Abstract
Little is known about the efficiency of biochar in the safety of food production in arid and semiarid contaminated soils. A field experiment in the semiarid region of Upper Egypt was conducted to explore heavy metals uptake by zucchini (Cucurbita pepo L) plants as affected by the application of cow manure biochar (CMB). Three rates of CMB were added, i. e., 0, 4, and 8 tonnes ha−1. The roots and shoots of zucchini plants stored 75 and 78% of Zn and Cu, respectively, while 25 and 22% were transported to the edible portions. On the other hand, the roots and shoots of zucchini plants stored more than 99% of Pb and Cd, while less than 1% was transported to the edible portions. Cow manure biochar (CMB) minimized the values of bioaccumulation factor (BAC) for Zn, Cd, and Ni by 5.6, 21.9, and 27.9%, respectively, while these reductions in the case of translocation factor (TF) were 4.9, 7.5, 23, and 5.9%. Cow manure biochar at a rate of 4 tonnes ha−1 reduced the availability of Zn, Cu, Pb, Cd, and Ni by 13.3, 8.3, 13.8, 9.1, and 3.6%, respectively, compared to the control treatment. Cow manure biochar at a rate of 8 tonnes minimized Zn, Cu, Pb, Cd, and Ni concentrations in the edible parts of zucchini plants by 10, 17, 66, 20, and 26%, respectively, in comparison with the untreated soil. Biochar reduced the heavy metals bioavailability, moreover; biochar minimized the soil-root transfer and the root-shoot of toxic metals. It is recommended to add cow manure biochar to zucchini plants to reduce the accumulating of hazard metals in the edible tissues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water accounted for about 50–97% of plant and animal bodies; moreover, it is vital for all biological processes in plant and animal cells (Buchholz 1998). The fresh water recourses in the world are very limited and only 0.6% of the total world water resources is fresh water (Fakayode 2005). Fresh water resources have been decreased at an alarming rate and this problem enlarged in the arid and semiarid conditions where high evapotranspiration rates (Qadir et al. 2008; Eissa 2016). Large parts of the agriculture lands located near urban are irrigated by wastewaters because of the low availability of the fresh ones (Eissa 2016). The use of sewage wastewaters in irrigation provides the soil with nutrients and organic matter; moreover, it is an inexpensive system for wastewaters disposal (Ghosh et al. 2012; Eissa 2016).
Accumulation of heavy metals in the agricultural soils has largely resulted due to anthropogenic activities and the addition of fertilizers and best side (Khan et al. 2016; Rizwan et al. 2016). The quality of soil as well as the crop yield production is declined due to soil contamination (Eissa et al. 2016; Rizwan et al. 2016). The use of sewage wastewaters to irrigate plants is the main source of heavy metals contamination of soils and plants (Eissa and Ahmed 2016). In many developing countries, these water resources may, in most cases, use as diluted raw sewage (Eissa 2016). Wastewaters contain high levels of cadmium (Cd), nickel (Ni), led (Pb), chromium (Cr), and arsenic (As). These elements are not essential for plant and animal nutrition (Kanwar and Sandha 2000). The use of sewage wastewaters in the irrigation processes may cause remarkable increases in the heavy metals concentrations in the agriculture lands (Ghosh et al. 2012). Elevation of soil heavy metal content will lead to introduce the metals to the vegetables and cereals crops causing a potential health risk to different organisms (Gupta et al. 2011). Toxicity of trace elements in plants cultivated in metal-contaminated soils is significantly higher than in those grown on non-contaminated ones (Gupta et al. 2011). Lead and cadmium are the metals of most concern due to the potential toxicity for animals and plants (Wolnik et al. 1983). Lead is the most common trace element contaminant in the environment and a small amount of absorbed Pb may be toxic to organisms (Watanabe 1997). Zinc and copper are essential nutrients for plants and animals; however, when they are found in high levels they can be toxic (Marchner 1995). It is known that the hazard of pollutions storing in the soil, water, and air affects the quality of products and healthiness (Eissa et al. 2014; Antisari et al. 2015; Eissa and Ahmed 2016).
In situ immobilization of toxic elements is recommended for remediation of metal-contaminated soil because it is effective in reducing the metals bioavailability with low costs (Lee et al. 2009). It involves applying amendments to reduce metals availability in soil (Zhou et al. 2014). Recently, numerous amendments for metal-contaminated soil have been used, such as apatite, lime, zeolite, red mud, compost, and biochar (Lee et al. 2009; Zhou et al. 2014; Mahmoud and Abd El-Kader 2015; Yang et al. 2017; Gu et al. 2018). Stabilization mechanisms contain precipitation, complexes formation, surface adsorption, and ligands or ion exchange (Mahmoud and Abd El-Kader 2015). Biochar is among the most effective materials, as it contains plant nutrients, improves the physical and chemical properties of farmland, and is more cheaper than other materials (Rehman et al. 2016, 2017; Amin and Eissa 2017; Abbas et al. 2018). The capacity of cation adsorption per unit of carbon unit of biochar is higher than other organic materials (Sombroek et al. 1993). The high adsorption capacity of biochar may due to its higher negative charges and higher charges density, moreover, its great surface area (Liang et al. 2006; Rizwan et al. 2016; Rehman et al. 2017; Gu et al. 2018). Biochar has different mechanisms in immobilization of heavy metals including ion exchange, precipitation, pore filling, electrostatic interaction, portioning, and surface sorption (Rehman et al. 2016; Abbas et al. 2018; Rwizan et al. 2018). Summer squash is one of the most important crops of the family Cucurbitaceae and of highly polymorphic vegetable grown during summer in tropical and subtropical condition (Sharma 2010; Tartoura et al. 2014). The edible part of zucchini is the fruit and it contains high levels of vitamins and minerals (Tartoura et al. 2014). Little information is available about the quality of zucchini plants cultivated on a metal-contaminated soil.
The plant has a natural capability to uptake metals from soil and to transfers to the shoots tissues depending on the biological practices in which the metal is involved (Ximénez-Embún et al. 2002). Heavy metal is defined as a metal with a density > 5.0 g cm−3 (Seaward and Richardson 1990), like essential plant nutrients, heavy metals can be taken up by plants (Marchner 1995). Some heavy metals are plant nutrients, e.g., Zn, Cu, and Mn and others are not essential nutrients such as Cd, Pb, and Ni (Marchner 1995).
Elements transport has a special interest because of the implications for the remediation of contaminated sites by plants (Eissa et al. 2013; Eissa 2015). But the available acknowledge about the transport processes for an element from soil to roots and from roots to shoots are still rudimentary in most cases (Park et al. 2012). The investigation of the translocation of metal from the soil to the plant and determine the places of accumulation at harvest stage helps greatly in making the right decision in the management of metal-polluted soils (Eissa 2014; Eissa et al. 2014; Eissa and Ahmed 2016).
Biochar has high ability in reducing metals availability and uptake by plants. So, this research work hypothesizes that biochar application may be an effective tool in enhancing the safety of food production in metal-contaminated soils. The current research was conducted to investigate the uptake and translocation of toxic metals by zucchini plants cultivated on a metal-polluted soil as affected by cow manure biochar application.
Materials and methods
Biochar production and characterization
Cow manure was collected from the Agricultural Experimental Station farm of the Faculty of Agriculture, (Assiut University, Egypt, 27° 12 N latitude and 31° 09 E longitude). The collected manure was air-dried then was oven-dried at 70 °C for 2 days. The dried cow manure was pyrolyzed (400 °C). Then, the produced biochar was crushed by a stainless steel mill. The total organic carbon in the biochar was determined by using the loss-on-ignition method (Ball 1964). Two-gram sample of biochar was digested in H2SO4 and HClO4 (1:1 v/v), then were analyzed for the total nitrogen, Zn, Cu, Cd, and Pb content. The pH of biochar was measured by a digital pH meter in a 1:10 suspension. The electrical conductivity (EC) was estimated in 1:10 extract using the salt bridge method (Burt 2004). The main chemical characteristics of cow manure biochar are shown in Table 1.
Field experiments
Three rates of cow manure biochar (CMB) were used in the current field trails (0, 4, and 8 tonnes ha−1). The experimental design was (RCBD) randomize complete block design with three replicates. The plot area was 20 m2. The field trials were carried out at Arab Elmadabegh village, Assiut, Egypt which are positioned at 27° 12′ 16.67″ N latitude and 31° 09′ 36.86″ E longitude. The experimental site has a semiarid condition without any rainfall during the period of the experiment. The average day and night temperature were 30–35 and 15–18 °C, respectively, and the relative humidity was 25–30%. The soil in this village has been irrigated by raw sewage water for more than 60 years and was classified as Arenosols. Table 2 displays the main characteristics of the studied site. Zucchini (Cucurbita pepo L cv Hybrid Fadwa) was cultivated in 2017 and 2018 growing seasons. Zucchini was seeded on ridges at 30 × 60 cm. After 2 weeks, plants were thinned to one plant per hill. The total number of plants was 55,000 ha−1. The plants were fertilized by 200, 150, and 100 kg ha−1 of urea (46% N), potassium sulfate (50% K2O), and superphosphate according (15% P2O5) based on the Ministry of Agriculture and Land Reclamation (Egypt). All the amount of P and K fertilizers was added before cultivation, while the urea fertilizer was added at three equal doses (1 week after germination, after 30 and 45 days of cultivation). All other recommended agriculture practices were taken according to the same annual recommendations. Zucchini plants were irrigated by sewage wastewaters every 10 days (Table 3 shows the main chemical characterizations).
Ten whole plants of zucchini were collected after 60 days of transplanting. Each plant sample was divided to root, shoot, and fruit. Each plant sample was washed twice with tap water. Then, washed with a solution of 0.1% HCl and then with 0.01% (Tween 80) to clean samples from the inorganic deposit. Finley, the samples were rinsed by distilled water, air-dried; oven-dried at 70 °C to a constant weight, ground, and then were kept for chemical analysis.
Chemical analysis of plant and soil
Physiochemical characteristics of the studied soil were determined according to Burt (2004) as they are presented in Table 1. The available heavy metals (Zn, Cu, Pb, Cd, and Ni) were extracted from the soil sample using a 0.005 M DTPA (diethylen triamine penta acetic acid) solution buffered at pH 7.3 as described by Lindsay and Norvell (1969).To determine the total heavy metals, the soil sample was digested according to the procedure given by the Arsenic and Beryllium (1996). The soil sample was air-dried and sieved with a 2-mm diameter sieve and kept for analysis. The metals in the soil and plant digest as well as DTPA soil extract were measured by using the inductivity coupled plasma emission optical emission spectrometry (ICP–OES thermo iCAP 6000 series). The ground plant samples were digested using concentrated acids of HNO3 and HClO4. A reference material was evaluated during soil and plant analysis for quality control and assurance.
Calculation of metals transfer
The bioaccumulation factor (BAF) was estimated to assess the transfer of metals from soil to root by the equation:
To assess metal transfer from root to shoot, translocation factor (TF) was calculated according to the equation:
The transfer of metal from shoots to fruit (SFT) was determined by the equation:
In the three above equations, the concentrations of metal expressed as mg kg−1.
Data analysis
The one-way ANOVA was run by SPSS software (version 15) to test the statistically significant difference between the two plants. Moreover, Duncan test (at 5% probability) was performed to compare between means.
Results
Effect of cow manure biochar on heavy metals levels in the studied site
The heavy metals content in the experimental site was analyzed and the data are presented in Table 1. The total concentration of Zn, Cu, Pb, Cd, and Ni in the studied site was 700 to 400, 350, 10, and 100 mg kg−1, respectively. The total content of the soil heavy metal determines the pollution level. The available concentrations of Zn, Cu, Pb, Cd, and Ni in the studied site were 8.8, 6.2, 4.5, 0.55, and 1.50 mg kg−1, respectively.
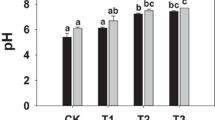
The application of cow manure biochar significantly minimized the bioavailability of Zn, Cu, Pb, Cd, and Ni in the experimental site (Fig. 1). Cow manure biochar at a rate of 4 tonnes ha−1 caused reductions in the bioavailability of Zn, Cu, Pb, Cd, and Ni by 13.3, 8.3, 13.8, 9.1, and 3.6%, respectively, compared to CMB0.
Toxic elements distribution in the tissues of zucchini plants
The concentrations of Zn, Cu, Pb, Cd, and Ni in the roots, shoots, and fruits of zucchini plants were determined and the data are presented in Table 4. The concentrations of Zn in the root of the studied plant ranged between 90 and 110 mg kg−1, while in the shoot these concentrations ranged from 70 to 90 mg kg−1. The root of zucchini plants stored 30–31% of the total Zn taken up by the whole plants, while the shoot tissues accumulated 43–46% (Table 5). The edible parts of zucchini stored 23–27% of the total Zn taken up by the whole plants. The concentration of Zn in the fruits of zucchini plants ranged between 54 and 60 mg kg−1.
Copper (Cu) concentration in the root and shoot of zucchini plants varied from 50 and 75 mg kg−1 (Table 4). The maximum value of Cu concentration was noted in the root, while the lowest concentration was obtained in the shoots. The roots of zucchini plants uptake 32–34% of the total Cu accumulated in the whole plants, while the shoots tissues stored about 45–47%. The concentrations of Cu in the edible parts of zucchini plants ranged between 30 and 35 mg kg−1, moreover, the fruits stored 21–24% of the total Cu accumulated in the whole plants.
Lead (Pb) content of the root and shoots of zucchini plants differed between 10 and 120 mg kg−1. The concentrations of Pb in the roots of zucchini plants were 105–120 mg kg−1, while in the shoots these concentrations were 10–15 mg kg−1. The roots of zucchini plants stored 81–85% of the total Pb taken up by the whole plants, while the shoot tissues absorbed 15–19% (Table 5). Pb concentrations in the fruits of the tested plants ranged between 0.34 and 1.0 mg kg−1. The Pb accumulated in the fruits accounted for 0.40–0.92% of the total Pb absorbed by the whole plant.
The roots and shoots of zucchini plants contained Cd levels varied between 3.0 and 13.0 mg kg−1. The concentrations of Cd in the shoots of zucchini plants were 3.2–4.5 mg kg−1; while in the roots were 10–13 mg kg−1. The roots of zucchini plants stored about 61–64% of the total Cd absorbed by the whole plants, while the shoot tissues stored about 35–38%. Cd concentrations in the fruits of the tested plants ranged between 0.07 and 0.10 mg kg−1 and this accounted for about 0.64–0.73% of the total Cd in the whole plant.
Nickel (Ni) levels in the roots and shoots of zucchini plants varied from 1.0 to 15 mg kg−1. The concentrations of Ni in the shoots of zucchini plants were 1.0–1.2 mg kg−1; while in the roots were 10–15 mg kg−1 (Table 4). The root of zucchini plant contained a very higher concentration of Ni compared to the shoot. The roots of zucchini plants stored about 82–84% of the total Ni absorbed by the whole plants, while the shoot tissues stored about 12–14% (Table 5). Ni concentration in the fruits of the tested plants ranged between 0.35–0.50 mg kg−1 and this accounted for about 3.60–4.15% of the total Ni in the whole plant.
In general, the roots of zucchini plants contained concentrations of Zn, Cu, Pb, Cd, and Ni than shoots. The concentrations of the investigated metals in the roots of the tested plant were found to decrease in the order: Pb > Zn > Cu > Ni > Cd, while in the shoots these concentrations were found to decrease in the order: Zn > Cu > Pb > Cd > Ni. The concentrations of investigated metals edible parts of zucchini plants were found to decrease in the order: Zn > Cu > Pb > Ni > Cd. The roots and shoots of zucchini plants stored 75 and 78% of Zn and Cu, respectively, while 25 and 22% was transported to the edible portions. On the other hand, the roots and shoots of zucchini plants stored more than 99% of Pb and Cd, while less than 1% was transported to the edible portions.
Soil-plant transfer of heavy metals
The behavior of heavy metal accumulation by zucchini plants was investigated by the calculation of the soil-root, root-shoot, and shoot-fruit transfer of the studied metals and the data are shown in Figs. 2, 3 and 4. The bioaccumulation factor (BAF) was calculated from the metal in the root divided by the available metal in the soil. The BAC is used to assess the soil-root transfer of metal (Eissa and Ahmed 2016). The values of BAC ranged between 7.78 and 30 and these values differed between the investigated metals (Fig. 2). The values of BAC of Cd and Pb were than those of Zn and Cu. The BAC values were found to decrease in the order Pb > Cd > Zn = Cu > Ni.
The translocation factor (TF) was calculated from the metal in the shoot divided by that found in the root (Fig. 3). The TF is used to assess the root-shoot transfer of metal (Eissa and Ahmed 2016). In the present research, the values of TF ranged between 0.08 and 0.82 and these values differed significantly from metal to metal. The TF values were found to decrease in the order Zn > Cu > Cd > Pb > Ni. The highest TF value was recorded in the case of Zn and Cu, while the lowest ones were found in the case of Pb, Cd, and Ni.
The shoot-fruit transfer (SFT) was calculated from the metal in the fruit divided by that found in the shoot (Fig. 4). The SFT is used to assess the shoot-fruit transfer of metal (Eissa and Ahmed 2016). In the current study, the values of SFTF ranged between 0.02 and 0.77 and these values differed significantly from metal to metal. The SFT values were found to decrease in the order Zn > Cu > Ni > Pb > Cd. The highest SFT value was recorded in the case of Zn and Cu, while the lowest ones were found in the case of Pb, Cd, and Ni.
Effect of cow manure biochar on heavy metals uptake and translocation
The concentrations of Zn in the root, shoot, and fruits of zucchini plant were significantly (P < 0.05) affected by the application of cow manure biochar. The higher Zn concentrations were recorded in the zucchini plants grown on control soil. The application of CMB caused a notable decrease in the concentration of Zn in the different tissues of the tested plant. CMB8 minimized the Zn concentration in the root, shoots, and fruits of zucchini plants by 18, 22, and 10%, respectively, in comparison to the untreated soil.
Cu concentrations in the root, shoot, and fruits of the tested plant were significantly (P < 0.05) affected by the addition of cow manure biochar. The higher Cu concentrations were obtained in the zucchini plants grown on the untreated soil. Cow manure biochar application caused a remarkable lessening in Cu concentrations in the different tissues of the zucchini plants. CMB8 reduced the Cu concentrations in the roots, shoots, and fruits of zucchini plants by 10, 17, and 17%, respectively, compared to the control soil.
Cow manure biochar significantly (P < 0.05) affected in the concentrations of Pb in the root, shoot, and fruits of zucchini plants. The lowest Pb concentrations were recorded in the zucchini plants grown on the soil mended with CMB8. The application of CMB caused a remarkable lessening in Pb concentrations in the different tissues of the zucchini plants. CMB8 reduced the Pb concentrations in the roots, shoots, and fruits of zucchini plants by 13, 34, and 66%, respectively, in comparison to the untreated soil.
The application of CMB caused a notable decrease in the concentration of Cd in the different tissues of the tested plant. CMB8 minimized the Cd concentrations in the roots, shoots, and fruits of zucchini plants by 24, 29, and 20%, respectively, compared to the control soil. The application of CMB caused a notable decrease in the concentration of Ni in the different tissues of the tested plant. CMB8 minimized the Ni concentrations in the roots, shoots, and fruits of zucchini plants by 30, 20, and 26%, respectively compared to the control soil.
Cow manure biochar application significantly reduced the bioaccumulation factor of Zn, Cd, and Ni. CMB8 minimized the BAC of Zn, Cd, and Ni by 5.6, 21.9 and 27.9%, respectively in comparison with the untreated soil. The translocation factor of the tested heavy metals was significantly affected by the application of cow manure biochar. The application of CMB caused remarkable decreases in the root-shoot transfer of Zn, Cu, Pb, and Cd. The application of CMB8 reduced the TF values of Zn, Cu, Pb, and Cd by 4.9, 7.5, 23, and 5.9%, respectively, in comparison with control soil.
Discussion
Heavy metals in the studied soil and wastewaters
The importance of total heavy metals content in soils is due to that the high concentrations of the toxic metals are the first problem facing the plant growth in the contaminated soils. The total concentrations of all the investigated metals (Zn, Cu, Pb, Ni, and Cd) in the soil were above the maximum permissible limits recorded by EU (2002) and USEPA (1997). According to the United States Environmental Protection Agency (USEPA 1997) and European Union Standards (EU 2002), the studied soil is a metal-contaminated soil. The guidelines of both agencies confirmed that the maximum permissible Zn, Cu, Cd, Ni, and Pb levels are 300, 140, 3, 50, and 300 mg kg−1 of soil. These obtained values confirmed that the soil under the study is contaminated with these heavy metals. The obtained results of the current research were found by Eissa and Ahmed (2016). The long-term use of the treated and untreated wastewaters in irrigation was reported to cause a significant buildup of the heavy metals in the soils (Ghosh et al. 2012). The levels of Zn, Cu, and Cd in the tested water were higher than the permissible limits of the irrigation water according to the FAO (1985).
Toxicity of heavy metals in the tissues of zucchini plants
The roots of zucchini plants contained higher levels of Zn, Cu, Pb, Ni, and Cd rather than the shoots and the edible parts. These results were confirmed by Eissa (2016) who found that the concentration of Zn, Cu, Pb, Ni, and Cd in the root of zucchini grown on a metal-contaminated soil was higher than those of the shoots and the edible parts. According to WHO/FAO (2007) and EU (2006), the maximum permissible Zn, Cu, Cd, and Ni limits for human consumption is between 60–80, 40, 0.20, and 1.50 mg kg−1 dry weights, respectively. Thus, the concentration of Zn, Cu, Cd, and Ni in the edible part of zucchini was less than the allowable level and these plants are safe for human consumption. According to WHO/FAO (2007) and EU (2006), the maximum Pb permissible level for human consumption is 0.3 mg kg−1 dry weight. The concentrations of Pb in the edible parts of zucchini were 0.35–1.0 mg kg−1. Thus, the concentrations of Pb in the edible plant parts were higher than this permissible level.
The shoots of zucchini plants usually use in animal feed especially in the arid regions (Eissa 2016). The data in Table 4 presents the concentration of toxic elements in the shoots of zucchini. The maximum allowable levels of elements in the animal forage are 10–100 mg kg−1 for Pb, 20–100 mg kg−1 for Cu, 250–1000 mg kg−1 for Zn, and 10 mg kg−1 for Cd (NRC 2005). Exploring the results in Table 4, it seems that the concentration of elements in the shoot of the studied plants was below these limits, and could therefore safely be used as a source of forage for livestock production.
. Soil-plant transfer of heavy metals
The data of the current study indicated that the values of Cd and Pb soil-root transfer were the highest ones. The high rate of the soil-root transfer in the case of Cd and Pb may be due to the high mobility of these metals compared to the other studied metals (Eissa 2017). The values of the root-shoot transfer of the investigated metals were less than one indicating that zucchini plants absorbed more metals in the roots and transferred fewer concentrations to the shoots (Eissa and Ahmed 2016). In general, the root-shoot transfer of Zn and Cu was higher than that of Pb, Cd, and Ni. The obtained results from the current research were confirmed by Eissa (2016). The higher rate of the TF values in the case of Zn and Cu may be due to they are essential micronutrients (Marchner 1995) while Pb, Cd, and Ni are toxic metals. The plants tend to accumulate the heavy metal in the roots and transfer a few concentrations to the shoots (Wozny 1995; Voutsa et al. 1996). Kadukova et al. (2004) found that the Pb stored in the plant roots was 93–98% from the total Pb absorbed the whole plant. Moreover, Wozny (1995) reported that the plant roots can absorb Pb 3–50 times more than the shoots. This may explain the high levels of Cd and Pb in the roots of zucchini plants. The current study indicated that the transport of heavy metals from the shoots to the edible parts was found to decrease in the order Zn = Cu > Ni > Cd > Pb. Zinc (Zn) and Cu transport within plants faster than Cd and Pb. Cadmium and Pb have low mobility throughout the plant tissues (Voutsa et al. 1996; Kadukova et al. 2004; Eissa 2016; Eissa et al. 2016).
Effect of cow manure biochar on soil-plant transfer of heavy metals
The cow manure biochar decreased the concentration of Zn, Cu, Pb, Cd, and Ni in the tissues of zucchini plant in comparison to the untreated soil. The current study confirmed that the cow manure biochar is an effective tool in reducing the uptake of toxic elements by zucchini plants. Sui et al. (2018) found that the application of 20 t ha−1 of biochar minimized the concentrations of Cd and Pb by 16 and 29% in the grain of wheat (Triticum aestivum L.).The biochar has been widely investigated for its ability to minimize the heavy metals uptake through depressing the metal availabilities by adsorption and pH-driven fixation reactions (Tang et al. 2013; Sui et al. 2018). The data shown in Fig. 1 confirmed that the addition of biochar decreased the bioavailability Zn, Cu, Pb, Cd, and Ni by 13.3, 8.3, 13.8, 9.1, and 3.6%, respectively, compared to the control soil. Equivalent results were obtained by (Tang et al. 2013; Rizwan et al. 2016; Sui et al. 2018). Most of these research papers confirmed that the biochar affected the heavy metal detoxification in the plants at soil level through element immobilization, soil pH modification, and development in physical and biological properties of the soil (Rizwan et al. 2016). Moreover, the biochar application to the soil increases the negative charge and this will cause the attraction between elements cations and soil particles more stable (Tang et al. 2013).
In the present research, the reduction in the heavy metals uptake by zucchini plants may be obtained through three mechanisms, i. e., reducing the soil metal availability, minimizing the root-shoot (TF), and reducing the soil-root transfer (BAF). The cow manure biochar (CMB) minimized the values of BAC for Zn, Cd, and Ni by 5.6, 21.9, and 27.9%, while these reductions in the case of TF were 4.9, 7.5, 23, and 5.9%. The biochar amendment significantly reduced the root-to-shoot and the root-to-leaf transfer of Pb in rice plants by 72.0 and 72.8%, respectively (Li et al. 2016). This reduction in the metals transfer may be due to the precipitation of these elements in the root tissues (Eissa et al. 2014; Li et al. 2016; Eissa and Ahmed 2016). Biochar has different mechanisms in immobilization of heavy metals including ion exchange, precipitation, pore filling, electrostatic interaction, portioning, and surface sorption (Rehman et al. 2016; Abbas et al. 2018; Rwizan et al. 2018).
Conclusion
It is recognized that the metal-contaminated resources should be avoided in human food production. However, under the limited resources, we may be forced to use these lower qualities of soils recourses. The guidelines of the optimum use of these resources must be obtained from field studies. The present research provided a detailed example of the biochar impact on heavy metals uptake under arid conditions. The uptake and translocation of toxic elements by zucchini plant were tested based on 2-year field studies. The cow manure biochar reduced the heavy metals uptake by zucchini plants through three mechanisms, i. e., reducing the soil metal availability, minimizing the root-shoot, and the soil-root transfer. Biochar reduced the bioavailability of Zn, Cu, Pb, Cd, and Ni by 13.3, 8.3, 13.8, 9.1, and 3.6%, respectively, compared to the untreated soil. Biochar minimized the values of BAC for Zn, Cd, and Ni by 5.6, 21.9, and 27.9%, while these reductions in the case of TF were 4.9, 7.5, 23 and 5.9%. It may be concluded that cow manure biochar is recommended for the safety of food production from metal-contaminated soil.
References
Abbas Z, Ali S, Rizwan M, Zaheer IE, Malik A, Riaz MA, Shahid MR, Rehman MZU, Al-Wabel MI (2018) A critical review of mechanisms involved in the adsorption of organic and inorganic contaminants through biochar. Arab J Geosci 11(448). https://doi.org/10.1007/s12517-018-3790-1
Amin AA, Eissa MA (2017) Biochar effects on nitrogen and phosphorus use efficiencies of zucchini plants grown in a calcareous sandy soil. J Soil Sci Plant Nutr 17(4):912–921
Antisari LV, Orsini F, Marchetti L, Vianello G, Gianquinto G (2015) Heavy metal accumulation in vegetables grown in urban gardens. Agron Sustain Dev 35:1139–1147
Arsenic AM, Beryllium AM (1996) Method 3050B acid digestion of sediments, sludges, and soils 10 Scope and Application https://www.epa.gov/homeland-security-research/epa-method-3050b-acid-digestion-sediments-sludges-and-soils. Accessed 26 November 2018
Ball DF (1964) Loss-on-ignition as an estimate of organic matter and organic carbon in non-calcareous soils. J Soil Sci 15:84–92
Buchholz RA (1998) Principles of environmental management. The Greetings of Business Englewood Cliffs: Prentice Hall
Burt R (2004) Soil survey laboratory methods manual. Soil Survey Investigations Report No. 42, Version 4.0, Natural Resources Conservation Service, United States Department of Agriculture https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcseprd1026807.pdf. Accessed 5 Dec 2018
Eissa MA (2014) Performance of river saltbush (Atriplex amnicola) grown on contaminated soils as affected by organic fertilization. World Appl Sci J 30:1877–1881
Eissa MA (2015) Impact of compost on metals phytostabilization potential of two halophytes species. Int J Phytorem 17(7):662–668
Eissa MA (2016) Phosphate and organic amendments for safe production of okra from metal-contaminated soils. Agron J 108(2):540–547. https://doi.org/10.2134/agronj2015.0460
Eissa MA, Ahmed EM (2016) Nitrogen and phosphorus fertilization for some Atriplex plants grown on metal-contaminated soils. Soil Sediment Contam Int J 25(4):431–442
Eissa MA, Ghoneim MF, Elgharably GA, AbdElRazek M (2014) Phytoextraction of nickel, lead and cadmium from metals contaminated soils using different field crops and EDTA. World Appl Sci J 32:1045–1052
Eissa MA, Nafady M, Ragheb H, Attia K (2013) Effect of soil moisture and forms of phosphorus fertilizers on corn production under sandy calcareous soil. World Appl Sci J 26(4):540–547
Eissa MA, Ahmed EM, Reichman S (2016) Production of the forage halophyte Atriplex amnicola in metal-contaminated soils. Soil Use Manag 32:350–356
EU European Union (2002) Heavy metals in wastes. European Commission on Environment http://ec.europa.eu/environment/waste/studies/pdf/heavy metals report.pdf. Accessed 26 November 2018
EU. European Union (2006) Commission regulation EC. No. 1881/2006 of 19 December setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364/5. https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF. Accessed 26 November 2018
Fakayode SO (2005) Impact of industrial effluents on water quality of the receiving Alaro River in Ibadan, Nigeria. Ajeam-Ragee 10:1–13
FAO (1985) Water quality for agriculture. Paper no. 29 (Rev. 1) UNESCO, publication, Rome (Anonymous, Annual Progress Report (2000–03). NATP-MM project on “use of urban and industrial effluents in agriculture”. CSSRI, Karnal-Haryana, India, 2004
Ghosh AK, Bhatt MA, Agrawal HP (2012) Effect of long-term application of treated sewage water on heavy metal accumulation in vegetables grown in northern India. Environ Monit Assess 1842:1025–1036
Gu JF, Zhou H, Yang WT, Peng P, Zhang P, Zeng M, Liao B (2018) Effects of an additive (hydroxyapatite–biochar–zeolite) on the chemical speciation of Cd and As in paddy soils and their accumulation and translocation in rice plants. Environ Sci Pollut Res 25:8608–8619
Gupta SK, Scott C, Mitra A (2011) Advances in land resource management for 21st century. Soil Conservation Society of India, New Delhi, pp 446–446
Kadukova J, Papadontonakis N, Naxakis G,Kalogerakis N (2004) Lead accumulation by the salt-tolerant plant Atriplex halimus. In: Moutzouris C, Christodoulatos C, Dermatas D, Koutsospyros A, Skanavis C, Stamou A (eds) e-Proceedings of the International Conference on Protection and Restoration of the Environment VII, June 28–July 1, Mykonos, Greece
Kanwar JS, Sandha MS (2000) Waste water pollution injury to vegetable crops, a review. Agric Rev 212:133–136
Khan MU, Shahbaz N, Waheed S, Mahmood A, Shinwari ZK, Malik RN (2016) Comparative health risk surveillance of heavy metals via dietary food stuff consumption in different land-use types of Pakistan. Hum Ecol Risk Assess Int J 22:168–186
Lee SH, Lee JS, Choi YJ, Kim JG (2009) In situ stabilization of cadmium, lead, and zinc-contaminated soil using various amendments. Chemosphere 77:1069–1075
Li HH, Liu YT, Chen YH, Wang SL, Wang MK, Xie TH, Wang G (2016) Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci Rep 2016:6. https://doi.org/10.1038/srep31616
Liang B, Lehmann J, Solomon D (2006) Black carbon increases cation exchange capacity in soils. Soil Sci Soc Am J 70:1719–1730
Lindsay WL, Norvell WA (1969) Equilibrium relationship of Zn+2, Fe3+, Ca2+ and H+ with EDTA and DTPA in soils. Soil Sci Soc Am J 33:62–68
Mahmoud E, Abd El-Kader N (2015) Heavy metal immobilization in contaminated soils using phosphogypsum and rice straw compost. Land Degrad Dev 26:819–824
Marchner H (1995) Mineral nutrition of higher plants. Academic Press, London
National Research Council (2005) Mineral tolerance of animals: Second Revised Edition, 2005. The National Academies Press, Washington, DC. https://doi.org/10.17226/11309 vet.unicen.edu.ar/ActividadesCurriculares/.../images/.../minerales%20NRC%202005.pdf Accessed 26 November 2018
Park W, Han KH, Ahn SJ (2012) Differences in root-to-shoot Cd and Zn translocation and by HMA3 and 4 could influence chlorophyll and anthocyanin content in Arabidopsis Ws and Col-0 ecotypes under excess metals. Soil Sci Plant Nutr 58:334–348
Qadir M, Wichelns DL, Raschid-Sally PG, McCornick P, Drechsel AB, Minhas PS (2008) The challenges of wastewater irrigation in developing countries. Agric Water Manag 15:110–117
Rehman MZ, Rizwan M, Ali S, Fatima N, Yousaf B, Naeem A, Sabir M, Ahmad HR, Ok YS (2016) Contrasting effects of biochar, compost and farmmanure on alleviation of nickel toxicity in maize (Zea mays L.) in relation to plant growth, photosynthesis and metal uptake. Ecotoxicol Environ Saf 133:218–225
Rehman MZ, Khalid H, Akmal F, Ali S, Rizwan M, Qayyum MF, Iqbal M, Khalid MU, Azhar M (2017) Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field. Environ Pollut 227:560–568
Rizwan M, Ali S, Qayyum MF, Ibrahim M, Rehman MZ, Abbas T, Ok YS (2016) Mechanisms of biochar-mediated alleviation of toxicity of trace elements in plants: a critical review. Environ Sci Pollut Res 23:2230–2248
Rwizan MJ, Oh S, Kim K, Kim SD (2018) Comparative sorption isotherms and removal studies for Pb(II) by physical and thermochemical modification of low-cost agro-wastes from Tanzania. Chemosphere 195:135–145
Seaward MRD, Richardson DHS (1990) Atmospheric sources of metal pollution and effects on vegetation. In: Shaw AJ (ed) Heavy metal tolerance in plants: evolutionary aspects. CRC Press, Boca Raton, pp 75–92
Sharma OP (2010) Plant taxonomy. Tata McGraw-Hill Education Pvt. Ltd. New York City, United States
Sombroek WG, Nachtergaele FO, Hebel A (1993) Amounts, dynamics and sequestering of carbon in tropical and subtropical soils. Ambio 22:417–426
Sui F, Zuo J, Chen D, Li L, Pan G (2018) Biochar effects on uptake of cadmium and lead by wheat in relation to annual precipitation: a 3-year field study. Environ Sci Pollut Res 25:3368–3377
Tang J, Zhu W, Kookana R, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116(6):653–659
Tartoura EA, El-Gamily EI, El-Waraky YB, Kamel M (2014) Effect of phosphorus fertilization and fruit thinning on seed production of summer squash plants. J Plant Prod Mansoura Univ 5:1807–1816
USEPA United State Environmental Protection Agency (1997) Exposure factors handbook. Volume II-Food Ingestion Factors. EPA/600//P-95/002Fa. Office of Research and Development, Washington, DC https://rais.ornl.gov/documents/EFH_Final_1997_EPA600P95002Fa.pdf. Accessed 26 November 2018
Voutsa D, Grimanis A, Samara C (1996) Trace elements in vegetables grown in an industrial area in relation to soil and air particulate matter. J Environ Pollut 9434:325–335
Watanabe MA (1997) Phytoremediation on the brink of commercialization. Environ Sci Technol 31:182–186
WHO/FAO (2007) Joint FAO/WHO food standard programme codex slimentarius commission 13th session. Report of the Thirty Eight Session of the .ene. Houston, United States of America, ALINORM 07/30/13. www.fao.org/input/download/report/686/al31_13e.pdf. Accessed 26 November 2018
Wolnik KA, Fricke FL, Capar SG, Braude GL, Meyer MW, Satzger RD, Bonnin E (1983) Elements in major raw agricultural crops in the United States. 1. Cadmium and lead in lettuce, peanuts, potatoes, soybeans, sweet corn and wheat. J Agric Food Chem 31:1240–244
Wozny A (1995) Lead in plant cells. Sorus, Poznan, Poland
Ximénez-Embún P, Rodríguez-Sanz B, Madrid-Albarrán Y, Cámara C (2002) Uptake of heavy metals by lupin plants in artificially contaminated sand: preliminary results. Int J Environ Anal Chem 82:805–813
Yang WT, Zhou H, Gu JF, Liao BH, Peng PQ, Zeng QR (2017) Effects of a combined amendment on Pb, Cd, and as availability and accumulation in rice planted in contaminated paddy soil. Soil Sediment Contam Int J 26:70–83
Zhou H, Zhou X, Zeng M, Liao BH, Liu L, Yang WT, We YM, Qiu QY, Wang YJ (2014) Effects of combined amendments on heavy metal accumulation in rice (Oryza sativa L.) planted on contaminated paddy soil. Ecotoxicol Environ Saf 101:226–232
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Implications of Biochar Application to Soil Environment under Arid Conditions
Rights and permissions
About this article
Cite this article
Eissa, M.A. Effect of cow manure biochar on heavy metals uptake and translocation by zucchini (Cucurbita pepo L). Arab J Geosci 12, 48 (2019). https://doi.org/10.1007/s12517-018-4191-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-4191-1