Abstract
The hydrogeochemistry of groundwater and environmental aspects of the Tanjero area (Sulaimani City, Kurdistan region, Iraq) were investigated statistically. Correlation analysis and cluster analysis revealed several indicators for the source of contaminations. The hydrochemical classification of the water samples determined Ca2+, Na+, and Mg2+ as dominant ions and K/Rb and Na/Cl ratios indicated water–rock interactions with several minerals (e.g., silicate and carbonate minerals). Sr, Ca, Mg, Rb, and K (1757, 117, 29.8, 7.23, and 10.1 μg/L, respectively) in the water samples correlate with each other and show higher concentrations in the wells around scrape and dump sites than the other wells. The water samples were classified according to a redox classification as well, and aerobic and intermediate anaerobic categories were recognized with regard to the reduction of dissolved oxygen and Mn (VI) ions with organic matter in the groundwater. Mn exceeds drinking water standards.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Background
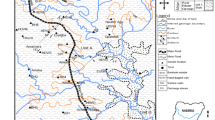
In the last decade, the industrial zone in the Tanjero area in Kurdistan (Northern Iraq) has been characterized by an expanding number of processing plants for industrial production. In addition, the increasing population in Sulaimani City has prompted the appearance of huge amounts of waste, summing up to 1000 tons per day (Rashid 2010). The location of the waste dump site and industrial activities is toward the southwest of Sulaimani City, 12 km from the downtown area (Fig. 1). Contamination sources such as waste, industrial deposits, uncased pit latrines, and fuel tanks are spread arbitrarily. Neither a concept for environmental safety nor a monitoring system is in place. The industrial remains are dumped uncontrolled in an enclosed unregulated landfill site, around 50 ha in area. Surface and groundwater in the region of interest (ROI) have been contaminated by different chemical components, causing changes in the quality of the pumped water and suitability for human utilization.
Climate
The climate of the ROI is characterized by rather cold (3 to − 7 °C) winters and long, hot dry summers, while fall and spring are short. In the mid-year, average daily temperatures can reach 45 °C. The rainy season (more than 125 mm per month of precipitation in the rainy season) for the most part begins in mid-October and finishes toward the start of May. The month with the highest amount of precipitation in the region is January (Stevanovic and Markovic 2003). The elevation of Sulaimani City ranges from 730 to 950 m above sea level (asl).
Geology
The ROI is composed of more than 140 m clastic rocks (mostly Tanjero Formation). The detailed geology is described by Budy et al. (1980). It comprises marl, marly limestone, claystone, siltstone, sandstone, occasionally conglomerate, clastic alluvium, sand, and silt.
The groundwater flow is directed from northwest to southeast of the ROI. The recharge area lies between Gowaizha mountain in the east and northeast of the ROI and the Sherkuzh anticline with the Baranan mountain in the southeast of the ROI (Rashid 2010) (Figs. 1 and 2). The groundwater samples were collected from wells belonging to two aquifers, Tanjero and Quaternary aquifers. The shallow Quaternary aquifer (0–25 m depth) represents one of the dominant aquifers in the ROI. The aquifer is composed of unconsolidated gravel and sand that yield a good quantity of water (Mustafa and Merkel 2015). The Tanjero aquifer represents a medium to deep aquifer (25–120 m) composed of alternating marly limestone, sandstone, marl, and occasionally conglomerate beds. The aquifer is highly deformed and fractured, which allows the formation of secondary porosity (Mustafa 2007). Regarding the Tanjero aquifer, it yields a good quantity of water in the sandstone and marly limestone facies. The majority of the wells in the ROI are drilled within the Tanjero aquifer (Mustafa and Merkel 2015).
Geological cross-section of ROI (modified after Stevanovic and Markovic 2003)
Tanjero is a perennial river formed by linking the Qiliasan and Kaniban streams at an altitude of 810 m asl flowing along the southern edge of Sulaimani City and discharging into the Darbadikhan Lake. This small river is contaminated from sewage effluents of Sulaimani City (NI 2008).
Redox framework background
Redox processes impact the water quality in the aquifers. Redox processes can influence the mobility status of the trace elements with toxic metals that are available naturally in aquifer materials and also on organic and inorganic constituents. These processes participate in the degradation or preservation of anthropogenic contaminants in the aquifers. The particulate organic carbon in groundwater systems acts efficiently as electron donors to the electron acceptors (McMahon and Chapelle 2008). The electron donor–acceptor reactions are represented in Fig. 3 (Sracek and Zeman 2004).
Significance of the work
There are some high concentrations of major and trace elements in the waters of the ROI. These could be an indicator for several sources of contamination and thus hazards for human health, animal health, and the environment of the ROI. Therefore, this study tries to address the concentration of these contaminants, their sources, and classify the available water resources in the ROI according to the presented results.
Materials and methods
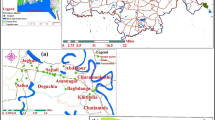
Fifteen water samples from groundwater and surface water of the ROI were gathered (Fig. 1). Twelve samples were collected from water wells in the industrial zone at different distances from the waste dump site. Samples 13 (southeast of the waste dump site and plants) and 14 (northwest of the waste dump site and refinery processing plants) were taken from the Tanjero river. A15 was collected from Sarchinar spring, 10 km upstream from the landfill site. Field parameters (pH, electrical conductivity, redox potential (Eh), dissolved oxygen (DO), and water temperature) were measured on-site using versatile devices (WTW: pH320, LF320, Multiline P4, and HACH: HQ40D). For major cation and anion analysis, a 100-mL water sample was collected in pre-cleaned polyethylene bottles. A 30 mL water sample was collected with a syringe and filtered with 0.2-μm film filters. Then, ultra-pure HNO3 (30%) acid was added to these samples for ICP–MS determination of trace elements. The analysis of significant cations, anions, and trace elements was performed in the laboratories of the Hydrogeology Department, Technische Universität Bergakademie Freiberg, Germany. Li, P, Si, Al, Mn, Fe, Ni, Zn, As, Se, Sr, Ba, Cr, V, Pb, and Rb as trace elements were analyzed by ICP–MS X-Series-2 (Thermo Scientific) either in direct mode or utilizing the collision mode. Cations (Ca2+, Mg2+, Na+, and K+) and anions (SO42−, NO3−, NO2−, PO43−, Cl−, and F−) were measured by ion chromatography (Metrohm: 850 Professional IC Metrohm with Metrosep C4–150 section and 2 mM dipicolinic corrosive eluent and Compact IC Pro 881 and Metrosep A sup 15–150 segment were utilized with 3 mM NaHCO3 and 3.5 mM Na2CO3 as the eluent). The total reproducibility of the IC and ICP–MS determinations was around 2 and 10%, respectively. Total inorganic carbon was analyzed by LiquiTOC (Elementar Analysen-Systeme GmbH).
The redox framework of McMahon and Chapelle (2008) was used for redox classification of the water samples by aerobic and anaerobic categories. The framework is based on the dissolved concentrations of five water-quality parameters (O2, NO3−, Mn2+, Fe2+, and SO42−). This framework has been discussed in detail by McMahon and Chapelle (2008). The statistical tests of the data were performed using SPSS version 11.0.0.
Results and discussion
Hydrochemistry of the water samples
Major ions
Depending on the results of the analyses that are shown in Table 1 and the dominance of major cations and anions, various hydrogeochemical types were recognized and are highlighted in Table 2. In some samples, Ca2+ is dominant, and in others, Na+ is dominant, and in the remaining samples, a mix of Ca2+, Na+, and Mg2+ is dominant.
The water samples are classified depending on the domination of Na+, Ca2+, and a mix of Ca2+, Na+, and Mg2+. This classification is a guide to show the groundwater quality with regard to using this groundwater for human consumption (Vasanthavigar et al. 2008). Na-water facies are mostly fresh waters (A1, A2, A6, A8, A11, and A12) but characterized by higher Na+ concentrations (135–222 mg/L) compared with the other samples (Table 1).
Ca2+ and Mg2+ in the water samples are impacted mainly from the sedimentary rocks of the aquifer in the ROI (Ali 2007) (Table 3).
Ratio of major and trace elements
Linear regression tests between major and trace elements were performed to evaluate the geochemical processes in the studied water samples (Table 2; Fig. 4). The K/Rb ratio shows higher values in A4, A7, A9, and A10 water samples than in the other samples. The high K/Rb ratios are typical for identification of areas where the overburden is enriched in calcite (Peltola et al. 2008).
A3, A4, A5, A7, and A9 water samples demonstrate high values of Sr, Ca, and Mg compared with the other water samples (Tables 1 and 4). These were water samples collected from wells near the municipal waste dump site (A3 and A4), near the scrape dump site (A5), or from wells from both sides of the Tanjero river (A7 and A9) (Table 2; Fig. 1). In Fig. 4c, Rb and K have higher values in A13 and A14 (4.4–7.23 μg/L and 7.8–10.1 mg/L for Rb+ and K+, respectively) compared with the other water samples. A13 and A14 are water samples from the Tanjero river, while the other samples are collected from wells and the spring.
Na domination and Na/Cl ratio
The Na/Cl ratio is higher in A1, A2, A6, A8, A10, A11, and A12 than in the other samples. The higher Na/Cl ratio shows that cation exchange occurred on the clay minerals in the groundwater aquifer (Edmunds et al. 2008). High Na/Cl ratio is due to silicate weathering as a source of Na+ (Meybeck 1987). Dissolution of Na-bearing minerals (e.g., albite) is possible, but the presence of minerals was not confirmed by the mineralogy of the aquifers. The high concentration of Na+ in A1, A2, A6, A8, A10, A11, and A12 indicates ion exchange in the aquifer, as concluded similarly by Raju et al. (2015) in New Delhi water. These samples were collected from wells inside houses, warehouses, or small factories. Uncased pit latrines or poorly constructed septic tanks exist in these locations as well. Anthropogenic sources for Na+ are possible as well. Sources of Na+ are suspicious and are not well identified in the groundwater of the area (Mustafa and Merkel 2015). The 1:1 Na/Cl relation is not correlated (R2 = 0.05), suggesting different Na+ sources. Increasing concentration of Na+ could be from sewage contamination (Mustafa 2007) or Sulaimani City waste dump.
The Na+ average values of the winter and summer samples were plotted in a Gibbs classification diagram (Fig. 5) (Gibbs 1970). The classification depends on the Na/Cl ratio (Table 2). According to Gibbs’ classification, the main sources of geochemical components in samples A3, A4, A5, A7, A9, A13, A14, and A15 is rock weathering (Figs. 5 and 6).
The potential sources of the Na+ in the ROI could be varied between the silicate weathered rocks and leaching of sewages and uncased pit latrines that distributed widely in the ROI.
Redox status framework
Trace element contamination and redox status are two bases for evaluation of trace element mobility and contamination processes. In this context, the redox framework of McMahon and Chapelle (2008) was used on the dissolved concentrations of O2, NO3−, Mn2+, Fe2+, and SO42−, and it was also used to classify the general categories of the water samples of the ROI.
Table 5 shows two dominant categories in the study area, which are the oxic and intermediate. The oxic category represents the aerobic conditions where dissolved oxygen acts as an electron acceptor. The intermediate water samples such as A4, A7, A9, A10, A13, and A14 represent moderate reduction where dissolved oxygen still exists but as well there is a considerable concentration of Mn (VI) (DeLaune and Reddy 2005). Most of the contaminated samples are listed in the oxic class and the others in the intermediate redox class. Intermediate redox condition (lower oxygen content) in the groundwater system of the ROI favors higher trace element contamination load. This may reflect the relation between the redox status and the contamination condition and can be used as a tracer.
A13 and A14 are water samples collected from the Tanjero river at locations where the sewage of Sulaimani City enters the water course with a high load of organic matter (Rashid 2010). A7, A9, and A10 are close to the banks of the Tanjero river. A4 is one of the wells close to the main dump site of the Tanjero industrial area and the dump site drainage, which contains significant BTEX concentrations compared with the other water samples (Kareem and Merkel 2015).
The dissolved organic carbon (DOC) analysis of the water samples in the ROI shows significantly elevated concentrations in water samples A4, A7, A9, A10, A13, and A14 compared with the other water samples (Table 6).
The occurrence of organic matter and nutrients combined with oxygen depletion constitutes an anaerobic environment (Yakushev and Newton 2012).
Oxygen and inorganic mineral ions could be reduced in these water samples because they could act as electron acceptors with organic matter such as human/animal waste and volatile organic carbon. After oxygen and nitrate ions are consumed by microorganisms, then Mn is normally used in the redox reaction chain (Stanton and Qi 1997).
The water samples A4, A7, A9, A10, A13, and A14 are considered as impacted by human activities, and these water samples are contaminated by BTEX (Kareem and Merkel 2015) that could be a reason of releasing the electrons in the redox reactions and availability of semi-reduction environment in these water samples. The summation of the trace element concentration in the human impact water samples in the ROI did not demonstrate a significant relation with redox oxic and anoxic categories comparing to the rest of water samples (Table 4).
Conclusions
Three groundwater classes were recognized in the ROI: Ca2+ dominated, Na+ dominated, and mixed type. The K/Rb ratio indicates enrichment in calcite and the Na/Cl ratio reflects the clay mineral enrichment in the aquifer. The sources of high Na+ concentration are geogenic and anthropogenic as well. Two redox categories are recognized in the water samples of the ROI. One category represents the aerobic environment with rather high O2 values and lack of organic matter in the groundwater. The other category is intermediate with lower O2 values and elevated Mn (IV) concentrations, which represent semi-reduced redox conditions due to organic matter and volatile organic carbons in the groundwater that originate from sewage wastes in the Tanjero river and BTEX leachate from the waste dump site into the groundwater. The Mn concentrations in the water samples that are close to the waste dump site or the Tanjero river make the water unsuitable for human consumption. Because untreated waste water is spilled into the Tanjero river and dumping of solid and liquid waste in the ROI happens without any technical measures, the contamination of the downstream groundwater contamination is rather moderate. Thus, it can be concluded that river water is not infiltrating to a great extent and that the groundwater recharge is very low. However, it cannot be excluded that contaminants are still in the unsaturated zone of the dump site and may enter groundwater in the near future, depending on the hydraulic properties of the aquifers and release of organic and inorganic contaminant flux to the unsaturated zone. The hydrological conditions need to be better addressed, especially the mechanisms of how, when, and where the river is in gaining or losing conditions to evaluate the contamination patterns. Regarding contamination risks, it can be concluded that water of the area is not save for domestic uses. Groundwater vulnerability and water resource managements should be more highlighted in the area. The inhabitants in the ROI need more sanitary projects with periodic monitoring and determine the main standards to build pit latrines in this area.
References
Ali SS (2007) Geology and hydrogeology of Sharzoor-Piramagroon basin-Sulaimani. Unpublished Ph.D. thesis. Belgrade University
Budy T, Jassim SZ, Kassab II (1980) Regional geology of Iraq 1:445
DeLaune RD, Reddy KR (2005) REDOX POTENTIAL. In: Redox potential. Elsevier
Edmunds WM, Shand P, Hart P, Ward RS (2008) The natural (baseline) quality of groundwater. UK pilot study, Malden, MA, Oxford, Blackwell 310
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 170:1088–1090
Kareem A, Merkel B (2015) The influence of volatile organic components on the stable isotopic composition of the groundwater in the Tanjero area—Kurdistan region. Iraq Freiberg Online Geoscience 43
Kareem A, Merkel B, Mustafa O (2014) Investigations of uranium and trace elements in groundwater of the Tanjero area, Kurdistan region, Iraq. Springer International Publishing, Cham, pp 811–819
McMahon PB, Chapelle FH (2008) Redox processes and water quality of selected principal aquifer systems. Ground Water 46:259–271
Meybeck M (1987) Global chemical weathering of surficial rocks estimated from river dissolved loads. Am J Sci 287(5):401–428
Mustafa O (2007) Impact of sewage wastewater on the environment of Tanjero River and its basin within Sulaimani City, NE Iraq. Unpublished M.Sc. Thesis, University of Baghdad
Mustafa O, Merkel B (2015) Classification of karst springs based on discharge and water chemistry in Makookkarst system, Kurdistan region, Iraq. Freiberg Online Geoscience 39:1–24
NI (2008) Survey of water quality, sediment, benthic macro invertebrate and fisheries for Qara Ali dam irrigation project (QDIP). Report. Environmental impact assessment, Nature Iraq field and lab
Peltola P, Brun C, Astroem M, Tomilina O (2008) High K/Rb ratios in stream waters—exploring plant litter decay, ground water and lithology as potential controlling mechanisms. Chem Geol 257:92–100. https://doi.org/10.1016/j.chemgeo.2008.08.009
Raju NJ, Chaudhary A, Nazneen S, Singh S, Goyal A (2015) Hydro-geochemical investigation and quality assessment of groundwater for drinking and agricultural use in Jawaharlal Nehru University (JNU), New Delhi, India. In: Raju N, Gossel W, Sudhakar M (eds) Management of Natural Resources in a changing environment. Springer, Cham
Rashid K (2010) Environmental implication of Tanjero waste disposal site of Sulaimani. Unpublished Ph.D, Sulaimani University, Sulaimani
Sracek O, Zeman J (2004) Introduction to environmental hydrogeochemistry. Masaryk University, Bern
Stanton J S, Qi L S (1997) Ground-water quality of the northern high plains aquifer. National Water-Quality Assessment Program, USGS
Stevanovic Z, Markovic M (2003) Hydrogeology of northern Iraq. Climate, Hydrology, Geomorphology, Geology. Ed. “Field documents” Vol. 1
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Rajiv Ganthi R, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2008) Application of water quality index for groundwater quality assessment: Thirumanimuttar sub-basin, Tamilnadu, India. Environ Monit Assess 171:595–609. https://doi.org/10.1007/s10661-009-1302-1
Yakushev E V, Newton A (2012) The handbook of environmental chemistry 22. Chemical structure of pelagic redox interfaces, observation. Vol. 1
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kareem, A., Mustafa, O. & Merkel, B. Geochemical and environmental investigation of the water resources of the Tanjero area, Kurdistan region, Iraq. Arab J Geosci 11, 461 (2018). https://doi.org/10.1007/s12517-018-3825-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-3825-7