Abstract
Fifty groundwater samples were collected from Al-Hasa to analyze the pH, electrical conductivity (EC, dS m−1), total dissolved solids (TDS), major anions (HCO3−, CO32−, Cl−, SO42−, and NO3−), major cations (Ca2+, Mg2+, Na+, and K+), and total hardness. The analyzed data plotted in the Piper, Gibbs, and Durov diagrams, and water quality index (WQI) were calculated to evaluate the groundwater geochemistry and its water quality. The results reveal that most of the investigated samples are Ca2+, Mg2+, SO42−, Cl− and Na+, and HCO3− water types using the Piper diagram. Na+ > Ca2+ > Mg2+ are the dominant cations, while Cl− > HCO3− > SO42− > CO32− are the dominant anions. Sodium adsorption ratio (SAR) values varied from 0.79 to 10; however, the Kelly ratio (KR) ranged between 0.1 and 2.2. The permeability index (PI) showed that well water is suitable for irrigation purposes with 75% or more of maximum permeability. The US salinity diagram revealed that the water quality classes of studied waters were CIII-SI, CIII-SII, and CIV-SII, representing height hazards of salinity and medium- to low-sodium hazard. The water quality index (WQI) results indicated that total dissolved solids are out of the drinking water standard limits in Saudi Arabia. The WQI revealed that 38% of the studied wells were considered as poor water (class III), 52% are found as very poor water class (IV), and 10% are unsuitable water for drinking class (V).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In Saudi Arabia, in general, and Al-Hasa oasis, in particular, the groundwater is mainly for drinking and irrigation. In the last two decades, exploitation of groundwater and other anthropogenic activities led to serious deterioration of groundwater and shrinking of the agricultural land (Aly et al. 2016). Water quality evaluation is vital for sustainability of water resources based on its physical, chemical, and hydro-geochemical properties (El-Sayed et al. 2012; Ledesma-Ruiz et al. 2014; Tiwari et al. 2015). The variation in groundwater hydro-geochemical process should be considered in the assessment of water quality, as the groundwater layer lithology may be affected by high concentration of some parameters (Kumar et al. 2006, 2009, 2016). In recent studies, hydro-geochemical reports provide more information in the consistency of hydro-geochemical processes influencing the quality and composition of groundwater (Aly et al. 2016; Tiwari and Singh 2014; Singh et al. 2013).
The water quality index (WQI) is a function to assess water quality and to help policymakers in reassuring the public on the quality of their water (Lateef 2011). The aim of the water quality index is to provide a simplified approach for assessment of drinking water quality. WQI defined the quality of water by merging all parameters measured or evaluated into a single number (Stigter et al. 2006; Saeedi et al. 2010). Numerous studies were carried out using WQI with different methods of calculation of the index and the weight values for each parameter (Horton 1965; Zagatto et al. 1998; Tiwari et al. 2014). Backman et al. (1998) used the WQI to identify the groundwater used for drinking purposes in central west Iran, and their results proved that the water is of good quality. Rokbani et al. (2011) used the WQI to assess the groundwater in EL-Khairat in Tunisia. The WQI was used to evaluate the water in Riyadh, Saudi Arabia (Al-Othman 2015). Al-Othman reported that WQI ranged from 34 to 513 with an average of 282, thereby indicating mild pollution at some sites. In India, WQI and hydro-chemical modeling were used to evaluate the groundwater quality in Palakkad District Kerala for drinking and irrigation purposes (Kumar et al. 2016). Furthermore, Kumar et al. (2015) made an estimate of specific differences of groundwater quality by the water quality index (WQI) in Tamil Nadu, India. According to them, the WQI results indicated that the majority of the samples are falling under excellent to good category and suitable for drinking water.
According to the Gibbs diagram, the predominant samples fall in the rock–water interaction dominance and evaporation dominance field. Moreover, the piper trilinear diagram shows that water samples are Na–Cl and mixed CaMg/Cl types. In Saudi Arabia, the groundwater in El-Saq aquifer is mostly identified as good quality water with rates of TDS 300–1000 mg L−1 (MAW 1984). The Poor quality water is due to the presence of basalt rock-covered areas, near the edge of the basement complex and valley outlets (Sharaf and Hussein 1997). El-Saq groundwater is categorized into six water types, and the most prevailing types are Ca(HCO3)2 or NaHCO3 and NaCl. The Na2SO4 type resides only in the basalt and paleo valley areas. Sharaf and Hussein (1997) explained that the Cl− and SO4− are the dominant anions; however, Ca2+ and Na+ are the dominant cations in the El-Saq aquifer. Sharaf and Hussein (1997), Abdel-Aal et al. (1997), and Aly et al. (2016) explained that the Saudi Arabia groundwater degradation in disquieting way is due to the increase of water salinity, as reported by numerous extensive groundwater research over the last few years. Furthermore, Al-Salamah et al. (2011) explained that the depletion of groundwater was clear in Saudi Arabia, and if this attrition continues insistently, the agricultural land may not be able to survive. It is a documented fact that the current excessive usage and drilling of groundwater in Saudi Arabia lead to extreme depletion of water resources, and there is a need to speed decision on some measure to reduce it. Accordingly, numerous measurements are already in place particularly about agricultural division. Thus, in the present work, groundwater of Al-Hasa oasis in the eastern part of Saudi Arabia was investigated for their suitability for drinking and irrigation purposes by comparing them with the standards (WHO 2011; Ayers and Westcot 1985). In addition, the WQI was also calculated to assess the suitability of water for drinking.
Materials and methods
Study area
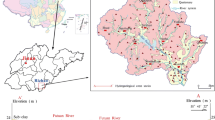
The Al-Hasa oasis is located in the eastern part of Saudi Arabia between 25° 29′ 58.8″ N–49° 43′ 29.2″ E and 25° 24′ 19.0″ E–49° 42′ 55.5″ N. The climate of the oasis is classified as hot and dry, desert climate. The average rainfall is 46 mm/year in the study area. The Al-Hasa climate is considered to be BSk (cold semi-arid climate) according to the Köppen-Geiger climate classification (Table 1).
The Al-Hasa oasis is the largest in the Kingdom of Saudi Arabia and one of the largest spring-fed oases in the world. On an area of about 12,000 ha, mostly date palms were cultivated in former times. The total number of date palm trees in the oasis exceeds 3 million (General Authority for Statistics 2015). The enormous size of the farming area was made possible by the immense volume of groundwater discharging from the underlying aquifers under natural artesian pressure. All urban areas are located in the traditional oasis. In addition, the oasis includes part of the giant Empty Quarter desert. The oasis population is over 1,200,000 (General Authority for Statistics 2015).
Geology
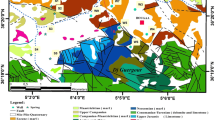
Quaternary deposits cover most of the surface in Al-Hasa. These deposits consist of Eolian sand, Sabkhas, playas, and fluvial deposits. The underlying strata are composed of cemented calcareous materials, soft gray green marl, and red beds. The red beds and the cemented calcareous materials are likely upper member of the Miocene and Pliocene Hofuf formation, while the gray green marl is almost belonging to the Miocene Dam formation. The land of the Al-Hasa oasis rises about 150 m from the sea and tends to the east side of the Gulf through the area of Kenzan (Al-Sayari and Zötl 2012). Today, the region is characterized by dry arid desert environment and Sabkha land. The Wadi region is geographically located between the coastal plain on the Arabian Gulf and the Al Saman plateau in the west. It is known for its abundance of water resources and trenches, which evaporates in the summer, leaving a saline layer on the surface. After a rain, the water remains above the surface to saturate the Sabkha (Matter et al. 2016; Chapman 1974).
Hydrogeology
The Al-Hasa region consists of several springs of natural water gathered to be a single-water stream like the river pours in the Arabian Gulf. This river was dried up but returned somewhat after the project of irrigation and drainage in the area of Al-Hasa. The springs in Al-Hasa belong to the huge Euphrates–Gulf–Rub Al Khali Basin. The Late Cretaceous and Tertiary formations of Aruma, Umm Er Radhuma, Rus, Dammam, and Neogene form a complex multi-aquifer system (Al-Sayari and Zötl 2012; MOWE 2007). The aquifers are partly interconnected. The Umm Er Radhuma aquifer and the Dammam aquifer are separated by the Rus formation that consists of evaporates, marls, and limestone. The groundwater system in the study area consists of four partly interconnected aquifers. These are as follows: (a) Neogene aquifer complex at the top—a mixture of karstified fractured bedrock aquifers and unconsolidated porous clastic aquifers; (b) Dammam aquifer complex—a partly karstified fractured bedrock aquifer; (c) Umm Er Radhuma aquifer—a karstified fractured bedrock aquifer; and (d) Aruma aquifer at the base—a karstified fractured bedrock aquifer of minor importance (Al Tokhais and Rausch 2008).
Chemical analysis
The groundwater samples were collected from 50 different locations that cover the Al-Hasa region during March 2016. In attempting to evaluate spatial variability in the water resource quality of the studied areas, samples were collected from different areas as shown in Fig. 1. The samples were stored in icebox and transferred immediately to King Saud University’s labs for analysis. The well samples were collected and analyzed for electrical conductivity, pH, cations, anions, and B. The EC was determined using EC-meter (dS m−1 at 25 °C) (Test kit Model 1500_20 Cole and Parmer). The pH was measured using a pH-meter (pH meter—CG 817). The soluble cations (Ca2+ and Mg2+) were determined by ethylene diamine tetra acetic acid (EDTA) titration method. The soluble Na+ and K+ were determined using flame photometer apparatus (Corning 400). The CO3− and HCO3− anions were determined by sulfuric acid (H2SO4) titration method, whereas the Cl− was determined by titration with silver nitrate (AgNO3) (APHA 1998). The sulfate (SO42−) was determined in suspended solution using the turbidity meter, and the nitrate (NO3−) was determined by the phenoldisulfonic acid method (APHA 1998). The B was determined using the azomethine-H method (Golterman 1969).
Accuracy of collected data
The accuracy of the collected results was verified by calculating the errors of ion balance; then, the standard solutions as blanks are usually run to check for possible errors in the analysis steps. The percentage of error in the data was calculated using the following equation (Appelo and Postma 1996):
An error of up to ± 2 percentage is acceptable, while any sample had error difference than this band should be taken for repeating the analysis again. About 98% of the samples were inside this range. This means that the resultant data quality is sufficient enough for calculation of WQI and determining type of water to draw a conclusion about water quality.
Calculation of water quality index
The calculation of WQI for estimating effect of physical and anthropogenic actions was based on numerous key elements in the chemistry of groundwater in the collected data. To estimate the WQI, weight has been assigned for each of the physico-chemical elements according to the elements based on the relative importance in the overall quality of water for drinking purposes. The weight value ranges between one and five. The maximum weight of 5 has been assigned for NO3− as the important element in this region; 4 for TDS, pH, EC, and SO42−; 3 for HCO3− and Cl−; and 1 for Ca2+, Na+, K+, and Mg2+ (Ramakrishnalah et al. 2009; Lateef 2011; Al-Hadithi 2012; Al-Omran et al. 2013). The relative weight was calculated by using the following equations:
where
- Wi :
-
the relative weight;
- wi :
-
the weight of each parameter;
- n :
-
the number of parameters.
The next step is the calculation of quality classification. The quality rating scale (qi) for each element is calculated by dividing the element concentration in each water sample by its respective standard (WHO 2011) (Table 2) multiplied by 100:
where
- qi :
-
the quality rating;
- Ci :
-
concentration of each chemical parameter in each sample (mg L−1);
- Si :
-
standard for each chemical parameter (mg L−1) according to the guidelines of the WHO (2011).
To calculate the final phase of the WQI, SI is determined first for each parameter. Total SI values give the water quality index for each sample.
where
- SIi :
-
the sub-index of ith parameter.
- qi :
-
the rating based on concentration of ith parameter;
- n :
-
the number of parameters.
Table 3 shows the limitations of WQI, which is, categorized by water types into five classes (Excellent water, Good water, Poor water, Very poor water, Water unsuitable for drinking).
Hydro-chemical characterization
The groundwater sample hydro-chemical categorization was estimated by using the major cations and anions. The obtained data are aggregated on the Piper and Durov diagrams using the Geochemistry Software AquaChem 2014.2 (AquaChem 2014) to classify the types of water. The United States salinity laboratory (Richards 1954) and Gibbs (Gibbs 1970) diagrams were also used in this research. Moreover, salinity hazard, sodium adsorption ratio (SAR), sodium percent (Na %), Kelly’s ratio (KR), and permeability index (PI) were estimated to investigate the suitability of groundwater for irrigation purposes.
Results and discussion
Evaluation of water quality for irrigation purposes
The major chemical analysis and calculation parameter records of groundwater samples were analyzed statistically, and the results of the minimum and maximum were tabulated in Table 4. The discussion was based on the following basic criteria: (a) cations and concentrations of Na+, Ca2+, and Mg2+ ions ranged between (11 up to 90), (0 up to 41.1), and (5.8 up to 23) meq L−1, consecutively (Table 4). The limit of these ions for irrigation water as suggested by Duncan et al. (2000) and Sharifi and Safari Sinegani (2012) is 4.0, 2.98, 7.0, and 0.8 meq L−1. On these permissible limits, only 2.0, 2.0, and 0.0% of the water samples are considered suitable for irrigation usage with respect to Ca2+, Mg2+, and Na+. The order of ionic concentrations for the cations is Na+ > K+ > Mg2+ > Ca2+. (b) The concentrations of anions, which are CO3, HCO3−, Cl−, NO3−, and SO4, lie between (0.02–1.51), (3.39–42.2), (4.8–23), (0.2–20), and (3.0–48.0) meq L−1, respectively, with a mean value of 0.51, 17.32, 9.00, 10.1, and 11.5 meq L−1 (Table 4). The maximum allowable (McKee and Wolf 1963; Ayers and Westcot 1985; Duncan et al. 2000; Sharifi and Safari Sinegani 2012) values of CO3−, HCO3−, Cl−, NO3−, and SO42− in irrigation water are 0.1, 5.5, 7.0, 17.0, and 10.0 meq L−1, respectively. Water is considered suitable for use in irrigation with respect to HCO3−, Cl−, and SO42−, consecutively. The order of the concentration of ions is Cl− > SO42− > HCO3−. The pH values of the water samples ranged between 7.15 and 8.06. The limit of maximum pH value of irrigation water ranged between 6.5 and 8.4 (Ayers and Westcot 1985). This means that 99.5% of the studied water samples were within safe limit with respect to pH since only one sample has pH = 8.6 (Ayers and Westcot 1985). The risk of salinity was measured by electrical conductivity (EC, dS m−1), which is a measure of the ability of water to transport electric current. It characterizes the quantity of soluble salts (TDS). Therefore, in this study, the risk of salinity was evaluated by the electrical conductivity which differs in 1.98–11 dS m−1, which is beyond classification of the FAO, as proposed by Ayers and Westcot (1985) for the salinity hazard. The results indicate that 65% of the studied wells are classified as moderate water salinity and the remaining wells classified as severe water salinity. These findings are in covenant with Aly et al. (2016) who indicated the high salinity of well water of Riyadh region in the central of Saudi Arabia. One of the most important criteria in determining the risk of sodium hazard is sodium adsorption ratio (SAR) (Todd and Mays 2005). The excessive sodium content in water sample reduces the soil infiltration and available water to plants. The excessive sodium content can also cause sodium toxicity in sensitive plants, causing marginal leaf burn and defoliation. The abundance of sodium in water may immobilize other nutrient ions, particularly Ca, Mg, and K (Sharifi and Safari Sinegani 2012). The SAR is calculated as follows:
Table 4presents the SAR data of the groundwater samples. The results have ranged from 0.79 to 10 with the mean of 3.86. All values were lower than 11. It can be classified as excellent water for irrigation (Richards 1954). There is another criterion that can be used to categorize water, and this criterion is Kelly’s ratio, which is calculated by measuring the concentration of Na+ against Ca2+ and Mg2+ using the following formula:
The value of KR is above one, and it gives a clear sign that the water is polluted with alkali hazards; thus, it is unusable for agricultural purposes (Karanth 1987). The Kelly’s ratio of the studied samples ranged from 0.1 to 2.2 with a mean value of 0.12 (data shown in Table 4); those results came in the same direction of result obtained by Al-Omran et al. (2005) for the Riyadh region. Values of our findings for the KR indicate the suitability for studied water for agricultural purposes. Sodium percent (Na %) was used for evaluation of the relevance of irrigation water (Wilcox 1955). High percentage of Na+ with respect to total cations in irrigation water causes desperation of soils and impairing of soil permeability (Richards 1954), where 60% of (Na%) is the maximum value for irrigation water. The sodium percentage was calculated for the samples using the following equation:
All concentrations are presented in meq L−1. The Na% of the samples ranged from 8 to 152% with a mean of 45%. Wilcox (1955) has classified the water according to some parameters (for example, Na% and EC), and according to his classification diagrams, the values of our sample are classified as excellent to good and good to permissible classes. The relationship between Na% and EC of the studied samples is shown in Fig. 2, with about 76% of the samples falling in the classification class of good to permissible (based on Wilcox (1955) classification). This means that 76% of water samples can be safely used for irrigation purposes.
Another parameter used to assess the suitability of water for irrigation purposes is permeability index (PI). Doneen (1964) classified irrigation water based on the permeability index using the following equation:
where all concentration is in meq L−1.
Soil permeability is affected by long-term use of irrigation water with abundance concentration of CO3, HCO3, and Na. According to Doneen (1964), irrigation water is classified in three PI categories. Categories 1 and 2 are types of water suitable for irrigation with 75% or more of maximum permeability, while category 3, with 25% of maximum permeability, is unsuitable for irrigation purposes. On these bases, all of the well water in the oasis fall in categories 1 and 2, implying that the well water is suitable for irrigation purposes with 75% or more of maximum permeability (Domenico and Schwartz 1990) as shown in Fig. 3.
Classes of salinity and alkalinity hazard
In the present study, the US Salinity Laboratory’s diagram (Richards 1954) is used to evaluate the water samples in terms of salinity and sodium hazards (Fig. 4). The salinity and alkalinity hazard classes of the studied water samples were CIII-SI, CIII-SII, and CIV-SII. The results suggested that these water samples are considered high-salinity hazard and low- to medium-sodium hazard. The excessive amount of dissolved salts is a major problem for irrigation water in the oasis. This high-salinity hazard water cannot be used for irrigation of many crops in the oasis without any kind of treatments, such as desalinization, leaching requirements, mixing water with less salty water, good quality-treated sewage water, or salt-tolerant plants (Lauchli and Epstein 1990).
Hydrochemical aspects
Gibbs’ diagrams
The controlling mechanism of the water chemistry in terms of dissolved ions present in water as evaporation–crystallization dominance, rock–weathering dominance, and atmospheric–precipitation dominance can be assessed by plotting hydrochemical data according to the variation in the ratios of Na+/(Na+ + Ca2+) and Cl−/(Cl− + HCO3−) as function of TDS (Gibbs 1970). The Gibbs plotting (Fig. 5) of chemical analysis data of the groundwater sample points of the studied area was mainly around the chemical weathering of rock-forming mineral zone and evaporation; therefore, it indicated that the water quality of these water was mainly controlled by weathering and evaporation. Evaporation increases the salinity by increasing sodium and chlorine and, thus, increasing total dissolved solids. The rock–water interaction is the main source of ion solution to control groundwater chemistry. It includes the process of interaction between the water–rock chemical weathering of rocks and the dissolution of the precipitation of secondary carbonates. Evaporation greatly increases the concentration of ions formed by chemical weathering, resulting in the increase of the salinity. The existing groundwater sampling points in the Gibbs diagram in the evaporation domain suggest an increase of Na and Cl ions and consequent higher water salinity due to the use of drainage water, high application of fertilizers, and sewage-treated water in the region.
In the Schoeller diagram (Fig. 6), there is a predominance of chlorine, magnesium, calcium, and sodium which influences the tendencies towards the chloride/sodium–magnesium–calcium facies.
Piper’s diagram
Cations and anions of groundwater samples in the Al-Hasa area were plotted on a Piper tri-linear diagram (Piper 1944) (Fig. 7). This scheme provides a convenient way to classify and identify the groundwater type, based on the cation and anion composition of different water samples (Aly and Benaabidate 2010; Semerjian 2011; Baba et al. 2008). Most of the well water samples (96%) have Ca2+, Mg2+, SO42−, and Cl− water types, and the remaining (4%) is Na+ and Cl− water type in the groundwater of the oasis.
Durov’s diagram
The chemical data of the well water samples in the Al-Hasa oasis are plotted on a Durov diagram (Fig. 8). Durov’s diagram helps the interpretation of the hydrochemical processes occurring in the groundwater system and can indicate the mixing of different water types and ion exchanges. The Durov diagram revealed that most of the water samples fall in fields 4 and 5, the zones of high-water salinity. The well water samples belonging to field 4 suggests the presence of SO4 and Ca2+ as dominant types of water, indicating gypsum-bearing sedimentary aquifer and that the groundwater is affected by oxidation of pyrite and other sulfide minerals. Samples located in zone 5 indicate that the mixing processes of two or more facies might exist and these finds are in agreement with Aly et al. (2016) for the Riyadh region.
Evaluation quality of water for drinking purposes
The statistical analysis of the 50 wells in the Al-Hasa oasis was done to identify the parameters that assess the drinking water quality of the wells in the oasis (deviating from the WHO drinking water standard) (Table 3). It is clear that the mean for most parameter tested is exceeding the acceptable limits of the drinking water standard used, such as the TDS, Mg, Ca, HCO3, Cl, and NO3. The calculated WQI values for the well waters in the Al-Hasa oasis ranged from 120 to 429 (Fig. 9), showing that 38% of the studied wells were considered poor water (class III), 52% are very poor water class (IV), and 10% are unsuitable water for drinking class (V). The reasons for the high WQI values obtained for this study area were high values of TDS, Mg2−, Ca2, HCO3 −, Cl−, and NO3−. A very high correlation coefficient between these values was also reported by Aly et al. (2016) and Deshpande and Aher (2012). The main reason of high nitrate concentrations in all water may be due to over application of fertilizer on the agricultural land in the oasis (Aly et al. 2016).
Conclusions
In this study, 50 well water samples from the Al-Hasa oasis, east of Saudi Arabia, were evaluated to assess the suitability of groundwater for irrigation and drinking purposes and its geochemistry. The results revealed that the studied wells are considered saline hazard water with no sodium hazards as indicated by the sodium adsorption ratio (SAR), Kelly’s ratio (KR), and permeability index (PI). The results of the SAR values have ranged from 0.79 to 10 with the mean of 3.86. All values were lower than 11. The samples can be classified as excellent water for irrigation. The hydrochemical analysis concluded that the well waters of the oasis correspond mainly to Mg–Ca/SO4–Cl water type. To calculate WQI for drinking purposes, ten parameters were taken into account, such as pH, soluble solids, calcium, magnesium, sodium, potassium, bicarbonate, chloride, sulfate, and nitrate. The results show that 38% of the studied wells are considered poor water class (III), 52% are very poor water class (IV), and 10% are unsuitable water for drinking class (V).
References
Abdel-Aal SI, Sabrah RE, Rabie RK, Abdel-Magid HM (1997) Evaluation of groundwater quality of irrigation in central Saudi Arabia, Arab Gulf. J Sci Res 15:361–377
Al Tokhais AS Rausch R (2008) The hydrology of Al Hassa springs. The 3rd international conference on water resources and arid environment, Riyadh, Saudi Arabia
Al-Hadithi M (2012) Application of water quality index to asses suitability of groundwater quality for drinking purposes in Ratmao-Pathri Raso watershed, Haridwar District, India. Am J Sci Ind Res. https://doi.org/10.5251/ajsir.s012.3.6.395.402
Al-Omran AM, Falatah AM, Al-Matrood SS (2005) Evaluation of irrigation well water quality in Riyadh region, Saudi Arabia. J King Abdulaziz Univ 16(2):23–40
Al-Omran MA, El-Maghraby SE, Aly AA, Al-Wabel IM, Al-Asmari ZA, Nadeem ME (2013) Quality assessment of various bottled waters marketed in Saudi Arabia. Environ Monit Assess 185(8):6397–6406. https://doi.org/10.1007/s10661-012-3032-z
Al-Othman A (2015) Evaluation of the suitability of surface water from Riyadh Mainstream Saudi Arabia for a variety of uses. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.01.001
Al-Salamah IS, Ghazaw YM, Ghumman AR (2011) Groundwater modeling of Saq Aquifer in Buraydah, Al Qassim for better water management strategies. Environ Monit Assess 173(1-4):851–860. https://doi.org/10.1007/s10661-010-1428-1
Al-Sayari SS, Zötl JG (Eds.) (2012) Quaternary period in Saudi Arabia: 1: sedimentological, hydrogeological, hydrochemical, geomorphological, and climatological investigations in central and eastern Saudi Arabia. Springer Science & Business Media
Aly AA, Benaabidate L (2010) Salinity of water resources in the Siwa Oasis: monitoring and diagnosis. In: Brikle, Torres A (eds) Water–rock interaction. Taylor & Francis Group, London ISBN 978-0-415-60426-0
Aly AA, Al-Omran AM, Alwabel M, Almahaini A, Alamari M (2016) Hydrochemical and quality of water resources in Saudi Arabia groundwater: a comparative study of Riyadh and Al-Hasa regions. Proc Int Acad Ecol Environ Sci 3(1):42–51
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington DC
Appelo CAJ, Postma D (1996) Geochemistry, groundwater and pollution. A.A. Balkema, Rotterdam
AquaChem (2014) A professional application for water quality data analysis, plotting, reporting, and modeling, p 390
Ayers RS, Westcot DW (1985) Water quality for agriculture. Irrigation and drainage paper 29 FAO, Rome
Baba A, Erees FS, Hicsonmez U, Cam S, Ozdilek HG (2008) An assessment of the quality of various bottled mineral water marketed in Turkey. Environ Monit Assess 139(1-3):277–285. https://doi.org/10.1007/s10661-007-9833-9
Backman B, Bodis D, Lahermo P, Rapant S, Tarvainen T (1998) Application of a groundwater contamination index in Finland and Slovakia. Environ Geol 36(1–2):55–64. https://doi.org/10.1007/s002540050320
Chapman RW (1974) Calcareous duricrust in Al-Hasa, Saudi Arabia. Geol Soc Am Bull 85(1):119–130. https://doi.org/10.1130/0016-7606(1974)85<119:CDIASA>2.0.CO;2
Deshpande SM, Aher KR (2012). Evaluation of groundwater quality and its suitability for drinking and agriculture use in parts of Vaijapur, District Aurangabad, MS, India. Jour Chem Sci 2(1):25–31.
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology, 2nd edn. Wiley, New York
Doneen LD (1964) Notes on water quality in agriculture. Department of Water Sciences and Engineering University of California Davis, Water science and Engineering
Duncan RR, Carrow RN, Huck M (2000) Understanding water quality and guidelines management (an overview of challenges for water usage on golf courses for the 21 century). USGA Green Section Record, 14–24
El-Sayed MH, Abo El-Fadl MM, Shawky HA (2012) Impact of hydrochemical processes on groundwater quality, Wadi Feiran, South Sinai, Egypt. Aust J Basic Appl Sci 6(3):638654
General Authority for Statistics (2015) https://www.stats.gov.sa/en
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Science 17:1088–1090
Golterman HL (1969) Methods for chemical analysis of fresh waters. ERIC Number: ED064114
Horton RK (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–305
Karanth KR (1987) Ground water assessment, development and management. Tata McGraw Hill Publishing Company Ltd., New Delhi, p 720
Kumar M, Ramanathan AL, Rao MS, Kumar B (2006) Identification and evaluation of hydrogeochemical processes in the groundwater environment of Delhi, India. J Environ Geol 50(7):1025–1039. https://doi.org/10.1007/s00254-006-0275-4
Kumar M, Kumari K, Singh UK, Ramanathan AL (2009) Hydrogeochemical processes in the groundwater environment of Muktsar, Punjab: conventional graphical and multivariate statistical approach. Environ Geol 57(4):873–884. https://doi.org/10.1007/s00254-008-1367-0
Kumar SK, Logeshkumaran A, Magesh NS, Godson NS, Chandrasekar N (2015) Hydro-geochemistry and application of water quality index (WQI) for groundwater quality assessment, Anna Nagar, part of Chennai City, Tamil Nadu, India. Appl Water Sci 5(4):335–343. https://doi.org/10.1007/s13201-014-0196-4
Kumar SS, Marender B, Dhakate R, Sankaran S, Kumar KR (2016) Assessment of groundwater quality for drinking and irrigation use in shallow hard rock aquifer of Pudunagaram, Palakkad District, Kerala. Appl Water Sci 6(2):149–167. https://doi.org/10.1007/s13201-014-0214-6
Lateef KH (2011) Evaluation of groundwater quality for drinking purpose for Tikrit and Samarra cities using water quality index. Eur J Sci Res 58(4):472–481
Lauchli A Epstein E (1990) Plant responses to saline and sodic conditions. In: Tanji KK (ed) Agricultural salinity assessment and management ASCE New York ASCE manuals and reports on engineering practice no 71:113–137
Ledesma-Ruiz R, Pastén-Zapata E, Parra R, Harter T, Mahlknecht J (2014) Investigation of the geochemical evolution of groundwater under agricultural land: a case study in northeastern Mexico. J Hydrol 521:410–423. https://doi.org/10.1016/j.jhydrol.2014.12.026
Matter A, Mahjoub A, Neubert E, Preusser F, Schwalb A, Szidat S, Wulf G (2016) Reactivation of the Pleistocene trans-Arabian Wadi ad Dawasir fluvial system (Saudi Arabia) during the Holocene humid phase. Geomorphology 270:88–101. https://doi.org/10.1016/j.geomorph.2016.07.013
MAW (Ministry of Agriculture and Water) (1984) Water atlas of Saudi Arabia. MAW, Riyadh
McKee JE, Wolf HW (1963) Water quality criteria. The Resource Agency of California. 2nd Ed. State Water Quality Control Board. Public no. 3A
MOWE (Ministry of Water and Electricity) (2007) The annual report from ministry of water and electricity. Riyadh, Saudi Arabia.
Piper AM (1944) A graphic procedure in the geochemical interpretation of water analysis. Trans Am Geophys Union 25:914–928 of the A.S.C.F, 607
Ramakrishnalah CR, Sadas hivalah C, Ranganna G (2009) Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka state, India. E-J Chem 6(2):523–530. https://doi.org/10.1155/2009/757424
Richards LA (1954) Diagnosis and improvement of saline and alkali soils. USDA hand book, no. 60, 160
Rokbani K, Mouna MG, Rachida B (2011) Use of geographical information system and water quality index to assess groundwater quality in El Khairat Deep Aquifer (Enfidha, Tunisian Sahel). Iran J Energy Environ 2(2):133–144
Saeedi M, Abessi O, Sharifi F, Meraji H (2010) Development of groundwater quality index. Environ Monit Assess 163(1-4):327–335. https://doi.org/10.1007/s10661-009-0837-5
Semerjian LA (2011) Quality assessment of various bottled waters marketed in Lebanon. Environ Monit Assess 172(1-4):275–285. https://doi.org/10.1007/s10661-010-1333-7
Sharaf MA, Hussein MT (1997) Groundwater quality in the Saq aquifer, Saudi Arabia. Hydrological Sciences-Journal-des Sciences Hydrologiques 4(5):683–696
Sharifi Z, Safari Sinegani AA (2012) Arsenic and other irrigation water quality indicators of groundwater in an agricultural area of Qorveh Plain, Kurdistan, Iran. Am Eurasian J Agric Environ Sci 12(4):548–555
Singh AK, Beenu R, Tiwari AK, Mahato MK (2013) Evaluation of hydrogeochemical processes and groundwater quality in the Jhansi district of Bundelkhand region, India. Environ Earth Sci 70(3):1225–1247
Stigter TY, Ribeiro L, Carvalho Dill AM (2006) Application of a groundwater quality index as an assessment and communication tool in agro-environmental policies—two Portuguese case studies. J Hydrol 327(3-4):578–591. https://doi.org/10.1016/j.jhydrol.2005.12.001
Tiwari AK, Singh AK (2014) Hydrogeochemical investigation and groundwater quality assessment of Pratapgarh district, Uttar Pradesh. J Geol Soc India 83(3):329–343. https://doi.org/10.1007/s12594-014-0045-y
Tiwari AK, Singh PK, Mahato MK (2014) GIS-based evaluation of water quality index of groundwater resources in West Bokaro Coalfield, India. Curr World Environ 9(3):843–850. https://doi.org/10.12944/CWE.9.3.35
Tiwari AK, Singh AK, Singh AK, Singh MP (2015) Hydrogeochemical analysis and evaluation of surface water quality of Pratapgarh District, Uttar Pradesh, India. Appl Water Sci 7(4):1609–1623. https://doi.org/10.1007/s13201-015-0313-z
Todd DK, Mays LW (2005) Groundwater hydrology, 3rd edn. Wiley, Hoboken, p 656
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization
Wilcox LV (1955) Classification and use of irrigation waters. US Department of Agriculture, Circular 969, Washington, DC, USA
Zagatto PA, Lorenzetti ML, Perez LS, Menegon JR, Buratini SV (1998) Proposal for a new water quality index. Verhandlundgen der Int Vereinigung f¨ur Theoretische und angewandte Limnol 26:2449–2451
Acknowledgments
The authors are grateful to the Deanship of the Scientific Research, King Saud University and the Agricultural Research Center, College of Food and Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Al-Omran, A.M., Mousa, M.A., AlHarbi, M.M. et al. Hydrogeochemical characterization and groundwater quality assessment in Al-Hasa, Saudi Arabia. Arab J Geosci 11, 79 (2018). https://doi.org/10.1007/s12517-018-3420-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-018-3420-y