18G, Kingdom of Saudi Arabia, with a Geographic map, compiled by F. J. fuller: Saudi Arabian Deputy Ministry for Mineral Resources Open-File Report USGS-OF-02-4, scale 1:250,000; text, 60p
Greenwood, WR, Stoeser, DB, Fleck, RJ, Stacey, JS (1982) Late Proterozoic island-arc complexes and tectionic belts in the southern part of the Arabian Shield, Kingdom of Saudi Arabia; Saudi Arabian Deputy Ministry for Mineral Resources Open-File Report USGS-OF-02-8, 46p
Handa BK (1964) Modified classification procedure for rating irrigation waters. Soil Sci 98:264–269
Handa BK (1965) Modified Hill-piper diagram for presentation of water analysis data. Curr Sci 34:131–314
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. USGS Water Supply Paper 2254:117–120
Ibrahim MN (2019) Assessing groundwater quality for drinking purpose in Jordan: application of water quality index. J Ecol Eng 20(3):101–111
Kelley WP (1940) Permissible composition and concentration of irrigation waters. Proc ASCE 66:607
Khan R, Jhariya DC (2017) Groundwater quality assessment for drinking purpose in Raipur City, Chhattisgarh using water quality index and geographic information system. J Geol Soc India 90:69–76
Mogren, S, Al-Ghamdi, A, Mukhopadhyay, M (2014) Central Arabia Salt Basin inferred by Gravity Modeling. GeoCanada 2010 – Working with the Earth, pp 1–4
Nag SK, Lahiri A (2012) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Dwarakeswar watershed area, India. Am J Clim Chang 01(04):217–230. https://doi.org/10.4236/ajcc.2012.14019
National Research Council (1979) Iron. University Park Press, Baltimore
Ramesh K, Elango L (2011) Groundwater quality and its suitability for domestic and agricultural use in Tondiar river basin, Tamil Nadu, India. Environ Monit Assess. https://doi.org/10.1007/s10661-011-2231-3
Ramesh K, Bhuvana JP, Li T (2012) Hydrochemical characteristics of groundwater for domestic and irrigation purposes in Periyakulam Taluk of Theni District, Tamil Nadu. I Res. J Environ Sci 1:19–27
Sarath Prasanth SV, Magesh NS, Jitheshlal KV, Chandrasekar N, Gangadhar K (2012) Evaluation of groundwater quality and its suitability for drinking and agricultural use in the coastal stretch of Alappuzha District, Kerala, India. Appl Water Sci 2(3):165–175. https://doi.org/10.1007/s13201-012-0042-5
Sawyer GN, McCarty DL (1967) Chemistry of sanitary engineers, 2nd edn. McGraw Hill, New York, p 518
Schmidt, DL, Hadley, DG, Stoeser, DB (1979) Late Najd province, Kingdom of Saudi Arabia, in Evolution and mineralization of the Arabian-Nubian Shield, southern Najd province, Kingdom of Saudi Arabia, in Evolution and mineralization of the Arabian-Nubian Shield: Oxford, Pergamon (King Abdul-Aziz University, Institute of Applied Geology Bulletin3), v. 2, p. 41–58
Selvakumar S, Chandrasekar N, Kumar G (2017) Hydrogeochemical characteristics and groundwater contamination in the rapid urban development areas of Coimbatore, India. Water Resourc Indust 17:26–33
Spanos T, Ene A, Xatzixristou C, Papaioannou A (2015) Assessment of groundwater quality and hydrogeological profile of Kavala area, northern Greece. Rom J Physiol 60(7–8):1139–1150
Subba Rao N (2001) Geochemistry of groundwater in parts of Guntur district, Andhra Pradesh, India. Environ Geol 41(5):552–562. https://doi.org/10.1007/s002540100431
Subyani A (2005) Hydrochemical identification and salinity problem of ground-water in Wadi Yalamlam basin, Western Saudi Arabia. J Arid Environ 60(2005):53–66
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley 31, New York, p 315
Van Duijvenboden W, Matthijsen (1989) Integrated criteria document nitrate. Bilthoven, National Institute for Public Health and the Environment (RIVM Report No. 758473012)
WHO (2004) WHO guidelines for drinking-water quality, 4th edn. World Health Organization
WHO (2011) Nitrate and nitrite in drinking water, Background document for development of WHO Guidelines for Drinking-water Quality. WHO/SDE/WSH/07.01/16/Rev/1
Wilcox LV (1955) Classification and use of irrigation waters, USDA, circular 969. USA, Washington DC
Zaidi FK, Kassem OM, Al-Bassam AM, Al-Humidan S (2015) Factors governing groundwater chemistry in Paleozoic sedimentary aquifers in an arid environment: a case study from Hail Province in Saudi Arabia. Arab J Sci Eng. https://doi.org/10.1007/s13369-014-1534-4
Funding
Deanship of Scientific Research at King Saud University provided funding for this research group No. RGP-1437-041.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Abdullah M. Al-Amri
Rights and permissions
About this article
Cite this article
Alfaifi, H.J., Kahal, A.Y., Abdelrahman, K. et al. Assessment of groundwater quality in Southern Saudi Arabia: case study of Najran area. Arab J Geosci 13, 101 (2020). https://doi.org/10.1007/s12517-020-5109-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-020-5109-2